低品位钼精矿钙化焙烧的反应机理
来源期刊:中国有色金属学报(英文版)2016年第11期
论文作者:甘敏 范晓慧 陈许玲 吴程骞 季志云 王送荣 汪国靖 邱冠周 姜涛
文章页码:3015 - 3023
关键词:钼精矿;钙化焙烧;反应机理;热力学研究;相变
Key words:molybdenum concentrate; calcification roasting; reaction mechanism; thermodynamic analysis; phase transformation
摘 要:研究了钙基添加剂对低品位钼精矿焙烧性能的影响。结果表明,钙基添加剂可与钼精矿反应生成CaSO4和CaMoO4。450 °C时MoS2开始氧化,500 °C以上生成CaMoO4和CaSO4,600~650 °C时钙化反应基本完成;进一步提高焙烧温度有利于CaMoO4的生成,但会降低焙烧过程固硫率和钼保留率。钙基添加剂焙烧效果依次为Ca(OH)2>CaO>CaCO3。随着Ca(OH)2用量的增加,钼保留率和固硫率均呈上升趋势,但过多的钙基添加剂会使酸浸过程硫酸的消耗增加,Ca(OH)2与钼精矿适宜的质量比为1:1。在650 °C下焙烧90 min时,低品位钼精矿钙化焙烧过程中钼保留率为100%、固硫率为92.92%,经硫酸浸出后钼的浸出率达到99.12%。
Abstract: The effects of Ca-based additives on roasting properties of low-grade molybdenum concentrate were studied. The results show that calcium-based additives can react with molybdenum concentrate to form CaSO4 and CaMoO4. The initial oxidation temperature of MoS2 is 450 °C, while the formation of CaMoO4 and CaSO4 occurs above 500 °C. The whole calcification reactions are nearly completed between 600 and 650 °C. However, raising the temperature further helps for the formation of CaMoO4 but is disadvantageous to sulfur fixing rate and molybdenum retention rate. Calcification efficiency of Ca-based additives follows the order: Ca(OH)2>CaO>CaCO3. With increasing the dosage of Ca(OH)2, the molybdenum retention rate and sulfur-fixing rate rise, but excessive dosages would consume more acid during leaching process. The appropriate mass ratio of Ca(OH)2 to molybdenum concentrate is 1:1. When roasted at 650 °C for 90 min, the molybdenum retention rate and the sulfur-fixing rate of low-grade molybdenum concentrate reach 100% and 92.92%, respectively, and the dissolution rate of molybdenum achieves 99.12% with calcines being leached by sulphuric acid.
Trans. Nonferrous Met. Soc. China 26(2016) 3015-3023
Min GAN, Xiao-hui FAN, Xu-ling CHEN, Cheng-qian WU, Zhi-yun JI, Song-rong WANG, Guo-jing WANG, Guan-zhou QIU, Tao JIANG
School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
Received 29 October 2015; accepted 10 March 2016
Abstract: The effects of Ca-based additives on roasting properties of low-grade molybdenum concentrate were studied. The results show that calcium-based additives can react with molybdenum concentrate to form CaSO4 and CaMoO4. The initial oxidation temperature of MoS2 is 450 °C, while the formation of CaMoO4 and CaSO4 occurs above 500 °C. The whole calcification reactions are nearly completed between 600 and 650 °C. However, raising the temperature further helps for the formation of CaMoO4 but is disadvantageous to sulfur fixing rate and molybdenum retention rate. Calcification efficiency of Ca-based additives follows the order: Ca(OH)2>CaO>CaCO3. With increasing the dosage of Ca(OH)2, the molybdenum retention rate and sulfur-fixing rate rise, but excessive dosages would consume more acid during leaching process. The appropriate mass ratio of Ca(OH)2 to molybdenum concentrate is 1:1. When roasted at 650 °C for 90 min, the molybdenum retention rate and the sulfur-fixing rate of low-grade molybdenum concentrate reach 100% and 92.92%, respectively, and the dissolution rate of molybdenum achieves 99.12% with calcines being leached by sulphuric acid.
Key words: molybdenum concentrate; calcification roasting; reaction mechanism; thermodynamic analysis; phase transformation
1 Introduction
Molybdenum concentrate is commonly extracted after MoS2 is oxidized to high-valence molybdenum [1]. Two kinds of oxygenolysis processes for molybdenum concentrates are usually applied. One is pyrometallurgy, the other refers to hydrometallurgy which has been developed since 1970s [2]. Pyrometallurgy includes the process of oxidizing roasting, alkali fusing method and additive roasting [3]. In China, oxidizing roasting process is the most widely applied process, which commonly roasts molybdenum concentrates in rotary kiln, multiple hearth furnace, flash furnace etc. However, for low-grade molybdenum concentrate with abundant impurities such as calcium, magnesium, copper, iron and lead, low-melting-point compounds can easily be generated during oxidization roasting, which will lead to agglomeration and even restrict the thorough oxidization of MoS2. Meanwhile, molybdates that are indissolvable in ammonia and further impede the recovery of molybdenum are easy to form. In addition, the low SO2 concentration in roasting flue gas makes its recovery costly and causes environmental pollution [4]. Hydrometallurgical processing refers to oxygen pressure process [5-7], nitric acid decomposition [8,9], sodium hypochlorite oxidation [10,11], electro-oxidation [12,13] and bioleaching method [14,15]. Although hydrometallurgy has the advantage of solving SO2 emission problem, lacking low-cost oxidant makes it difficult to solve the problem thoroughly. Meanwhile, some impurities like metal compounds are easy to dissolve, which increases the difficulties of the follow-up purification. As a result, the high cost of leaching and purification restricts the development of hydrometallurgy to process low-grade molybdenum concentrates mostly [16,17].
Consequently, the key point of low-grade molybdenum concentrate utilization lies in improving the oxidation efficiency of molybdenum and resolving SO2 pollution problem. Benefiting from the effects of calcium-based additives during roasting, calcification roasting is able to fix sulfur and inhibit the volatilization of MoO3 together with SO2, and the generated CaMoO4 can be fully dissolved in acid. This method is prospective to solve current problems in oxidizing roasting and hydrometallurgical processing. So, the selection of additives for calcification roasting was investigated in this work, and the mechanisms of sulfur fixing and MoS2 oxidization during calcification roasting were revealed, based on which a novel method for effectively handling low-grade molybdenum concentrate was provided.
2 Experimental
2.1 Materials
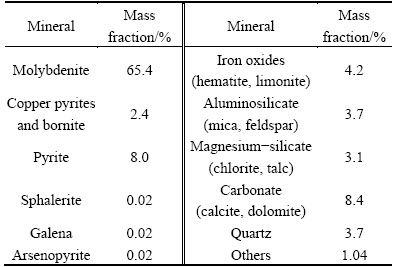
The chemical composition of molybdenum concentrate is shown in Table 1. The molybdenum content is 39.27%, which is lower than that of standard-molybdenum concentrate with Mo content above 45%. The impurity substances mainly consist of SiO2, CaO, MgO, and iron-containing minerals. According to the mineral compositions of molybdenum concentrate (Table 2), the major component is molybdenite, which takes up 65.4%, followed by sulfide ores including copper pyrites, bornite and iron pyrite. There are also iron oxides mainly consisting of limonite and hematite, silicon gangue minerals composed of aluminosilicate, magnesium-silicate and quartz, and carbonates composed of calcite and dolomite.
Table 1 Chemical composition of molybdenum concentrate (mass fraction, %)
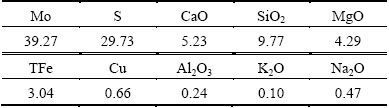
Table 2 Mineral compositions of molybdenum concentrate
The mineral dissemination characteristics of molybdenum concentrate are investigated by optical microscope, as can be seen in Fig. 1. Molybdenite mainly appears as monomer particle with sizes of 0.02-0.20 mm, which are scale-like, slab-like, strip and irregular particles. Few molybdenite particles are interlocked by pyrites otherwise coated by large particles of copper pyrites, which indicates that molybdenite and other sulfide ores are embedded closely. Most of calcite, quartz and silicate have been dissociated from molybdenite.
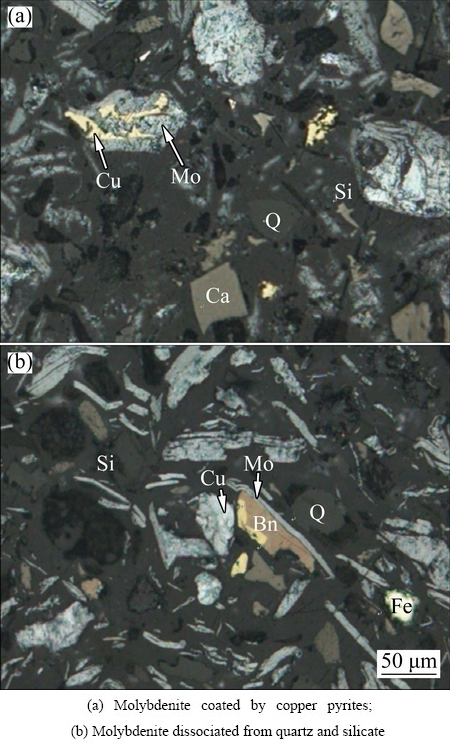
Fig. 1 Dissemination characteristics of main minerals in molybdenum concentrate (Mo—Molybdenite; Cu—Copper pyrite; Bn—Bornite; Ca—Calcite; Q—Quartz; Fe—Pyrite; Si—Silicate)
2.2 Methods and evaluation indicators
The flow diagram of experiments, which simulated the oxidation process of molybdenum concentrate, is depicted in Fig. 2. The raw material was dried at 70 °C in the oven till its mass remained unchanged. 50 g molybdenum concentrates and moderate quantity of calcium-based additives were weighed to be mixed at a certain proportion. Calcium-based additives applied in the experiments included CaCO3, CaO and Ca(OH)2, which were analytical reagents.
Molybdenum concentrates and calcium-based additives were mixed thoroughly, and then roasted in a muffle furnace. After being roasted for a prescribed time, the molybdenum calcine was cooled in inert atmosphere. The phases and microstructures in roasted products were studied by using the modern microcosmic detecting equipments such as X-ray diffraction (XRD) and scanning electronic microscope (SEM). XRD measurements were performed on the samples with a powder diffractometer(Rigaku D/M4X 2500) using a Cu Kα radiation. The range of scanning angle (2θ) was from 5° to 100° and the scanning speed was 0.2156 (°)/s. SEM (JEOL JSM-5600) with energy dispersive X-ray (EDX) was used to analyze particle morphology and major chemical distribution in typical minerals.
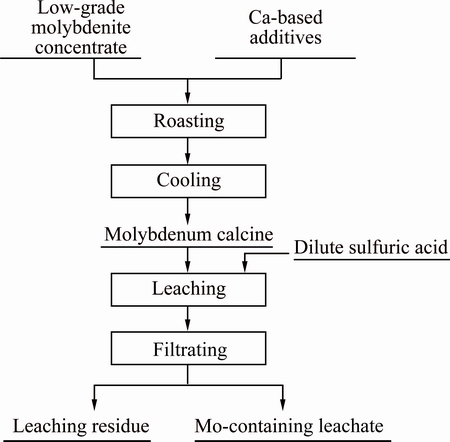
Fig. 2 Flow diagram of calcification roasting-acid leaching
Roasted products were ground into 0.074 mm with mass fraction above 80%, and then samples were obtained for examining the contents of molybdenum and sulfur. Another 5 g calcine was added into flasks containing dilute sulfuric acid for leaching molybdenum. The acid leaching conditions were set up as follows: sulfuric acid concentration of 70 g/L, temperature of 90 °C, time of 2 h, and liquid-solid ratio of 5:1. After leaching, the suspended solution was filtrated using a vacuum filter, and then the content of molybdenum in the residue was analyzed.
The evaluation indicators of roasting-acid leaching process mainly contain the leaching rate of molybdenum (αMo), the retention rate of molybdenum (ηMo) and the sulfur-fixing rate βS, which can be calculated as follows:
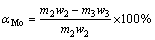 (1)
(1)
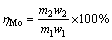 (2)
(2)
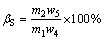 (3)
(3)
where m1, m2 and m3 stand for the mass of molybenite concentrate, molybdenum calcine and leaching residue, respectively, g; w1, w2 and w3 stand for the mass fractions of molybdenum in molybenite concentrate, molybdenum calcine and leaching residue, respectively, %; w4 and w5 stand for the mass fractions of sulfur in molybenite concentrate and molybdenum calcine, respectively, %.
3 Results and discussion
3.1 Thermodynamics of calcification roasting process
During the roasting process of molybdenum concentrate, the reactions of MoS2 oxidation, calcium molybdate generation and sulfur-fixing reaction occur potentially. And the oxidation of MoS2 is shown as: (2/7)MoS2 + O2(g) = (2/7)MoO3 + (4/7)SO2 (g), 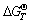 = -300.83 + 0.05T (kJ/mol). When the temperature is lower than 6000 K, MoS2 oxidation reaction is spontaneous because of
= -300.83 + 0.05T (kJ/mol). When the temperature is lower than 6000 K, MoS2 oxidation reaction is spontaneous because of 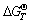 <0. As calcium-based additives were mixed with molybdenum concentrate, calcium-based additives can react with the oxidative products of MoS2 like MoO3 and SO2. The thermodynamic conditions of the formation reactions of molybdate and the sulfation reactions are shown in Fig. 3. If the temperature is below 798 K, the easy tendencies of different calcium-based additives reacting with MoO3 and SO2 to generate CaMoO4 and CaSO4 can be ranked as CaO>Ca(OH)2> CaCO3. While at the temperature of above 798 K, the order changes to be Ca(OH)2> CaO>CaCO3. As can be observed from the thermodynamic curves, CaO, MgO, FeO and CuO react with SO2 below 1150 K, and the products are CaSO4, MgSO4, FeSO4 and CuSO4, respectively. However, the reaction tendency decreases successively. CuSO4 is resolved into CuO and SO2 above 1150 K, and FeSO4 will be decomposed above 1300 K. But CaSO4 and MgSO4 are only decomposed at high temperatures, which proves that sulfur-fixing reactions are qualified to proceed during the calcification roasting process.
<0. As calcium-based additives were mixed with molybdenum concentrate, calcium-based additives can react with the oxidative products of MoS2 like MoO3 and SO2. The thermodynamic conditions of the formation reactions of molybdate and the sulfation reactions are shown in Fig. 3. If the temperature is below 798 K, the easy tendencies of different calcium-based additives reacting with MoO3 and SO2 to generate CaMoO4 and CaSO4 can be ranked as CaO>Ca(OH)2> CaCO3. While at the temperature of above 798 K, the order changes to be Ca(OH)2> CaO>CaCO3. As can be observed from the thermodynamic curves, CaO, MgO, FeO and CuO react with SO2 below 1150 K, and the products are CaSO4, MgSO4, FeSO4 and CuSO4, respectively. However, the reaction tendency decreases successively. CuSO4 is resolved into CuO and SO2 above 1150 K, and FeSO4 will be decomposed above 1300 K. But CaSO4 and MgSO4 are only decomposed at high temperatures, which proves that sulfur-fixing reactions are qualified to proceed during the calcification roasting process.
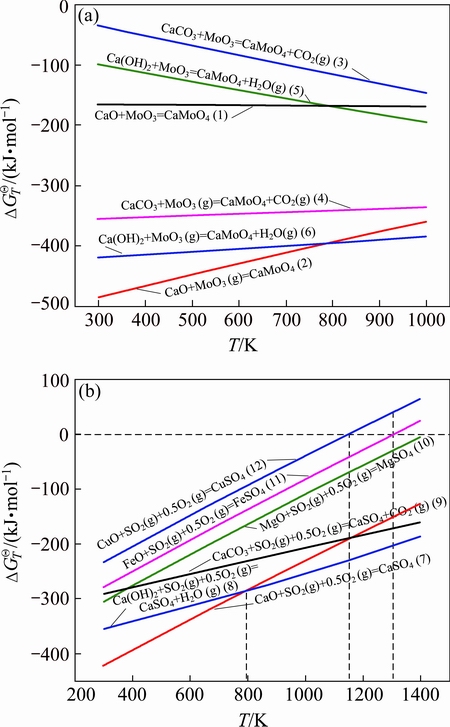
Fig. 3 Relationship between  -T for formation of molybdate and sulfate
-T for formation of molybdate and sulfate
Although there are differences in reactive ability of three calcium-based additives, the formation of molybdate and the sulfation reaction occur spontaneously in the temperature range of 400-1300 K. Therefore, the oxidation and calcification reactions are carried out simultaneously according to thermodynamic analysis.
3.2 Effects of calcium-based additives on roasting
As adding calcium-based additives with their theoretical quantities (theoretical amount means that molybdenum and sulfur convert to CaMoO4 and CaSO4 completely, and theoretical amounts of CaO, Ca(OH)2 and CaCO3 are the mass ratios of 0.64:1, 0.84:1 and 1.13:1 compared with molybdenum concentrate), the effects of roasting temperature on the roasting-leaching process of molybdenum concentrate at the roasting time of 90 min are given in Fig. 4. With temperature rising, the retention rate of molybdenum decreases gradually. Especially when the temperature increases to above 750 °C, the retention rate of molybdenum is observed to decrease apparently. The reason is that MoS2 is oxidized to MoO3 rapidly and part of MoO3 volatilized before reacting with calcium-based additives at high temperatures. The loss of molybdenum is relatively small below 700 °C. Using Ca(OH)2 achieves the highest retention rate of molybdenum, which nearly reaches 100% at 650 °C. Differences in the sulfur-fixing rate of these additives are studied, adsorption efficiency firstly increases and then decreases with the temperature rising. According to its effects, the sulfur-fixing rate of different additives can be ranked as Ca(OH)2>CaO>CaCO3, and the sulfur-fixing rate achieves the maximum at 650-700 °C. However, the sulfur-fixing rate is lower when using theoretic dosage of additives. During the experiments of leaching molybdenite by acid, the leaching rate increases with the rise of roasting temperature, but then the leaching rate increases at a slower pace above 700 °C. Taking the effects of three additives on retention rate of molybdenum, sulfur-fixing rate and leaching rate of molybdenum into consideration, 650 °C is suitable for roasting.
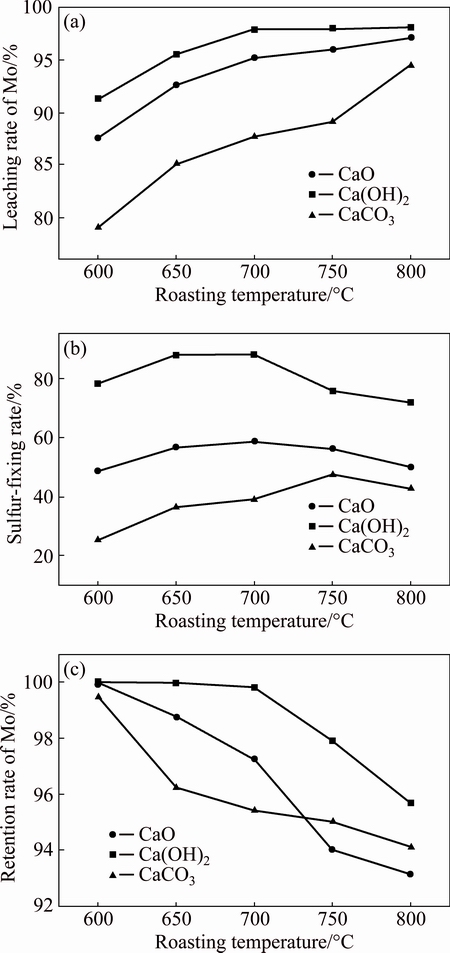
Fig. 4 Effects of roasting temperature on calcification roasting and acid leaching
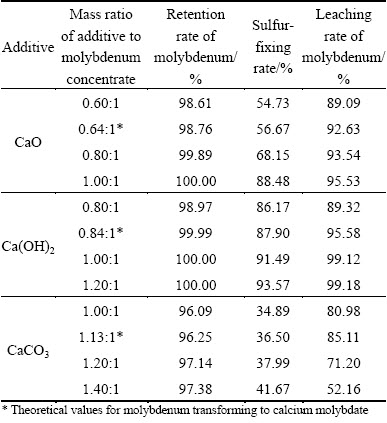
Figure 4 shows that the sulfur-fixing rate stays at a low level when using the theoretic dosage of additives, thus it is necessary to add more additives. When roasting molybdenum concentrate at 650 °C for 90 min, the effects of different additives on roasting-leaching rate are studied, and the results are shown in Table 3. On one hand, with increasing the dosage of Ca(OH)2 or CaO, the retention rate and the leaching rate of molybdenum and the sulfur-fixing rate tend to increase; on the other hand, increasing the dosage of CaCO3 improves both the retention rate of molybdenum and the sulfur-fixing rate, while the leaching rate of molybdenum increases firstly and then decreases for the reason that excessive CaCO3 consumes more acid, which leads to lower leaching rate of molybdenum.
According to the retention rates and the leaching rates of molybdenum, it can be concluded that all of the calcium-based additives can react with MoO3 to generate CaMoO4, and the reaction ability can be ranked as Ca(OH)2>CaO>CaCO3. From the sulfur-fixing rate, it is believed that the sulfur-fixing efficiencies of Ca(OH)2 and CaO are better than that of CaCO3, and the sulfur-fixing efficiencies of Ca(OH)2 are better than that of CaO. The sulfur-fixing rate with the addition of CaCO3 is only 41.67% even though the dosage of CaCO3 is 1.4 times of molybdenum concentrate, which indicates that the sulfur-fixing efficiency of CaCO3 is inferior to that of other additives. When the mass ratio of Ca(OH)2 to MoO3 is 1:1, the retention rate of molybdenum reaches 100%, the sulfur-fixing rate attains 92.92%, and the leaching rate of molybdenum is 99.12%.
Table 3 Effects of dosages of calcium-based additives on calcification roasting and acid leaching
3.3 Phase evolution during calcification roasting process
In order to study the effects of additives on the particle morphology of calcine and the formation of products, energy dispersive X-ray spectrometric microanalyzer (EDS) with SEM is employed and the scanning pictures are shown in Fig. 5. In this study, the roasting process of molybdenum concentrate is performed under conditions of adding additives at theoretic ratios and roasting at 650 °C for 90 min. With the addition of CaCO3, the original particle morphology is not changed after roasting, but some new phases appear on the surface and a few particles bond mildly. With the addition of CaO, crystalline transformation is observed mainly to be needle-like and granular, and other morphology of the complex interwoven structure is formed after sintering. With the addition of Ca(OH)2, two kinds of particles with different crystalline forms appear, one is developed from aggregated particles, and the other is small monomer bean shaped particles.

Fig. 5 SEM images of roasted products with different calcium- based additives
For revealing the types of products after roasting, the energy spectrum analyses of different zones in the calcine are applied, and the results are shown in Fig. 6. With the addition of Ca(OH)2, well-crystallized CaSO4 and CaMoO4 appear in Zones A and B in (Fig. 6(a)). With the addition of CaO, the sulfur-fixing rate is evaluated to be lower. According to the analyses of Zones C and D in Fig. 5(b), the main component is CaMoO4 and there are still a few of CaSO4 and CaO. With the addition of CaCO3, CaSO4 and CaMoO4 in roasted product are detected in Zones E and F, whose content of CaSO4 is less than that with the addition of CaO. Illustrated by the results of SEM, reactions run smoothly and well-crystallized particles of CaMoO4 and CaSO4 are formed with the addition of Ca(OH)2. In addition, the generation of CaMoO4 becomes relatively difficult and the sulfur-fixing rate decreases obviously during the roasting process with the addition of CaCO3 or CaO.
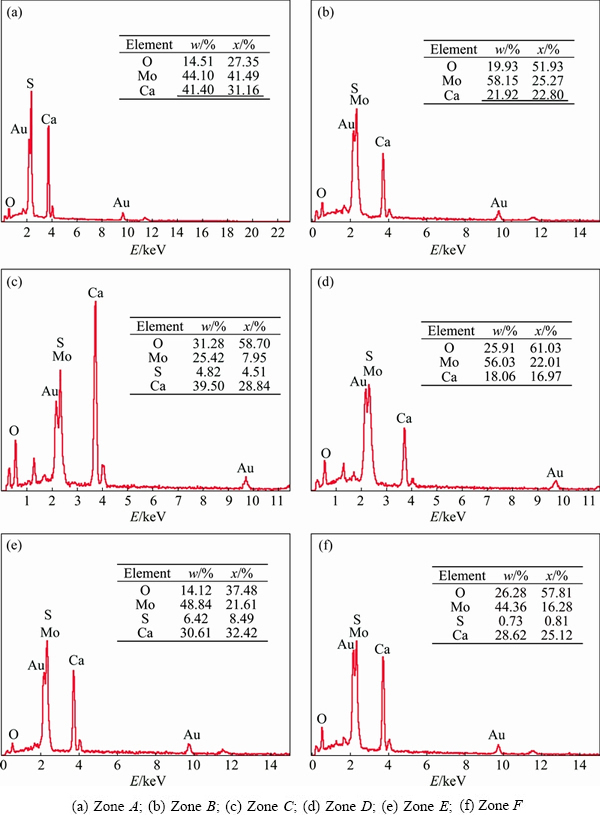
Fig. 6 SEM-EDAX patterns of different zones in Fig. 5 in calcine
The roasted products, which come from the molybdenum concentrate that is roasted at 650 °C for 90 min with theoretic quantities of additives, are investigated by means of XRD and the patterns are shown in Fig. 7, from which it can be seen that the common phases of the calcines are CaMoO4, CaSO4 and Mg3(Si4O10)(OH)2. The intensities of the CaSO4 diffraction peaks with different additives can be ranked as Ca(OH)2>CaO>CaCO3, which indicates that calcium- based additives are convinced to have the function on sulfur fixation. This agrees with the thermodynamic results.
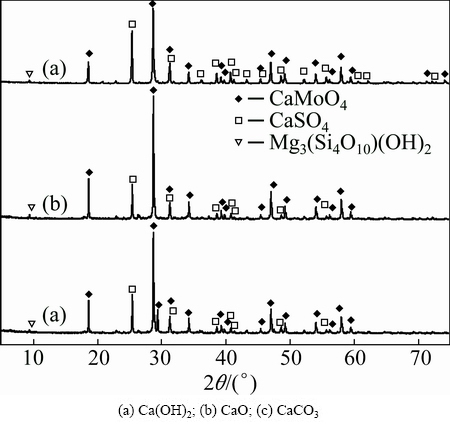
Fig. 7 XRD patterns of roasted products with different additives
The retention rates of molybdenum are 98.76%, 99.99% and 96.25% with the addition of CaO, Ca(OH)2 and CaCO3, respectively (Table 3). High retention rate of molybdenum indicates that the solid-solid reactions or gas-solid reactions between MoO3 or MoO3 (g) and additives can run smoothly. The sulfur-fixing rates being 56.67%, 87.90% and 36.50% respectively indicate that reactions between Ca(OH)2 and SO2 are able to proceed more sufficiently. The leaching rates of Mo in calcine are 92.63%, 95.58% and 85.11%, respectively. The phase analyses also sustain the fact that a large amount of calcium molybdate, which can be dissolved in acid, generates under the roasting temperature. Therefore, the most suitable calcium-based additive is Ca(OH)2.
Ca(OH)2 is the product of CaO hydration. Ca(OH)2 colloid particles are formed in the process of hydration, which makes larger specific surface area of Ca(OH)2 than that of CaO. Therefore, reactions are easily to proceed on the kinetics because of the larger contact area with molybdenum concentrate. Whereas, compared with CaCO3, thermodynamics trend of reaction between Ca(OH)2 and MoO3, SO2 is larger. Moreover, the decomposition temperature of CaCO3 is high, it starts to decompose at 530 °C and decompose acutely at 900 °C to form CaO. As a consequence, the calcification efficiency of adding Ca(OH)2 to roast molybdenum concentrate is the best.
Molybdenum concentrates with equivalent mass of Ca(OH)2 are roasted for 90 min at different temperatures, the phase compositions of the products are analyzed, and the patterns are shown in Fig. 8.
The molybdenum phase is mainly composed of MoS2 at about 400 °C, where the oxidation reaction does not start yet. When the temperature increases to 450 °C, the product of MoO3 appears through the oxidization of MoS2, and Ca(OH)2 begins to decompose where the diffraction peak of CaO appears. The height diffraction peaks of MoS2 and MoO3 drop significantly while the diffraction peaks of CaMoO4 and CaSO4 appear at 500 °C. It can be known that MoO3 and SO2, which derive from the oxidization of MoS2, begin to react with CaO. When the temperature is above 600 °C, the main diffraction peaks are those of CaMoO4 and CaSO4, and MoS2 is completely transformed into CaMoO4.
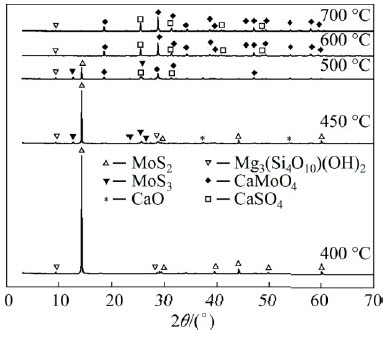
Fig. 8 XRD patterns of roasted products at different temperatures
The reaction process between molybdenum concentrate and Ca(OH)2 is further proved by thermal gravity analysis. According to the TG-DSC curves (Fig. 9), the evaporation of moisture and the volatilization of something volatile go on before 400 °C. When the temperature ranges from 400 to 470 °C, the oxidization of sulfide and the decomposition of Ca(OH)2 occur. When the temperature surpasses 470 °C, three exothermic peaks appear at 530.5, 574.4 and 640.9 °C, respectively, where the reaction speed of MoO3 oxidation and the generation speed of CaMoO4 and CaSO4 are the fastest. As it has been introduced in Figs. 8 and 9, the transformation process is as follows: Ca(OH)2 decomposition→MoS2 oxidation→molybdate and sulfate generation.

Fig. 9 Thermogravimetric curves of molybdenite concentrate and Ca(OH)2 in air
3.4 Calcification roasting-acid leaching process
The quality flow chart of calcium-based roasting and acid-leaching process is shown in Fig. 10. According to the analysis of the calcine and the leaching residue, which is roasted from the molybdenum concentrates with addition of equivalent mass of Ca(OH)2 at the roasting temperature of 650 °C for 90 min, the leaching conditions are set up as follows: concentration of sulfuric acid of 70 g/L, leaching at 90 °C for 120 min, ratio of liquid to solid 5:1. It can be concluded that molybdenum is hard to be lost or volatilized during the roasting process. The sulfur-fixing rate is 91.49%, which indicates that little sulfur is volatilized into the smoke as SO2, and it can be absorbed by alkali. After acid leaching, the surplus grade of molybdenum attains 0.2%, the leaching rate of molybdenum reaches 99.12%, and the total loss is only 0.88% in the whole process.
The phase compositions of calcine and the leaching residue are studied by XRD analysis as shown in Fig. 11.
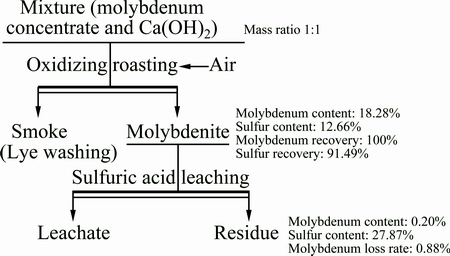
Fig. 10 Quality flow chart of of calcium-based roasting and acid leaching process
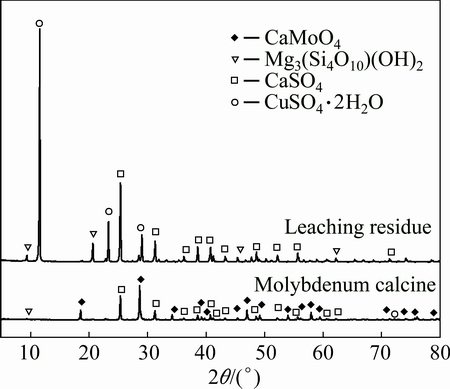
Fig. 11 XRD patterns of leaching residue obtained with acid leaching
MoS2 disappears after roasting, and the main phases of calcine are CaMoO4 and CaSO4. The characteristic peaks of CaMoO4 disappear, and the peaks of CaSO4 are markedly strengthened after acid leaching, indicating that calcium molybdate is completely dissolved. The main components of the leaching residue are CaSO4, gypsum (CaSO4·2H2O) and talc (Mg3(Si4O10)(OH)2). As a conclusion, molybdenum in low-grade molybdenum concentrate can be recycled completely by calcification roasting-acid leaching process.
4 Conclusions
1) During the roasting process of molybdenum concentrate with calcium-based additives, additives are able to react with MoO3 and SO2 to form CaMoO4 and CaSO4. Adding Ca(OH)2 can achieve the best roasting effects and sulfur-fixing rate, which is followed by CaO, and then CaCO3. The microstructure of calcine shows that the intact grains of CaMoO4 and CaSO4 are formed with the addition of Ca(OH)2, while adding CaO or CaCO3 is unable to achieve the above effect.
2) Higher roasting temperature contributes to the formation of CaMoO4 while it causes the decrease of both sulfur-fixing rate and retention rate of molybdenum. The phase evolution indicates that MoS2 starts to be oxidized at 450 °C, while CaMoO4 and CaSO4 start to form at 500 °C, and the main phase compositions of calcine are CaMoO4 and CaSO4 at temperatures of 600-650 °C, at which the calcification reaction is nearly completed.
3) With increasing the dosage of Ca(OH)2, the retention rate of molybdenum and the sulfur-fixing rate tend to grow with more acid consumed. The appropriate mass ratio of Ca(OH)2 to molybdenum concentrate is 1:1. By roasting the molybdenum concentrate at 650 °C for 90 min with appropriate dosage of Ca(OH)2, the retention rate of molybdenum during the roasting process achieves 100%, the sulfur-fixing rate attains 92.92%, and the leaching rate of molybdenum achieves 99.12%.
References
[1] GUPTA C K. Extractive metallurgy of molybdenum [M]. London: CRC Press, 1992: 15-30.
[2] JENNINGS P H, STANLEY R W, AMES H L. Development of a process for purifying molybdenum concentrate [C]//Proceedings of the second International Symposium on Hydrometallurgy. New York: AIME, 1974: 868-883.
[3] XIANG Tie-gen. Molybdenum metallurgy [M]. Changsha: Central South University Press, 2002: 1-20. (in Chinese)
[4] GAN Min, FAN Xiao-hui, ZHANG Lin. Reaction behavior of low grade molybdenum concentrates in oxidation roasting process [J]. The Chinese Journal of Nonferrous Metals, 2014, 24(12): 3315-3322. (in Chinese)
[5] PENG Jian-rong, YANG Da-jin, CHEN Jia-xi, YAN Jiang-feng. Experimental study on alkaline leaching of crude molybdenite under pressure of oxygen [J]. Chinese Journal of Rare Metals, 2007, 31(6): 110-113. (in Chinese)
[6] WANG Si-fu, WEI Chang, DENG Zhi-gan, LI Cun-xiong, LI Xin-bing, WU Jun, WANG Ming-shang, ZHANG Fan. Extraction of molybdenum and nickel from Ni-Mo ore by pressure acid leaching [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(10): 3083-3088.
[7] KETCHAM J. Pressure oxidation process for the production of molybdenum trioxide from molybdenite: United States, US6149883 [P]. 2000-11-21.
[8] ZHANG Qi-xiu, ZHAO Qin-sheng. Molybdenum tungsten metallurgy [M]. Beijing: Metallurgical Industry Press, 2005: 26-32. (in Chinese)
[9] MANOJ K, MANKHAND T R, MURTHY D S R, MUKHOPADHYAY R, PRASAD P M. Refining of a low-grade molybdenum concentrate [J]. Hydrometallurgy, 2007, 86(3): 56-62.
[10] WARREN I H, MOUNSEY D M. Factors influencing the selective leaching of molybdenum with sodium hypochlorite from copper/ molybdenum sulphide minerals [J]. Hydrometallurgy, 1983, 10(3): 343-357.
[11] ANTONIJEYIC M M, PACOVIC N V. Investigation of molybdenite oxidation by sodium dichromate [J]. Minerals Engineering, 1992, 5(2): 223-233.
[12] CAO Zhan-fang, ZHONG Hong, JIANG Tao, LIU Guang-yi, WANG Shuai. Selective electric-oxidation leaching and separation of Dexing molybdenum concentrates [J]. The Chinese Journal of Nonferrous Metals, 2013, 23(8): 2290-2295. (in Chinese)
[13] FU Jian-gang, ZHONG Hong, HUANG Yong-ping, PU Xiang-ming. Indirect electro-oxidation of molybdenite by Mn3+/Mn2+ [J]. Journal of Central South University (Science and Technology), 2004, 35(5): 797-801. (in Chinese)
[14] ROMANO P, BLAZQUEZ M L, ALGUACIL F J, MUNOZ J A, BALLESTER A, GONZALEZ F. Comparative study on the chalcopyrite bioleaching of a molybdenum concentrate with mesophilic and thermophilic bacteria [J]. FEMS Microbilology Letters, 2001, 196(1): 71-75.
[15] GREGORY J O, THOMAS R C. Bioleaching of molybdenite [J]. Hydrometallurgy, 2006, 82(3): 133-136.
[16] LI Hong-gui, HUO Guang-sheng, SUN Pei-mei, ZHAO Zhong-wei, LI Yun-jiao, SU Peng-tuan. Developing new reagent for selectively precipitation of molybdenum from tungstate solution [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(1): 184-187.
[17] CAO Zhan-fang, ZHONG Hong, LIU Guang-yi. Electric-oxidation kinetics of molybdenum concentrate in acidic NaCl solution [J]. The Canadian Journal of Chemical Engineering, 2009, 87(6): 939-944.
甘 敏,范晓慧,陈许玲,吴程骞,季志云,王送荣,汪国靖,邱冠周,姜 涛
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:研究了钙基添加剂对低品位钼精矿焙烧性能的影响。结果表明,钙基添加剂可与钼精矿反应生成CaSO4和CaMoO4。450 °C时MoS2开始氧化,500 °C以上生成CaMoO4和CaSO4,600~650 °C时钙化反应基本完成;进一步提高焙烧温度有利于CaMoO4的生成,但会降低焙烧过程固硫率和钼保留率。钙基添加剂焙烧效果依次为Ca(OH)2>CaO>CaCO3。随着Ca(OH)2用量的增加,钼保留率和固硫率均呈上升趋势,但过多的钙基添加剂会使酸浸过程硫酸的消耗增加,Ca(OH)2与钼精矿适宜的质量比为1:1。在650 °C下焙烧90 min时,低品位钼精矿钙化焙烧过程中钼保留率为100%、固硫率为92.92%,经硫酸浸出后钼的浸出率达到99.12%。
关键词:钼精矿;钙化焙烧;反应机理;热力学研究;相变
(Edited by Wei-ping CHEN)
Foundation item: Project (51304245) supported by the National Natural Science Foundation of China; Project (2014T70691) supported by the Postdoctoral Science Foundation of China; Project (2015CX005) supported by the Innovation Driven Plan of Central South University, China; Project supported by the Hunan Provincial Co-innovation Center for Clean and Efficient Utilization of Strategic Metal Mineral Resources, China
Corresponding author: Xiao-hui FAN; Tel: +86-13508480582; E-mail: csufanxiaohui@126.com
DOI: 10.1016/S1003-6326(16)64432-1

