Gypsum crystallization and potassium chloride regeneration by reaction of calcium chloride solution with potassium sulfate solution or solid
来源期刊:中国有色金属学报(英文版)2010年第4期
论文作者:彭翠 张福利 郭占成
文章页码:712 - 720
Key words:crystallization; gypsum; KCl regeneration; calcium removal
Abstract:
Gypsum crystallization along with the simultaneous regeneration of KCl was investigated by the reaction of CaCl2 solution with K2SO4. Well developed sheet structure gypsum crystals were produced when K2SO4 solution was added into the CaCl2 solution by slow titration or in multiple stages over 2-8 h followed by 2 h equilibration. In order to regenerate KCl solution as concentrated as possible, K2SO4 solid was added into the given CaCl2 solution instead of K2SO4 solution, obtaining gypsum crystals with almost the same quality by multistage addition with [SO42-]/[Ca2+] molar ratio no larger than 0.8. However, impurity of K2SO4·CaSO4·H2O was detected by XRD and was further confirmed by SEM-EDS in the produced crystals when the [SO42-]/[Ca2+] ratio increased to 1.1. It is proved that appearance of the double sulfate is attributed to the relatively high concentration of K2SO4. So, it is essential to properly control the [SO42-]/[Ca2+] ratio and make sure [Ca2+] in excess to suppress the solubility of CaSO4 even at the expense of low calcium removal rate.
基金信息:the National Natural Science Foundation of China
Key Project of the Ministry of Education of China

PENG Cui(彭 翠), ZHANG Fu-li(张福利), GUO Zhan-cheng(郭占成)
Key Laboratory of Ecological and Recycle Metallurgy,
University of Science and Technology Beijing, Beijing 100083, China
Received 6 July 2009; accepted 17 December 2009
Abstract: Gypsum crystallization along with the simultaneous regeneration of KCl was investigated by the reaction of CaCl2 solution with K2SO4. Well developed sheet structure gypsum crystals were produced when K2SO4 solution was added into the CaCl2 solution by slow titration or in multiple stages over 2-8 h followed by 2 h equilibration. In order to regenerate KCl solution as concentrated as possible, K2SO4 solid was added into the given CaCl2 solution instead of K2SO4 solution, obtaining gypsum crystals with almost the same quality by multistage addition with [SO42-]/[Ca2+] molar ratio no larger than 0.8. However, impurity of K2SO4·CaSO4·H2O was detected by XRD and was further confirmed by SEM-EDS in the produced crystals when the [SO42-]/[Ca2+] ratio increased to 1.1. It is proved that appearance of the double sulfate is attributed to the relatively high concentration of K2SO4. So, it is essential to properly control the [SO42-]/[Ca2+] ratio and make sure [Ca2+] in excess to suppress the solubility of CaSO4 even at the expense of low calcium removal rate.
Key words: crystallization; gypsum; KCl regeneration; calcium removal
1 Introduction
Potassium chloride is one of the most important potassium fertilizers, and also is the major raw material of non-chloride potassium fertilizers. In the past four years, it is remarkable that price of KCl have been increased by about 1 000 yuan per ton, while the price of K2SO4 only increased by about 200-250 yuan per ton. Moreover, the effective potassium content of KCl is higher than that of the same amount of K2SO4.
On the other hand, CaCl2 is a common impurity in the industrial practice of KCl production. A typical example is: in the process of recovering KCl from the electrostatic precipitator sintering dust of iron and steel making by water leaching[1-2], KCl, NaCl and CaCl2 are detected as the three major constituents in the water leaching solution. Undoubtedly adverse effect of CaCl2 on quality of KCl product would be caused if the mixture solution was directly evaporated and crystallized for KCl recovery. So, it is necessary to remove Ca2+ first. In view of the situation analysis mentioned above, an alternative precipitating agent K2SO4 is thus selected according to the following reaction:
CaCl2(aq)+K2SO4(aq or s)+2H2O→CaSO4·2H2O(s)+2KCl(aq) (1)
Taking K2SO4 as the precipitator, not only Ca2+ concentration could be decreased, but potassium could be finally left in the solution and be regenerated in the form of KCl, which is suitable for those systems which aim at obtaining pure KCl and are obsessed with impurity of CaCl2.
AL-OTHMAN and DEMOPOULOS[3] did some work on gypsum crystallization and HCl regeneration by reaction of CaCl2 solution with H2SO4 in a relatively concentrated CaCl2 solution of 3-3.5 mol/L with 2.6-8 mol/L H2SO4 as precipitating agent[3], which shows higher requirement to the equipments compared with a neutral system.
In industrial practice, crystallization process will be followed by solid-liquid separation in order to obtain the final product. The energy consumption in such operation is a decisive factor that depends on the properties of the crystals produced[4]. The crystal quality can be improved by supersaturation control[5-7]. In this work, supersaturation is controlled by the addition method of K2SO4 solution or solid.
The objective of this work is to systematically investigate the different reaction conditions such as rate of K2SO4 addition, CaCl2 and K2SO4 concentration, temperature and stirring speed on the quality of produced crystals. Meanwhile, it is also very important to regenerate KCl as concentrated as possible and check the Ca2+ concentration in the end of the reaction. In order to purify the KCl solution, Ca2+ concentration is better to be decreased to the lowest. However, it is found impracticable to decrease Ca2+ concentration and at the same time increase KCl concentration by adding excessive K2SO4 solid into the given CaCl2 solution.
2 Experimental
All the experiments were performed at constant temperature range of 20-60 ℃ using 1 L Pyrex, baffled reactor equipped with an agitator and a temperature controller.
In the first part of the work, K2SO4 solution with a limited dosage was added into the given CaCl2 solution under different conditions: variable addition rate, variable concentration in the range of 0.2-0.4 mol/L, different total molar ratio of [SO42-] to [Ca2+] either 0.6 or 0.8, whereas calcium chloride solution (200 mL) was in the concentration range of 0.90-0.99 mol/L. These conditions were chosen in such a way to keep always an amount of unreacted calcium chloride in the final solution to suppress the solubility of CaSO4 in the regenerated solution[8]. When different K2SO4 solution concentrations were used, the solution was added in different volumes to ensure always that the same amount of solids was produced in all experiments. The addition time of the K2SO4 solution was varied between two cases that all the solution was added at once and up to 4 h of addition either by titration or in small doses (stages). After the addition of the K2SO4 solution, the mixture obtained was continued to be agitated at constant temperature for the purpose of crystal ripening. This equilibration period was varied from 0 to 8 h. The effect of temperature and stirring speed on crystal quality was as well examined in the range of 20-60 ℃ and 0-300 r/min, respectively. All the produced crystals were observed by SEM (Scanning electron microscopy) in an appliance of JSM-35F; and concentrations of K+ and Ca2+ in the solution were measured by flame photometer FP640 and titration with EDTA respectively. Calcium removal is the molar fraction of Ca2+ removed through the production of gypsum.
In the second part, to obtain an ideal KCl concentration in the regenerated solution, K2SO4 solid instead of solution was added into the given CaCl2 solution under the optimized condition in the first part of the work. Variation of Ca2+ concentration in the solution under different addition stages was monitored by sampling. In addition, in an attempt to decrease Ca2+ concentration in the solution as low as possible, [SO42-]/[Ca2+] ratio was increased on purpose, of which KCl concentration regenerated was compared with the theoretical value calculated based on the stoichiometry of the chemical reaction presented in Eq.(1). Then, XRD (X-ray diffractometry) and SEM-EDS (Scanning electron microscopy with X-ray energy dispersive spectrometry) were resorted to explain the deviation between the practical KCl concentration in the regenerated solution with the theoretical value using an appliance model M21 from MAC Co. Ltd, Japan for XRD and CAMBRIDGE S-360, Tracer Northern for SEM-EDS.
3 Results and discussion
3.1 Effect of concentration of K2SO4 solution
The concentration of the precipitating agent (in this case, K2SO4) and the addition method have a great impact on supersaturation, hence, on the quality of produced crystals[9], which is better seen by referring to Eq.(2):
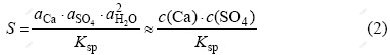
where S is the saturation ratio[5], a and c are activity and concentration respectively, and Ksp is the solubility product of gypsum.
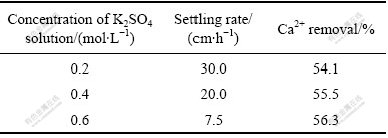
One of the objectives of this work is the regeneration of KCl out of the CaCl2 solution. For a given concentration of CaCl2 solution, it is obvious that K2SO4 should be used as concentrated as possible, even in the solid phase, which will be discussed in the later sections. Here, K2SO4 solution with different concentrations was investigated as titrant to precipitate the calcium ions. The morphologies of obtained gypsum crystal at different initial concentrations of K2SO4 solution are shown in Fig.1. As expected, particle size of the gypsum obtained with 0.2 mol/L K2SO4 as titrant is to some extent larger than that with 0.6 mol/L K2SO4, which can also be explained by Eq.(2). Higher titrant concentration decides higher supersaturation degree, thus a large number of crystal nucleus were produced instantaneously, eventually leading to small crystal particle. The measured settling rates of the crystals obtained with initial titrant concentrations of 0.2, 0.4 and 0.6 mol/L are 30, 20 and 7.5 cm/h, respectively (see Table 1), which is probably due to the decrease of crystal particle size with elevation of the titrant concentration.
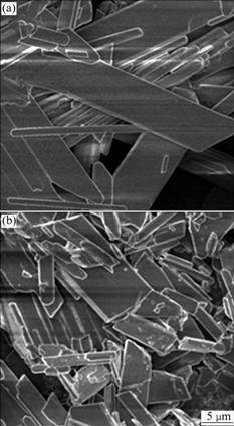
Fig.1 Effect of K2SO4 concentration on gypsum crystal morphology: (a) 0.2 mol/L (b) 0.6 mol/L (Conditions: 200 mL 0.95 mol/L CaCl2, titration for 2 h followed by 2 h equilibration, [SO42-]/[Ca2+] ratio 0.6, 20 ℃)
Table 1 Effect of concentration of K2SO4 solution on settling rate and calcium removal
3.2 Effect of addition method
Three methods of K2SO4 addition were investigated: all the precipitating agent added at once, K2SO4 solution added by titration and added in stages. In all the cases, the XRD analysis of the crystals obtained confirmed the presence of only gypsum (DH phase).
The effects of addition method of K2SO4 solution on the induction time, settling rate and calcium removal are listed in Table 2. And SEM morphologies of the produced gypsum with different addition methods are shown in Fig.2.
Since induction period is considerably affected by the level of supersaturation[10], the induction time examined in this test showed the expected results. When the precipitating solution was added by titration, the settling rate of the crystals increased with longer addition time, and the calcium removal increased as well. For example, the setting rate was increased from 8 cm/h when the K2SO4 solution was added by 1 h titration to 30 cm/h with 4 h of titration, and the calcium removal increased from 66.6% when all the K2SO4 solution was added at once to 75.8% with 4 h titration. The improved results with titration can be understood upon evaluation of their crystal size and morphological characteristics in Fig.2.
Table 2 Effect of addition method of K2SO4 solution on induction time, settling rate and calcium removal (Conditions: 381.0 mL of 0.4 mol/L K2SO4 added to 200 mL 0.95 mol/L CaCl2 at [SO42-]/[Ca2+] of 0.8 at 20 ℃)
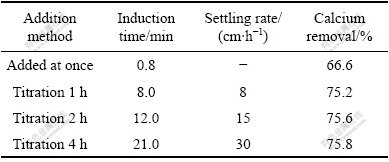
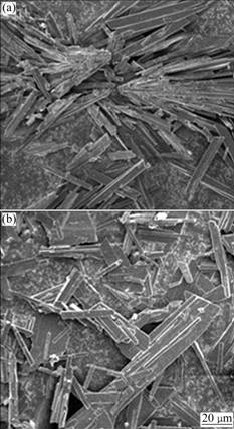
Fig.2 SEM morphologies of produced gypsum with K2SO4 solution added at once (a) and titration for 2 h (b) (Conditions: 381.0 mL of 0.4 mol/L K2SO4 added to 200 mL 0.95 mol/L CaCl2 at [SO42-]/[Ca2+] of 0.8 at 20 ℃)
It is clear that, when K2SO4 solution was added at once, the clustered gypsum crystals, with a “broom” appearance, were obtained, as shown in Fig.2(a). However, the crystals obtained with gradual addition of the solution, i.e. by titration for 2 h as seen in Fig.2(b) or by 4 h of titration (not shown) were well developed and dispersed. Since there was no much difference in gypsum crystal and calcium removal between 2 and 4 h titration, it was decided to conduct most of the subsequent titration tests with 2 h addition period of K2SO4 solution.
On the other hand, equilibration time has a noticeable effect on the crystal morphological characteristic, as shown in Fig.3. It can be easily seen that equilibration benefits crystal ripening[10].
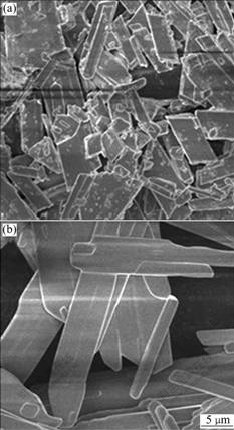
Fig.3 Effect of equilibration on gypsum crystal morphology: (a) Without equilibration; (b) 2 h of equilibration (Conditions: 285.8 mL of 0.4 mol/L K2SO4 added to 200 mL 0.95 mol/L CaCl2 at [SO42-]/[Ca2+] of 0.6 at 20 ℃)
3.3 Effect of temperature
The effect of temperature on the morphology of produced gypsum is illustrated in Fig.4. As shown in Fig.4, the particle size of the crystal increases with temperature increasing, which is due to the increase in growth rate. So, it is not difficult to understand the increase of solid settling rate with temperature increasing. The result obtained is the same as that of some researches done in gypsum crystallization by reaction of calcium chloride solution with sulfuric acid[3].
The temperature range investigated is just 20-60 ℃, so, further examination is needed beyond this temperature range in the future work. Researchers have done a lot of works on the phase conversion of calcium sulfate dihydrate to hemihydrates or anhydrate in various systems as temperature elevated. MARSHALL and SLUSHER[11] investigated thermodynamics of calcium sulfate dihydrate in aqueous sodium chloride solutions in temperature range of 0-110 ℃, finding that no phase conversion of calcium sulfate in this temperature range.
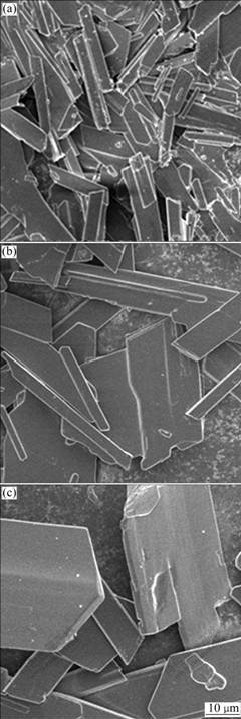
Fig.4 Effect of temperature on gypsum crystal morphology: (a) 20 ℃; (b) 40 ℃; (c) 60 ℃ (Conditions: 382 mL of 0.4 mol/L K2SO4 added to 200 mL 0.95 mol/L CaCl2 for 2 h followed by 2 h equilibration at [SO42-]/[Ca2+] of 0.8)
3.4 Effect of stirring speed
In the crystallization process, stirring is another important factor affecting quality of the crystal obtained. A fast stirring speed may break crystals into fragments, narrow the metastable region and increase secondary nucleation rate, causing crystal particles with small size. And too slow stirring is not advantageous for slurry suspension. On the other hand, approximate stirring intensity favors the diffusion of molecules, eliminating partial over-concentration and benefitting the formation of even supersaturation degree. Effect of stirring speed on the morphology of the produced gypsum in this test was investigated and the results are shown in Fig.5. It can be clearly seen that the needle like crystal clusters are obtained when no stirring is taken. However, when stirring speed is 300 r/min, the crystals seem to be broken. So, 200 r/min is considered as a proper stirring speed.
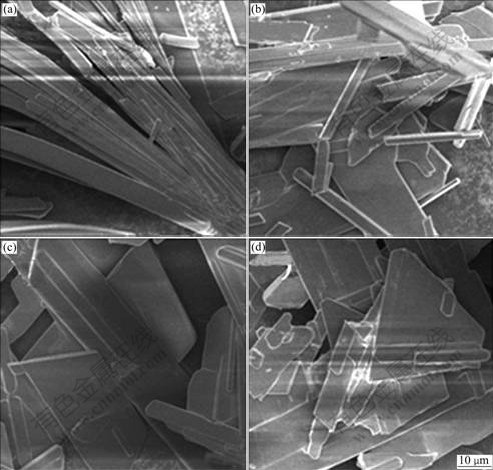
Fig.5 Effect of stirring speed on morphology of produced gypsum: (a) 0 r/min; (b) 100 r/min; (c) 200 r/min; (d) 300 r/min (Conditions: 382 mL of 0.4 mol/L K2SO4 added to 200 mL 0.95 mol/L CaCl2 for 2 h followed by 2 h equilibration at 40 ℃ with [SO42-]/[Ca2+] of 0.8)
3.5 Step-wise addition method
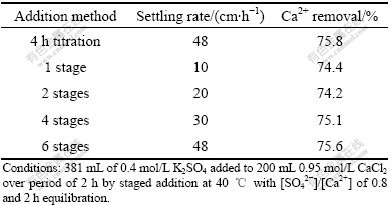
It is demonstrated that good quality gypsum crystal is produced by titration method under proper condition; however, it is not so suitable for industrial application as step-wise addition method[5]. With dual purpose of verifying that the step-wise method gives equivalent results to titration and determining the optimum number of steps (or stages) required to approximate the titration procedure, a new set of tests was conducted. In each experiment, 2 h of equilibration was applied after the limited reactant was consumed. The results in terms of crystal quality, settling rate and calcium removal are shown in Fig.6 and Table 3, respectively.

Fig.6 Gypsum morphology at different addition stages: (a) 1 stage; (b) 2 stages; (c) 4 stages; (d) 6 stages (Conditions: 381 mL of 0.4 mol/L K2SO4 added to 200 mL 0.95 mol/L CaCl2 over period of 2 h by staged addition at 40 ℃ with [SO42-]/[Ca2+] of 0.8 and 2 h equilibration)
It can be clearly seen that the produced gypsum has a larger particle size and a smoother surface with increasing addition stages. Furthermore, the settling rate increases apparently from 10 cm/h at 1 stage to 48 cm/h at 6 stages, which is equivalent to the settling rate value of titration addition method, as shown in Table 3. In addition, almost the same calcium removal is obtained in staged addition with that of titration addition method, confirming that it is feasible to take the step-wise addition method.
Table 3 Results of titration vs staged addition of K2SO4 solution
As discussed above, concentration of KCl regenerated from the reaction system is another very important term needed to pay great attention. On the other hand, concentration of the regenerated KCl is dependent on the concentration of K2SO4 added. As we know, concentration of saturated K2SO4 solution at room temperature is less than 0.7 mol/L, which decides that volume of the reaction system will be enlarged to a large extent if K2SO4 solution is taken as the precipitating agent of the calcium ions. So, it was decided to use K2SO4 in the form of solid. The step-wise addition method tests were also done with K2SO4 solid to precipitate the calcium ions, indicating that quality of crystals obtained was as good as that of crystals obtained with K2SO4 solution (figures not shown). In view of a dissolution process of K2SO4 solid before reacting with CaCl2, concentration of Ca2+ in the system is monitored with the passage of time at 1, 4 and 6 addition stages to investigate the equilibration time. The results are shown in Fig.7.
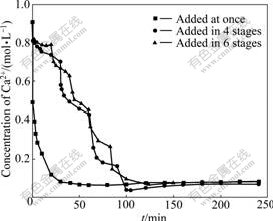
Fig.7 Concentration of Ca2+ vs time (Conditions: 33.3 g K2SO4 added to 200 mL 0.96 mol/L CaCl2 in 1, 4 and 6 stages within 2 h followed by another 2 h equilibration with [SO42-]/[Ca2+] of 1.0 at 40 ℃)
It is evident from Fig.7 that Ca2+ concentration decreases sharply when K2SO4 was added at once into the CaCl2 solution, and it decreases periodically and slowly when the same amount of K2SO4 was added in 4 and 6 stages. The more the stages are divided into, the closer the concentration—time curve to the equilibration curve is, which can also be explained by Eq.(2), and thus it is not difficult to understand the gradual increase of particle size of the produced crystal with increasing addition stages.
3.6 KCl regeneration
As mentioned earlier, one of the objectives of this work is to regenerate KCl from CaCl2 solutions by reaction with K2SO4. Fig.8 compares the theoretical KCl concentration with the actual one obtained from different [SO42-]/[Ca2+] ratios used to treat 200 mL 0.99 mol/L CaCl2 at 40 ℃. The theoretical concentration was calculated based on the stoichiometry of the chemical reaction presented in Eq.(1), assuming that all K2SO4 solid reacted and neglecting the volume variation brought by the addition of K2SO4 solid and the production of the precipitum.
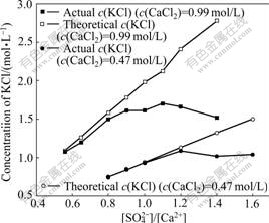
Fig.8 Actual and theoretical KCl concentration in regenerated solutions obtained at different [SO42-]/[Ca2+] ratios (Conditions: K2SO4 solid added to 200 mL 0.99 mol/L CaCl2 solution at 40 ℃)
As expected, Ca2+ concentration firstly decreased and then kept constant with increase of [SO42-]/[Ca2+] ratio, as shown in Table 4, partly because the solubility of gypsum increases with ionic intensity increasing in the solution. However, the actual KCl concentration did not increase with the increase of [SO42-]/[Ca2+] ratio from 0.8 to 1.4, even deviated from the theoretical value farther and farther, as shown in Fig.8, which is unexpected. In addition, the higher the initial CaCl2 concentration, the smaller the [SO42-]/[Ca2+] ratio which resulted in no derivation. To find out where the potassium went, XRD and SEM-EDS were resorted to analyze the obtained crystal. The XRD analysis was done to the crystals obtained by reaction of the given CaCl2 solution with K2SO4 solution or solid at different [SO42-]/[Ca2+] ratios. The results are shown in Fig.9. As illustrated in Fig.9, only gypsum phase was observed no matter K2SO4 solution or solid was added when [SO42-]/[Ca2+] was kept at 0.8; while a new phase syngenite (K2SO4? CaSO4·H2O)[12] appeared when the [SO42-]/[Ca2+] was 1.1. Now, it is easy to understand that KCl cannot be regenerated completely in the solution with [SO42-]/[Ca2+] increasing. For further verification of the new phase appearance, the produced crystal sample corresponding to Fig.9(c) was also subjected to SEM-EDS analysis. The results are shown in Fig.10. It can be clearly seen that there are some small pieces of particles consisting of calcium, potassium, sulfur, oxygen and hydrogen (element hydrogen cannot be detected using the present analyzer) attached on the surface of rodlike crystals of gypsum.
Table 4 Ca2+concentration variation with increasing [SO42-]/[Ca2+] ratios (initial Ca2+concentration of 0.99 mol/L )
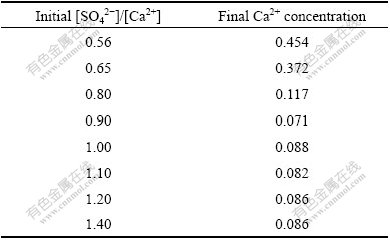
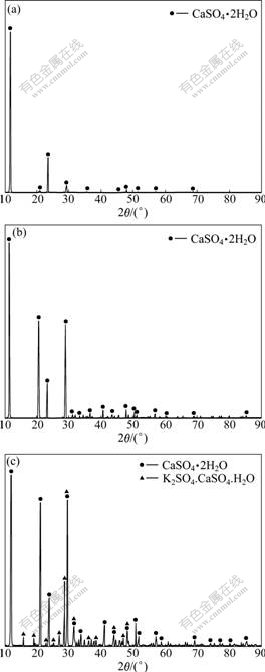
Fig.9 XRD analysis results of crystals obtained by reaction of 200 mL 0.99 mol/L CaCl2 solution with 0.4 mol/L K2SO4 solution with [SO42-]/[Ca2+] of 0.8 (a), K2SO4 solid with [SO42-]/[Ca2+] of 0.8 (b), and K2SO4 solid with [SO42-]/[Ca2+] of 1.1 (c)
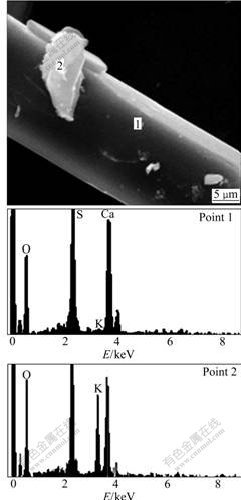
Fig.10 SEM-EDS analysis results of cystal obtained by reaction of 200 mL 0.99 mol/L CaCl2 solution with K2SO4 solid with [SO42-]/[Ca2+] of 1.1
Double sulfates of potassium and calcium including K2SO4·CaSO4·H2O, K2SO4·2CaSO4·3H2O and K2SO4·5CaSO4·H2O have been studied by many investigators as early as 1850s[13-15]. And the equilibrium conditions at different temperatures have been determined. ANDERSON and NESTELL[16] pointed out that required concentration of potassium sulfate for the equilibrium represented by the following equations varies with temperature:
5(CaSO4·2H2O)+K2SO4→K2SO4·5CaSO4·H2O+9H2O (3)
K2SO4·5CaSO4·H2O+4K2SO4+4H2O→5(K2SO4·CaSO4·H2O) (4)
As a result, if K2SO4 was selected as precipitating agent to remove Ca2+ in the solution, it is essential to appropriately control the addition amount of K2SO4, and make sure that the Ca2+ is in excess in the solution, especially for the solution which itself contains a certain concentration of K+.
4 Conclusions
1) Crystallization chemistry reaction of CaCl2 (0.47-0.99 mol/L)-K2SO4 (0.2-0.6 mol/L) can be controlled in such a way that the production of well-grown and clean gypsum (CaSO4·2H2O) crystals is obtained with the simultaneous regeneration of KCl in the temperature range of 20-60 ℃.
2) Well-developed sheet structure gypsum crystals are achieved through supersaturation control by addition of K2SO4 solution or solid in titration or multiple stages. Gypsum crystals grow with increasing addition stages and temperature in the range of 20-60 ℃. Stirring speed of 200 r/min is optimized as the proper speed in this reaction system.
3) K2SO4 solid instead of K2SO4 solution can also precipitate Ca2+ and produce good quality gypsum crystals by multiple stage addition with [SO42-]/[Ca2+] less than 0.8. But K2SO4·CaSO4·H2O is detected when [SO42-]/[Ca2+] is increased to 1.1 with the help of XRD and SEM-EDS. Appearance of the double sulfate is attributed to the relatively high concentration of K2SO4, so it is essential to properly control the [SO42-]/[Ca2+] ratio and make sure [Ca2+] in excess to suppress the solubility of CaSO4 even at the expense of low calcium removal percentage.
References
[1] PENG C, GUO Z C, ZHANG F L. Discovery of potassium chloride in the sintering dust by chemical and physical characterization [J]. ISIJ Int, 2008, 48: 1398-1403.
[2] PENG C, ZHANG F L, GUO Z C. Separation and recovery of potassium chloride from sintering dust of ironmaking works [J]. ISIJ Int, 2009, 49: 735-742.
[3] AL-OTHMAN A, DEMOPOULOS G P. Gypsum crystallization and hydrochloric acid regeneration by reaction of calcium chloride solution with sulfuric acid [J]. Hydrometallurgy, 2009, 96(1/2): 95- 102.
[4] FRANKE J, MERSMANN A. The influence of the operational conditions on the precipitation process [J]. Chem Eng Sci, 1995, 50: 1737.
[5] DEMOPOULOS G P. Aqueous precipitation and crystallization for the production of particulate solids with desired properties [J]. Hydrometallurgy, 2009, 96(3): 199-214.
[6] SYNOWIEC P M, BUNIKOWSKA B. Application of crystallization with chemical reaction in the process of waste brine purifying in evaporative sodium chloride production [J]. Ind Eng Chem Res, 2005, 44: 2273-2280.
[7] HAMDONA S K, ALHADAD O A. Influence of additives on the precipitation of gypsum in sodium chloride solutions [J]. Desalination, 2008, 228: 277-286.
[8] KUMAR A, SANGHAVI R, MOHANDAS V P. Solubility pattern of CaSO4·2H2O in the system NaCl+CaCl2+H2O and solution densities at 35 ℃: Non-ideality and ion pairing [J]. J Chem Eng Data, 2007, 52: 902-905.
[9] LOFFELMANN M, MERSMANN A. How to measure supersaturation [J]. Chem Eng Sci, 2002, 57: 4301-4310.
[10] MULLIN J W. Crystallization [M]. Oxford: Butterworth-Heinemann, 2001: 182-188.
[11] MARSHALL W L, SLUSHER R. Thermodynamics of calcium sulfate dihydrate in aqueous sodium chloride solutions, 0-110 ℃ [J]. J Phys Chem, 1966, 70: 4015-4027.
[12] BELLMANN F, MOSER B, STARK J. Influence of sulfate solution concentration on the formation of gypsum in sulfate resistance test specimen [J]. Cem Concr Res, 2006, 36: 358-363.
[13] CAMERON F K, BREAZEALE J F. Calcium sulfate in aqueous solutions of potassium and sodium sulphates [J]. J Phys Chem, 1904, 8: 335-340.
[14] ANDERSON E. Double salts of calcium and potassium and their occurrence in leaching cement mill flue dust [J]. Ind Eng Chem, 1919, 11: 327-332.
[15] CLARKE L, PARTRIDGE E P. Potassium sulfate from syngenite by high-temperature extraction with water [J]. Ind Eng Chem, 1934, 26: 897-903.
[16] ANDERSON E, NESTELL R J. The formation of the double salts of calcium and potassium sulfates at 100 ℃ [J]. Ind Eng Chem, 1920, 12: 243-246.
Foundation item: Project(50974018) supported by the National Natural Science Foundation of China; Project(108007) supported by Key Project of the Ministry of Education of China
Corresponding author: GUO Zhan-cheng; Tel: +86-10-82375042; E-mail: zcguo@metall.ustb.edu.cn
DOI: 10.1016/S1003-6326(09)60203-X

