Tb/Mg掺杂对羟基磷灰石纳米颗粒基因载体理化特性的影响
来源期刊:中国有色金属学报(英文版)2018年第1期
论文作者:陈良建 陈恬 曹君 刘蓓蕾 邵春生 周科朝 张斗
文章页码:125 - 136
关键词:羟基磷灰石纳米颗粒;基因载体;胞吞作用;掺杂;荧光标记
Key words:hydroxyapatite nanoparticles; gene vector; endocytosis; doping; fluorescence labeling
摘 要:羟基磷灰石纳米颗粒(HAnps)基因载体的转染效率与粒径、形貌、表面电荷、表面改性等有关。本研究通过水热合成法制备掺杂Tb/Mg的HAnps,观察Tb/Mg掺杂量对HAnps形貌、粒径、表面电荷、成分和细胞胞吞作用的影响。结果表明,掺Mg组的分散性优于掺Tb组的分散性。增加掺杂量使Tb-HAnps的粒径增大,而Mg-HAnps的粒径减小。掺Mg组的粒径及zeta电位均低于掺Tb组的。掺Mg量为7.5%的HAnps平均粒径约30 nm,呈相对均匀的细长杆状,而掺Mg量为10%的HAnps易于团聚。被胞吞入MG63细胞的Mg-HAnps-GFP呈点状分布于核周区域并形成荧光圈,而Tb-HAnps易于团聚。因此,作为基因载体,Mg-HAnps明显优于Tb-HAnps。
Abstract: Transfection efficiency of hydroxyapatite nanoparticles (HAnps) is relative to the particle size, morphology, surface charge, surface modifier and so on. This study prepared HAnps with doped Tb/Mg by hydrothermal synthesis method (HTSM) and investigated the effects of different Tb/Mg contents on the morphology, particle size, surface charge, composition and cellular endocytosis of HAnps. The results showed that Mg-HAnps possessed better dispersion ability than Tb-HAnps. With increasing doping content of Tb/Mg-HAnps, the granularity of Tb-HAnps increased, while that of Mg-HAnps declined. Both particle size and zeta potential of Mg-HAnps were lower than those of Tb-HAnps. 7.5% Mg-doping HAnps presented relatively uniform slender rod morphology with average size of 30 nm, while 10% Mg-doping HAnps were prone to agglomeration. Moreover, Mg-HAnps-GFP (green fluorescent protein) endocytosed by MG63 cells was dotted in the perinuclear region, while Tb-HAnps were more likely to aggregate. In conclusion, as gene vectors, Mg-HAnps showed enhanced properties compared to Tb-HAnps.
Trans. Nonferrous Met. Soc. China 28(2018) 125-136
Liang-jian CHEN1,2, Tian CHEN3, Jun CAO1, Bei-lei LIU1, Chun-sheng SHAO1, Ke-chao ZHOU2, Dou ZHANG2
1. Department of Stomatology, Third Xiangya Hospital, Central South University, Changsha 410013, China;
2. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
3. Xiangya School of Medicine, Central South University, Changsha 410013, China
Received 19 August 2016; accepted 18 July 2017
Abstract: Transfection efficiency of hydroxyapatite nanoparticles (HAnps) is relative to the particle size, morphology, surface charge, surface modifier and so on. This study prepared HAnps with doped Tb/Mg by hydrothermal synthesis method (HTSM) and investigated the effects of different Tb/Mg contents on the morphology, particle size, surface charge, composition and cellular endocytosis of HAnps. The results showed that Mg-HAnps possessed better dispersion ability than Tb-HAnps. With increasing doping content of Tb/Mg-HAnps, the granularity of Tb-HAnps increased, while that of Mg-HAnps declined. Both particle size and zeta potential of Mg-HAnps were lower than those of Tb-HAnps. 7.5% Mg-doping HAnps presented relatively uniform slender rod morphology with average size of 30 nm, while 10% Mg-doping HAnps were prone to agglomeration. Moreover, Mg-HAnps-GFP (green fluorescent protein) endocytosed by MG63 cells was dotted in the perinuclear region, while Tb-HAnps were more likely to aggregate. In conclusion, as gene vectors, Mg-HAnps showed enhanced properties compared to Tb-HAnps.
Key words: hydroxyapatite nanoparticles; gene vector; endocytosis; doping; fluorescence labeling
1 Introduction
Transfection, extensively used in laboratory and promising for clinical therapeutic application, is introducing functional foreign DNA into cell nucleus to repair missing cell function and enhance or silence gene expression. Naked DNA and siRNA are negatively charged, thus the electrostatic repulsion with the anionic cell membrane reduces their transfection efficiency [1]. Rapid clearance from the body and extracellular enzymatic degradation by plasma nucleases are the major reasons that make direct gene delivery an inefficient process [2,3]. Therefore, a suitable delivery vehicle is necessary for effective transfection.
Many different vectors have been investigated for transfection including viral [4], polymeric [5,6], liposomes [7] and inorganic vectors [8]. Among the inorganic vectors, hydroxyapatites (HAs) are able to encapsulate negatively charged genetic material by chelating calcium ions while forming calcium phosphate crystals [9,10]. HAs are not prone to enzymatic degradation in the physiological environment, comparing with organic or polymeric gene delivery systems [11,12]. Regardless of the Ca/P ratio, phase and crystallinity, HAs are relatively insoluble at physiological pH of 7.4, but soluble in acidic environment, e.g. below pH 6.5 [13], such as in endocytic vesicles [14], lysosomes [15] or around solid tumors [16]. Therefore, HAs are promising for gene delivery, due to their biocompatibility, biodegradability and encapsulating ability.
However, HAs’ utility in vivo is profoundly limited by the lack of tissue specificity and the uncontrollable growth in a physiological solution. Nanoparticles with 20-200 nm in diameter can be taken up by endocytosis, while phagocytosis is thought to take the predominance if their particle size is above this range [17]. Nanoparticles are prone to agglomerate, which changes their effective size “seen” by the cells. Currently, the preparation of HAnps is mainly carried out in liquid media including chemical co-precipitation, sol-gel and microemulsion method, etc. These methods have some shortages such as complex process, poor controllability and intermediate calcinations causing the agglomeration of HAnps. However, hydrothermal synthesis method (HTSM) can directly generate HAnps in a closed reactor, which has good controllability and can prevent particles from agglomerating in the course of high-temperature calcination [18]. Moreover, adjusting the process parameters of HTSM enable the growth of HA crystals controllable.
The gene loading efficiency of HAnps is closely related to chemistry, surface area, surface charge, crystallinity and local micro-environment [19]. In the synthesis process, the calcium element was relatively active, and could be easily replaced by other elements to generate different doped hydroxyapatite compounds [20]. The ionic radius and biological activity of terbium (Tb) and magnesium (Mg) were similar to those of calcium ion (Ca2+). Tb3+ doping played an important role in controlling the growth of hydroxyapatite and contributed to an especially strong luminescence which was beneficial to the study of the behavior of cellular endocytosis [21]. HANIFI et al [22] showed that Mg2+ doping into HAs increased the surface positive charge of the HAnps and hence increased their DNA loading capacity. Although doped HAnps are widly used as in vitro transfection agents, little attention has been paid to the composition and physical properties of the hydroxyapatite precipitate and their effects on transfection.
In this study, we took advantage of HTSM to prepare HAnps and adjusted different doping amounts of Tb/Mg to control the growth of HA crystals under the same reaction temperature, reaction time and dropping rate of (NH4)2HPO4. The processing parameters were optimized by doping different amounts of Tb/Mg, and their influence on dispersion, particles size, morphology, surface charge and biological characterization was also investigated. The effects of doped HAnps on cytotoxicity and endocytosis were assessed.
2 Experimental
2.1 Preparation of Tb/Mg-HAnps
1 mol/L Ca(NO3)2·6H2O, 1 mol/L Tb(NO3)3, 1 mol/L Mg(NO3)2 and 1 mol/L (NH4)2HPO4 solutions (Yacoo Chemical Reagent Co., Ltd., Suzhou, China) were prepared in deionized water respectively. HAnps were prepared by HTSM, with Ca/P molar ratio of 1.67. Tb/Mg-HAnps with different Tb/Mg-doped amounts were prepared by HTSM, with molar ratios of (Ca+Tb)/P and (Ca+Mg)/P of 1.67, and mass fractions of Tb/ (Ca+Tb) and Mg/(Ca+Mg) of 0, 2.5%, 5.0%, 7.5% and 10.0%, respectively. Meanwhile, surfactant PEG-2000 (4%, mass fraction) (Yacoo Chemical Reagent Co., Ltd., Suzhou, China) was added to 1 mol/L Ca(NO3)2·6H2O solution. The mixed solutions of Ca(NO3)2·6H2O and Tb(NO3)3·6H2O or Ca(NO3)2·6H2O and Mg(NO3)2·6H2O were prepared, followed by drop-wise addition of 1 mol/L (NH4)2HPO4 solution at a feeding rate of 0.12 mL/min during the stirring. Both suspensions were sonicated in ultrasonic oscillator (B200, Branson, USA) for 1 h. The two resulting suspensions were adjusted pH to 10 with ammonium solution, and then transferred to the kettles for hydrothermal synthesis at 170 °C for 3 h (Guang Ying Instrument Co., Ltd., Shanghai, China). After the reaction, the two resulting suspensions were naturally cooled to room temperature, washed with ethanol and centrifuged at 1000 r/min for 5 min. The precipitate nanoparticles were freeze-dried at -50 °C for 12 h in vacuum using a freeze-dryer (FD-1A-50, Boyikang Corp., Beijing, China). All of the chemicals used in the sample preparation were analytical grade, and used as received without further purification.
2.2 Characterization of samples
The nanoparticles were firstly characterized using XRD for phase analysis. The X-ray analysis was carried out using a X-ray powder diffractometer with Cu Kα radiation (λ=1.5418  ) over a range of 20°<2θ<80°. The operational voltage and current were kept at 40 kV and 250 mA, respectively. The as-prepared particles were morphologically characterized by transmission electron microscopy (TEM). For TEM, HAnps were applied to copper grids covered with a Formvar support film. Micrographs were recorded at 80000 magnification using a JEOL JEM-3010 TEM (JEOL, Tokyo, Japan). The zeta potential measurements (n=6) were made in deionized water solutions using a ZetaSizer Nano ZS (Malvern Instruments, MA, USA) fitted with a red laser (633 nm). Size data were analyzed for significance using the Mann–Whitney test (SPSS v15.0).
) over a range of 20°<2θ<80°. The operational voltage and current were kept at 40 kV and 250 mA, respectively. The as-prepared particles were morphologically characterized by transmission electron microscopy (TEM). For TEM, HAnps were applied to copper grids covered with a Formvar support film. Micrographs were recorded at 80000 magnification using a JEOL JEM-3010 TEM (JEOL, Tokyo, Japan). The zeta potential measurements (n=6) were made in deionized water solutions using a ZetaSizer Nano ZS (Malvern Instruments, MA, USA) fitted with a red laser (633 nm). Size data were analyzed for significance using the Mann–Whitney test (SPSS v15.0).
2.3 Preparation of Mg-HAnps-GFP
A standard curve was drawn according to the gradient concentration and the absorbance of the standard solution of green fluorescent protein (GFP). 10 mg Mg-HAnps were added to 5 mL phosphate- buffered saline (PBS, pH 7.4), mixed with 1 mL GFP standard solutions with different concentrations and incubated for 1 h. The aqueous suspension was centrifuged at 5000 r/min for 5 min to precipitate the nanoparticles and then the supernatant was removed. The optical density (OD) value of unabsorbed GFP of the supernatant was measured using a automatic microplate spectrophotometer (ELX800, BioTek Instruments, Inc., USA) at 505 nm. The experiment was repeated in triplicate for each group. Protein adsorption capacity η=(CT-CF)V/W, where CT and CF are the total amounts of GFP standard solution and free GFP of supernatant, respectively, V is the volume of the GFP solution (mL), and W is the quality of Mg-HAnps (g). Effects of Mg2+ doping amount, the initial concentration of GFP, the pH value, time and temperature on the adsorption capacity of GFP were evaluated. The aqueous suspensions of Mg-HAnps-GFP with different Mg doping amounts were centrifuged, freeze-dried and stored at 4 °C.
2.4 Fluorescence characteristics
Different Tb/Mg doping amounts of Tb-HAnps and Mg-HAnps-GFP powders were added to suspensions at a concentration of 2 mg/mL using PBS, respectively. The fluorescence characteristics were tested by fluorescence microscope (IX-71, Olympus instruments, Japan).
2.5 Cell cultures
The cell line used in the experiment was MG63 cell and L929 cells (Cell Experiment Center of Xiangya Medical College, CSU, China). MG63 cells and L929 cells were seeded in Rosewell Park Memorial Institute 1640 (RPMI1640, GIBCO BRL, America) culture medium, supplemented with 10% fetal bovine serum (FBS, Hangzhou Sijiqing Biological Engineering Co., Ltd., China) at 37 °C in a humidified incubator with 5% CO2. Cultures were supplied with fresh medium every two days. Confluent cells were detached by trypsin/ ethylenediamine tetraacetic acid (EDTA) (0.25% (w/v) trypsin/0.02% EDTA, Amresco, USA) and were subcultured in batches of 1:3 in the RPMI1640 culture medium containing 10% fetal bovine serum.
2.6 Cytotoxicity
The cytotoxicity of Tb/Mg-HAnps was evaluated by methylthiazolyldiphenyl-tetrazolium bromide (MTT) (Amresco, USA) assay. L929 cells were seeded in two 96-well plates at a density of 2.5×104 cell/mL in medium containing 10% FBS and incubated at 37 °C in a humidified 5% CO2 atmosphere for 24 h. The culture medium was removed, and experiment groups were replaced with Tb/Mg-HAnps suspension of a concentration 0.10, 0.25, 0.50, 0.75, 1.00, 1.50 and 2.00 mg/mL with a replication of 6 wells for each group. Positive and negative control groups were replaced with phenol and medium containing 10% FBS, respectively. After seeding for 3 d, each well was added with 10 μL MTT solution (5 mg/mL) and incubated at 37 °C for 4 h. Then, the supernatant liquid was abandoned and 150 μL dimethyl sulfoxide was added to each well. The absorbance was measured using a microplate reader (Bio-Tek Co, USA) at 490 nm. Cell viability percentage was calculated by relative growth rate (RGR).
2.7 Endocytosis
After plasma disinfection, 7.5% Tb3+ doped HAnps and 7.5% Mg2+ doped HAnps-GFP powders were added into suspensions at a concentration of 1.5 mg/mL with sterilized deionized water. They were filtered and centrifuged under clean benches. The precipitate was added to RPMI1640 medium containing 10% FBS and stored at 4 °C. Tb-HAnps and Mg-HAnps-GFP solutions with additive amounts of 0, 50, 100 and 150 μL were set as groups A, B, C and D, respectively, with three replications for each group. The MG63 cells were digested by 0.25% trypsin digestion liquid and terminated with GIBCO RPMI1640 culture containing 10% fetal bovine serum. 2 mL of MG63 cells suspension at a density of 2.5×104 cell/mL was seeded on each sample in a 24-well plate. Cultures were supplied with fresh medium for 48 h, and then cells were washed with PBS three times. Cell morphology and cell endocytosis behavior were observed under fluorescence microscope.
3 Results and discussion
3.1 XRD analysis
The XRD patterns of HAnps with different doping amounts of Mg2+ or Tb3+ are shown in Fig. 1. As shown in Fig. 1(a), the XRD patterns of all samples were similar when Mg2+ contents were in the range of 2.5%-7.5%, corresponding well to characteristic diffraction peaks of HA crystal (JCPDS No.2-6205). However, the main peaks of doped samples became weakened and broadened, which are attributed to the existence of Mg2+ ions in HA crystals and a certain degree of lattice distortion of crystals. The nanoparticles of 10% Mg2+ content sample showed different characteristic diffraction peaks and a new compound was identified as Ca18Mg2H2(PO4)14(JCPDS No. 0-1490). The XRD patterns of HAnps with different doping amount of Tb3+ are shown in Fig. 1(b). The main peaks of patterns were basically the same when Tb3+ contents were in the range of 2.5%-10.0%, corresponding well to HA crystal’s characteristic diffraction peaks (JCPDS No. 2-6205). With the increase of Tb content, new diffraction peaks appeared at 2θ values of 14°, 20°, 29°, 38° and 43°, while patterns without and with 2.5% Tb were absent of these peaks in the same positions. These peaks matched well with the diffraction peaks of TbPO4 (JCPDS No. 3-1565).
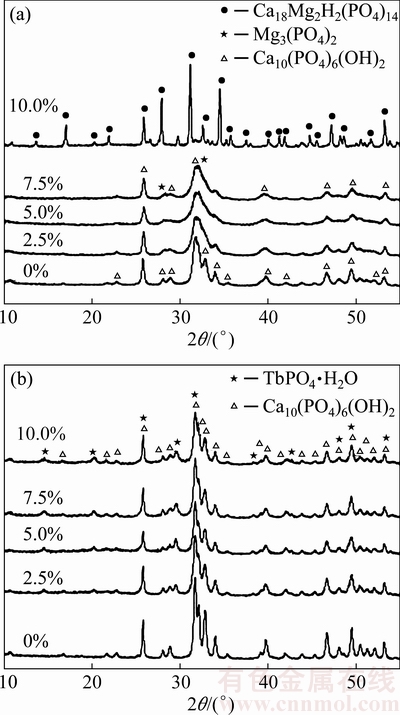
Fig. 1 XRD patterns of Mg-HAnps (a) and Tb-HAnps (b) with different Tb/Mg-doping contents
3.2 Transmission electron microscopy analysis
Figure 2 shows TEM images of HAnps with different Tb/Mg-doping amounts. The morphology of undoped HAnps was mainly short rod with good dispersion and the average particle size was about 50 nm (Figs. 2(a0), (b0)). When the doping amount of Tb3+ions ranged from 2.5% to 10.0%, particle sizes of HAnps increased from 50 to 70 nm with remarkable aggregation (Figs. 2(a1)-(a4)). At the doping amount of 2.5%-7.5%, Mg-HAnps’ morphologies were slender rod-like and the particle sizes decreased approximately from 50 to 30 nm (Figs. 2(b1)-(b4)), while no highly aggregated particles were observed. But Mg-HAnps particles showed clear agglomeration at 10.0% Mg. With the incorporation of Tb3+/ Mg2+ into the lattice structure of HAnps, the central Ca atom is substituted by Tb or Mg. The ionic radius (1.18 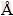 ) of Tb3+ is larger than that (0.99
) of Tb3+ is larger than that (0.99 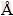 ) of Ca2+, while the ionic radius of Mg2+ (0.72
) of Ca2+, while the ionic radius of Mg2+ (0.72  ) is smaller than that of Ca2+. Substitution of Ca2+ by Tb3+ and Mg2+ distorted the HAnps lattice. This result is in agreement with the results of most scholars [21,23,24]. Due to the same valence of Mg2+ and Ca2+, Mg2+ doping was considered to occupy Ca2+ sites. Mg2+ replaced part of Ca2+ in HAnps crystal lattice, was capable of suppressing size growth of hydroxyapatite nanoparticles [25]. Besides, Mg2+ and Ca2+ had different ionic radii and the length of Mg-O was shorter than that of Ca-O. When defects and disorders occurred in the crystal structure of HA , the ratio of the major and minor axis changed, the crystallinity and grain size reduced, and the thermal stability decreased [26]. The results also showed that Tb-doped groups aggregated more strongly than Mg-doped groups, and the particle size of Mg-doped groups was smaller than that of Tb-doped ones, because the ionic radius of Tb3+ was larger than that of Mg2+, and Tb3+ tended to prefer sites with high coordination numbers [27]. The results indicated that doping of Tb/Mg had an influence on HAnps’ morphology, dispersion and average particle size, and doping of 7.5% Mg2+ ions made the HAnps’ morphology relatively uniform in a slender rod shape with an average size of 30 nm.
) is smaller than that of Ca2+. Substitution of Ca2+ by Tb3+ and Mg2+ distorted the HAnps lattice. This result is in agreement with the results of most scholars [21,23,24]. Due to the same valence of Mg2+ and Ca2+, Mg2+ doping was considered to occupy Ca2+ sites. Mg2+ replaced part of Ca2+ in HAnps crystal lattice, was capable of suppressing size growth of hydroxyapatite nanoparticles [25]. Besides, Mg2+ and Ca2+ had different ionic radii and the length of Mg-O was shorter than that of Ca-O. When defects and disorders occurred in the crystal structure of HA , the ratio of the major and minor axis changed, the crystallinity and grain size reduced, and the thermal stability decreased [26]. The results also showed that Tb-doped groups aggregated more strongly than Mg-doped groups, and the particle size of Mg-doped groups was smaller than that of Tb-doped ones, because the ionic radius of Tb3+ was larger than that of Mg2+, and Tb3+ tended to prefer sites with high coordination numbers [27]. The results indicated that doping of Tb/Mg had an influence on HAnps’ morphology, dispersion and average particle size, and doping of 7.5% Mg2+ ions made the HAnps’ morphology relatively uniform in a slender rod shape with an average size of 30 nm.

Fig. 2 TEM images of Tb-HAnps (a) and Mg-HAnps (b)
3.3 Zeta potentials of HAnps
The zeta potentials of HAnps in deionized water with different doping amounts of Tb/Mg are shown in Fig. 3. Zeta potential is thought to be representative of the electrostatic potential at the plane of shear [28]. As Tb/Mg-doping content increased, the zeta potentials of HAnps became higher. When Tb3+ doping amount ranged from 0 to 10.0%, the zeta potential of HAnps changed from -15.88 to -18.70 mV, compared with zeta potential of Mg-HAnps varying from -15.85 to -21.30 mV. Zeta potential was influenced by combination factors including the properties and size of the material. Surface conductivity is particularly important for highly charged particles with a very high specific surface area [29]. The results indicated that there was a certain correlation between particle size, dispersion and zeta potential.
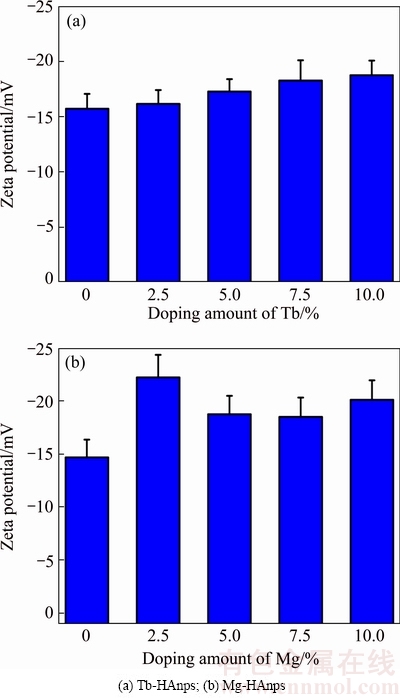
Fig. 3 Zeta potentials of different Tb/Mg doping amounts of Tb/Mg-HAnps
3.4 Cell cytotoxicity
HAnps have been constantly used as biomaterials for clinical purposes due to their excellent bio- compatibility and biodegradability. The cytotoxicity of Tb/Mg-HAnps was further assessed using MTT assay, as shown in Fig. 4. There was little difference in cell proliferation behaviors between the negative control (NC) group and HAnps samples doped with Tb3+ or Mg2+, while a significant difference was observed in the positive control (PC) group, showing a complete reduction in cell proliferation. Cytotoxicity grade was 0 or 1. The results showed that Tb/Mg-HAnps had no cytotoxicity.
3.5 Effects of factors on Mg-HAnps adsorption of GFP
The doping content of Mg2+ had influence on the HAnps adsorption of GFP. Figure 5(a) shows the calibration curve of GFP absorbance and concentration. GFP initial concentration affected the adsorption capacity of GFP (Fig. 5(b)). At the concentration of 0 to 0.7 mg/mL, the capacity for HA adsorption of GFP had a positive correlation with GFP initial concentration. Exceeding this concentration range, the adsorption curve began to flatten and reached saturation. As HAnps adsorption of GFP was reversible, increasing GFP initial concentration promoted the adsorption to reach balance, as well as the adsorption capacity. The adsorption capacity increased significantly with the increase of Mg-doping amount from 0 to 7.5%, but the adsorption capacity changed little at 10.0% Mg (Fig. 5(c)). HAnps and Mg-HAnps adsorption curves showed a rising trend within initial 30 min to 1 h and reached the peak at 1 h. The curves fell slightly and leveled off after 1 h. This indicated that the adsorption amount reached the maximum at 1 h (Fig. 5(d)). HAnps and Mg-HAnps adsorption curves of GFP showed a decline with increasing the pH value (Fig. 5(e)). This showed that the adsorption amount for GFP decreased with increasing the pH value. Since HAnps released hydroxide during the adsorption process, the adsorption reaction would be suppressed and the amount of adsorption reduced when the alkalinity became stronger with increasing pH level. HAnps and Mg-HAnps adsorption of GFP curves showed a rising trend with the increase of the temperature and leveled off at 37 °C. The curves turned downward when the temperature was above 37 °C (Fig. 5(f)). This showed that the adsorption amounts reached the maximum at 37 °C.
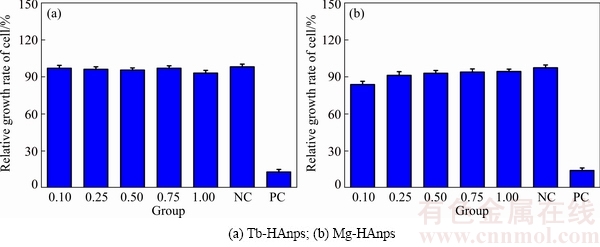
Fig. 4 MTT assay for Tb/Mg-HAnps on L929 cells
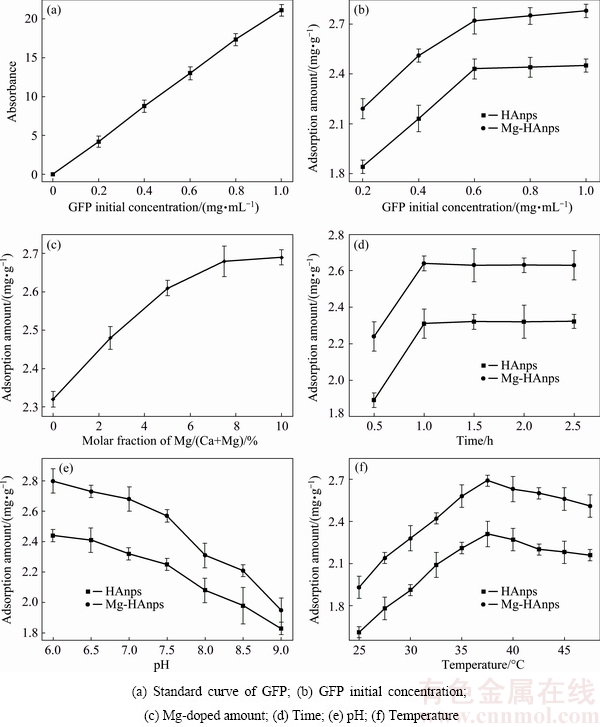
Fig. 5 Effects of factors on Mg-HAnps adsorption capacity of GFP
3.6 Fluorescence property of Tb-HAnps and Mg- HAnps-GFP
Fluorescence properties of Tb-HAnps- and Mg- HAnps-GFP are shown in Fig. 6 and Fig. 7 respectively. Tb-HAnps and Mg-HAnps-GFP produced green fluorescence under UV irradiation. With the increase of Tb/Mg-doping content, the number of fluorescent spots increased. But in HAnps without Tb-doped and Mg-HAnps unabsorbed of GFP, fluorescence was not observed. Among rare earth elements, Tb shows strong luminescence due to its energy excitation mode. Since Tb has unstable valence electron, it is easy to produce energy transition and emit green fluorescence under UV irradiation [30]. GFP can emit visible green light in the UV or blue light irradiation [31]. GFP can therefore be used as reporter protein to detect the Mg-HAnps endocytosis [32]. With increasing the doping content of Mg, the particle size of HAnps decreased and the surface area increased, so the adsorption amount of GFP increased and the fluorescence was enhanced. The results proved that HAnps obtained good fluorescence properties by doping Tb or adsorbing GFP.

Fig. 6 Fluorescence microscopic images of Tb-HAnps with different Tb contents

Fig. 7 Fluorescence microscopic images of HAnps-GFP Mg-HAnps, and Mg-HAnps-GFP with different Mg contents
3.7 Endocytosis property of sample with MG63 cell co-culture
The human osteosarcoma MG63 cells were co-cultured with different concentrations of Tb-HAnps and Mg-HAnps-GFP for 48 h. The cells morphology and endocytosis were observed under inverted microscope and fluorescence microscope, as shown in Figs. 8 and 9, respectively. In Tb-HAnps and Mg-HAnps-GFP groups, most MG63 cells stretched well under visible light with normal cell morphology. Intracellular accumulation density and irregular clump images matched well with the green fluorescent images, and the fluorescence intensity in cells increased with the increase of the concentration of HAnps. Moreover, Tb-HAnps particles were prone to agglomeration in co-culturing process, and aggregated obviously when the concentration of Tb-HAnps increased (Fig. 8), while Mg-HAnps-GFP endocytosed by MG63 cells were dotted in the perinuclear region (Fig. 9). Besides, the luminescence particles of Mg-HAnps-GFP groups were remarkably more than those of Tb-HAnps groups. The results may be attributed to the fact that both Mg-HAnps’ particle size and zeta potential were lower than those of Tb-HAnps. The particles size of the nanomaterials was considered to play a crucial role in the endocytic pathway. The standard dimension of nanodrugs was defined as 1-100 nm [33]. When the diameter is above 250 nm, phagocytosis is thought to predominate [34]. This experiment also confirmed Tb-HAnps (50-70 nm) and Mg-HAnps (30-50 nm) can be endocytosed by cells, and HAnps with smaller particle size is prone to endocytosis.
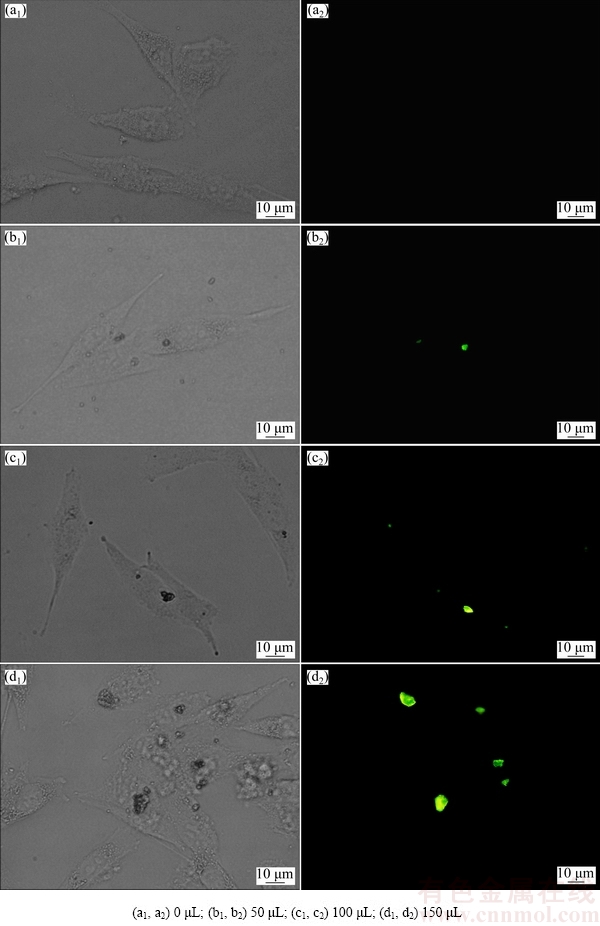
Fig. 8 Inverted and fluorescence microscopic images of Tb-HAnps with different volumes and co-cultured with MG63
In addition, positively charged particles are easier to be endocytosed by cells with negatively charged cell surface because of electrostatic adsorption. However, there was no evidence to prove that the endocytosis of nanoparticles with neutral or negatively charge was absolutely limited. In contrast, a higher uptake of negatively charged nanoparticles has been reported in HEK cells [35]. And nanoparticles’ structure and static composition were changed by the excess of cations when binding to serum proteins in vivo environment, which disrupted cell membrane transport mechanism [36].

Fig. 9 Inverted and fluorescence microscopic images of Mg-HAnps-GFP with different volumes and co-cultured with MG63
From the results above, it is speculated that there may be cellular pathways of negatively charged nanomaterials in a complex cell membrane transport mechanisms. This experiment also proved that Tb/Mg-HAnps with negative charge could be endocytosed by MG63 cells. It also showed that Tb/Mg-HAnps were non-cytotoxic and met the requirements of gene vectors.
4 Conclusions
1) The particle size (30-50 nm) and zeta potential of Mg-HAnps were smaller than those of Tb-HAnps (50-70 nm), and the morphology of HAnps doped with 7.5% Mg was relatively uniform and slender at average size of 30 nm.
2) Tb-HAnps and Mg-HAnps-GFP showed green fluorescence and good cytocompatibility in vitro.
3) Mg-HAnps were easier to be endocytosed by MG63 cells than Tb-HAnps.
4) It is necessary to carry out further researches in vivo to investigate Mg-HAnps as gene vectors.
References
[1] REISCHL D, ZIMMER A. Drug delivery of siRNA therapeutics: Potentials and limits of nanosystems [J]. Nanomedicine: Nanotechnol Biol Med, 2009, 5(1): 8-20.
[2] POUTON C W, SEYMOUR L W. Key issues in non-viral gene delivery [J]. Advanced Drug Delivery Reviews, 1998, 34: 3-19.
[3] XU Z P, ZENG Q H, LU G Q, YU A B. Inorganic nanoparticles as carriers for efficient cellular delivery [J]. Chem Eng Sci, 2006, 61: 1027-1040.
[4] HENDRIE P C, RUSSELL D W. Gene targeting with viral vectors[J]. Molecular Therapy, 2005, 12: 9-17.
[5] HAENSLER J, SZOKA F C. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture [J]. Bioconjugate Chemistry, 1993, 4: 372-379.
[6] GODBEY W T, WU K K, MIKOS A G. Poly(ethylenimine) and its role in gene delivery [J]. Journal of Controlled Release, 1999, 60: 149-160.
[7] LASIC D D, TEMPLETON N S. Liposomes in gene therapy [J]. Advanced Drug Delivery Reviews, 1996, 20: 221-266.
[8] CHOWDHURY E H, AKAIKE T. Bio-functional inorganic materials: An attractive branch of gene-based nano-medicine delivery for 21st century [J]. Current Gene Therapy, 2005, 5: 669-676.
[9] OKAZAKI M, YOSHIDA Y, YAMAGUCHI S, KANENO M, ELLIOTT J C. Affinity binding phenomena of DNA onto apatite crystals [J]. Biomaterials, 2001, 22: 2459-2464.
[10] EPPLE M, GANESAN K, HEUMANN R, KLESING J, KOVTUN A, NEUMANN S, SOKOLOVA V. Application of calcium phosphate nanoparticles in biomedicine [J]. J Mater Chem, 2010, 20: 18-23.
[11] STUBBS M, MCSHEEHY P M, GRIFFITHS J R, BASHFORD C L. Causes and consequences of tumour acidity and implications for treatment [J]. Mol Med Today, 2000, 6: 15-19.
[12] CHEUNG H Y, LAU T K, LU T P, HUI D. A critical review on polymer-based bioengineered materials for scaffold development [J]. Compos (Part B): Eng, 2007, 38: 291-300.
[13] ROY I, MITRA S, MAITRA A, MOZUMDAR S. Calcium phosphate nanoparticles as novel non-viral vectors for targeted gene delivery [J]. Int J Pharm, 2003, 250: 25-33.
[14] WANG S, MCDONNELL E H, SEDOR F A, TOFFALETTI J G. pH effects on measurements of ionized calcium and ionized magnesium in blood [J]. Arch Pathol Lab Med, 2002, 126: 947-950.
[15] TYCKO B, MAXFIELD F R. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin [J]. Cell, 1982, 28: 643-651.
[16] EPPLE M, KOVTUN A. Functionalized calcium phosphate nanoparticles for biomedical application [J]. Key Eng Mater, 2010, 441: 299-305.
[17] XIANG S D, SCHOLZEN A, MINIGO G, DAVID C, APOSTOLOPOULOS V, MOTTRAM P L,PLEBANSKI M. Pathogen recognition and development of particulate vaccines: Does size matter? [J]. Methods, 2006, 40: 1-9.
[18] BYRAPPA K, ADSCHIRI T. Hydrothermal technology for nanotechnology [J]. Progress in Crystal Growth and Characterization of Materials, 2007, 53: 117-166.
[19] BHOJ V G, CHEN Z J. Ubiquitylation in innate and adaptive immunity [J]. Nature, 2009, 458: 430-437.
[20] CAPUCCINI C, TORRICELLI P, SIMA F, BOANINI E, RISTOSCU C, BRACCI B, SOCOL G, FINI M, MIHAILESCU I N, BIGI A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response [J]. Acta Biomaterials, 2008, 4: 1885-1893.
[21] YANG C, YANG P P, WANG W X, WANG J, ZHANG M L, LIN J. Solvothermal synthesis and characterization of Ln (Eu3+, Tb3+) doped hydroxyapatite [J]. Journal of Colloid and Interface Science, 2008, 328: 203-210.
[22] HANIFI A, FATHI M H, MIR M S H, VARSHOSAZ J. Mg2+ substituted calcium phosphate nano particles synthesis for non viral gene delivery application [J]. J Mater Sci: Mater Med, 2010, 21: 2393-2401
[23] JALLOT E, NEDELEC J M, GRIMAULT A S, CHASSOT E, GRANDJEAN-LAQUERRIERE A, LAQUERRIERE P, LAURENT-MAQUIN D. STEM and EDXS characterization of physico-chemical reactions at the periphery of sol-gel derived Zn-substituted hydroxyapatites during interactions with biological fluids [J]. Colloids and Surfaces, 2005, 42: 205-210.
[24] SUN L J, NI P F, GUO D G, FANG C Q, WANG J, YANG F, HUANG X F, HAO Y Z, ZHU H, XU K W. Synthesis and characterization of Tb-incorporated apatite nano-scale powers [J]. Journal of Material Science Technology, 2012, 28: 773-778.
[25] WEBSTER T J, MASSA-SCHLUETER E A, SMITH J L, SLAMOVICH E B. Osteoblast response to hydrox-yapatite doped with divalent and trivalent cations [J]. Biomaterials, 2004, 25: 2111-2121.
[26] LAURENCIN D, ALMORA-BARRIOS N, LEEUW N H, GERVAIS C, BONHOMME C, MAURI F, CHRZANOWSKI W, KNOWLES J C, NEWPORT R J, WONG A, GAN Z, SMITH M E. Magnesium incorporation intohydroxyapatite [J]. Biomaterials, 2011, 32: 1826-1837.
[27] DOU X H, ZHAO W R, SONG E H, DENG L L, FANG X B, MIN H C. Photoluminescence, structural characterization and enegy transfer of NaBa1-x-yPO4: xCe3+, yTb3+ phosphors [J]. Journal of Rare Earths, 2012, 30: 739-743.
[28] USKOKOVIC V, ODSINADA R, DJORDJEVIC S, HABELITZ S. Dynamic light scattering and zeta potential of colloidal mixtures of amelogenin and hydroxyapatite in calcium and phosphate rich ionic milieus [J]. Archives of Oral Biology, 2011, 56: 521-532.
[29] LYKLEMA J, MINOR M. On surface conduction and its role in electrokinetics [J]. Colloids and Surfaces A, 1998, 140: 33-41.
[30] DUAN C K, KO C C, JIA G H, CHEN X Y, TANNER P A. 5d3-5d4 cross relaxation of Tb3+ in a cubic host lattice [J]. Chem Physics Letters, 2011, 506: 179-182.
[31] CAO A, YE Z, CAI Z, DONG E, YANG X, LIU G, DENG X, WANG Y, YANG S T, WANG H, WU M, LIU Y. A facile method to encapsulate protein in silica nanoparticles: Encapsulated green fluorescent protein as a robust fluorescence probe [J]. Chemical Physics Letters, 2010, 49: 3022-3025.
[32] LAFOLLA M A, MAZUMER M, SARDANA V, VELAUTHAPILLAI T, PANNU K, MCMILLEN D R. Dark proteins: Effects of inclusion body formation on quantification of protein expression [J]. Proteins, 2008, 72: 1233-1242.
[33] BONDI M L,AZZOLINA A, CRAPARO E F, LAMPIASI N, CAPUANO G, GIAMMONA G, CERVELLO M. Novel cationic solid-lipid nanoparticles as non-viral vectors for gene delivery [J]. J Drug Target, 2007, 15: 295-301.
[34] SAHAYG, ALAKHOVA D Y, KABANOV A V. Endocytosis of nanomedicines [J]. Journal of Controlled Release, 2010, 145: 182-195.
[35] ZHANG L W, MONTEIRO-RIVIERE N A. Lectins modulate multi-walled carbon cellular uptake in human epidermal keratinocytes [J]. Toxicol in Vitro, 2010, 24: 546-551.
[36] HARUSH-FRENKEL O, ROZENTUR E, BENITA S, ALTSCHULER Y. Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells [J]. Biomacromolecules, 2008, 9: 435-443.
陈良建1,2,陈 恬3,曹 君1,刘蓓蕾1,邵春生1,周科朝2,张 斗2
1. 中南大学 湘雅三医院 口腔科,长沙 410013;
2. 中南大学 粉末冶金重点实验室,长沙 410083;
3. 中南大学 湘雅医学院,长沙 410013
摘 要:羟基磷灰石纳米颗粒(HAnps)基因载体的转染效率与粒径、形貌、表面电荷、表面改性等有关。本研究通过水热合成法制备掺杂Tb/Mg的HAnps,观察Tb/Mg掺杂量对HAnps形貌、粒径、表面电荷、成分和细胞胞吞作用的影响。结果表明,掺Mg组的分散性优于掺Tb组的分散性。增加掺杂量使Tb-HAnps的粒径增大,而Mg-HAnps的粒径减小。掺Mg组的粒径及zeta电位均低于掺Tb组的。掺Mg量为7.5%的HAnps平均粒径约30 nm,呈相对均匀的细长杆状,而掺Mg量为10%的HAnps易于团聚。被胞吞入MG63细胞的Mg-HAnps-GFP呈点状分布于核周区域并形成荧光圈,而Tb-HAnps易于团聚。因此,作为基因载体,Mg-HAnps明显优于Tb-HAnps。
关键词:羟基磷灰石纳米颗粒;基因载体;胞吞作用;掺杂;荧光标记
(Edited by Wei-ping CHEN)
Foundation item: Project (2015WK3012) supported by the Hunan Provincial Science and Technology Department Project, China; Project (81571021) supported by the National Natural Science Foundation of China; Project (225) supported by the High Level Health Personnel in Hunan Province, China; Project (621020094) supported by the State Key Laboratory of Powder Metallurgy of Central South University, China; Project (20160301) supported by New Talent Project of the Third Xiangya Hospital of Central South University, China
Corresponding author: Liang-jian CHEN; Tel: +86-13507405799; E-mail: jian007040@sina.com
DOI: 10.1016/S1003-6326(18)64645-X

