J. Cent. South Univ. (2017) 24: 773-781
DOI: 10.1007/s11771-017-3479-8

Gaseous hydrogen storage thermodynamics and kinetics of RE–Mg–Ni-based alloys prepared by mechanical milling
ZHANG Yang-huan(张羊换)1, 2, YUAN Ze-ming(袁泽明)2, YANG Tai(杨泰)2, BU Wen-gang(卜文刚)2,
HOU Zhong-hui(侯忠辉)1, ZHAO Dong-liang(赵栋梁)2
1. Key Laboratory of Integrated Exploitation of Baiyun Obo Multi-Metal Resources,
Inner Mongolia University of Science and Technology, Baotou 014010, China;
2. Department of Functional Material Research, Central Iron and Steel Research Institute, Beijing 100081, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Central South University Press and Springer-Verlag Berlin Heidelberg 2017
Abstract: Nanocrystalline/amorphous LaMg11Ni+xNi (x=100%, 200%, mass fraction) composite hydrogen storage alloys were synthesized by ball milling technology. The effects of Ni content and milling time on the gaseous hydrogen storage thermodynamics and dynamics of the alloys were systematically investigated. The hydrogen desorption properties were studied by Sievert’s apparatus and a differential scanning calorimeter (DSC) connected with a H2 detector. The thermodynamic parameters (△H and △S) for the hydrogen absorption and desorption of the alloys were calculated by Van’t Hoff equation. The hydrogen desorption activation energy of the alloy hydride was estimated using Arrhenius and Kissinger methods. The results indicate that a variation in the Ni content has a slight effect on the thermodynamic properties of the alloys, but it significantly improves their absorption and desorption kinetics performances. Furthermore, varying milling time clearly affects the hydrogen storage properties of the alloys. All the as-milled alloys show so fast hydrogen absorption rate that the absorbed amount in 10 min reaches to at least more than 95% of the saturated hydrogen absorption capacity. Moreover, the improvement of the gaseous hydrogen storage kinetics of the alloys is found to be ascribed to a decrease in the hydrogen desorption activation energy caused by increasing Ni content and prolong milling time.
Key words: hydrogen storage; mechanical milling; activation energy; kinetics
1 Introduction
In recent decades, a great deal of efforts has been made for hydrogen being used as a fuel for vehicle applications. As a globally accepted clean and recyclable fuel, the wide applications of hydrogen fuel cell vehicles will fundamentally reduce both energy consumption and carbon dioxide emissions due to the fact that about one quarter of the world total energy is believed to be consumed by transport [1]. A key challenge is to find a method for hydrogen storage that brings on a high volumetric and gravimetric density. Although many hydrogen storage materials have been reported for the application goal, none of them can meet all the requirements of the performances proposed by US Department of Energy (DOE) for vehicular applications [2, 3]. In terms of hydrogen absorption capacity, Mg-based alloys are considered to a promising alternative for hydrogen fuel cell vehicle. Especially, the Mg-rich rare earth-Mg-based alloy has attracted increasing interest due to its gaseous hydrogen storage capacity of 3.7%-6.0% (mass fraction) [4] and the theoretical electrochemical capacity (over 1000 mA·h·g–1) [5], which is much higher than that of Mg–Ni alloy. However, the practical application of the alloys is seriously hindered by their relatively high hydrogen desorption temperatures, sluggish hydriding/dehydriding kinetics and extremely low electrochemical discharge capacity at room temperature as well as quite poor cycle stability either as the hydrogen storage materials of on-board use or as the negative electrode materials of Ni-MH battery. Therefore, researchers in this area still face a major challenge: how to improve the hydrogen storage kinetics of Mg-based alloys.
It has been well known that the hydrogenation/ dehydrogenation properties of Mg-based alloys can be improved by reducing the grain size to the nanometer scale [6] and adding small quantities of catalytically active transition elements, metal oxides, or rare earth elements also used as catalysts [7, 8]. Especially, mechanical milling (MM) [9] and melt spinning [10] are widely accepted techniques for producing amorphous and nanocrystalline Mg-based alloys with different compositions. YUAN et al [11] reported their investigation on the hydriding behavior of LaMg11Ni and found that the ball milling for 20 h makes hydrogen absorption capacity of LaMg11Ni increase by about 20%, and the kinetics of hydrogen absorption was markedly improved. ZHANG et al [12] reported that the as-spun Mg10NiR (R=La, Nd, Sm) alloys displayed superior hydriding and dehydriding kinetics markedly improved by adding different rare earth elements. POLETAEV et al [13] reported that the LaMg11Ni alloy solidified at the highest cooling rate exhibited the fastest hydrogenation kinetics, reaching maximum hydrogenation capacity of 5.02%. WANG et al [4] investigated the electrochemical hydrogen storage properties of ball-milled MmMg12 alloy with Ni powders, and the results indicated that with the Ni content in the alloy increasing from 150% to 200%, the first discharge capacity of the ball-milled sample was enhanced from 770 to 1200 mA·h·g–1.
It is well known that Ni can accelerate the amorphization process in the alloy and exert a very powerful catalytic action for the hydrogen absorption and desorption of the Mg-based alloy. Hence, Mg in the LaMg12 alloy was partially substituted by Ni in the present work. The nanocrystalline and amorphous LaMg11Ni+xNi (x=100%, 200%, mass fraction) alloys were prepared by mechanical milling, and the effects of Ni content and milling time on the gaseous hydrogen storage kinetics performances of the alloys have been investigated in detail.
2 Experimental
The experimental alloys with the chemical composition of the LaMg11Ni were prepared using a vacuum induction furnace in a helium atmosphere at a pressure of 0.04 MPa to prevent Mg from volatilizing. The molten alloy was poured into a cooled copper mould and then a cast ingot was obtained. The as-cast alloys were mechanically crushed into powder with a diameter of about 50 μm. The alloy powder is mixed with carbonyl nickel powder with a mass ratio of 1:1 and 1:2 respectively. Then the mixed powder was mechanically milled in a planetary-type mill and was handled in a glove box under Ar atmosphere to prevent the powder from oxidation during ball milling. Cr–Ni stainless steel ball and the powder with a weight ratio of 35:1 were put into Cr–Ni stainless steel vials together. The milling speed was 350 r/min and the duration time was 5, 10, 20, 40 and 60 h, respectively. For simplicity,the chemical composition of ball-milled alloys was defined as LaMg11Ni+xNi (x=100%, 200%, mass fraction). All gaseous hydrogen sorption capacities and electrochemical performances of the samples were calculated based on LaMg11Ni (excluding the mass of carbonyl nickel powder during ball milling) alloy.
The phase structures of the as-cast and milled alloys were determined by X-ray diffraction (XRD) (D/max/ 2400), using Cu Kα radiation filtered by graphite at 160 mA, 40 kV and 10 (°)/min. A Philips scanning electron microscopy (SEM) (QUANTA 400) linked with an energy dispersive spectrometer (EDS) was used for observing morphologies and analyzing chemical composition of the as-cast alloys. The powder samples of the as-milled alloys were observed by high resolution transmission electron microscope (HRTEM) (JEM- 2100F, operated at 200 kV) and their crystalline states were ascertained by electron diffraction (ED).
The hydrogen absorption and desorption kinetics and pressure-composition isotherms (P-C-T) curves of the alloys were measured using an automatically controlled Sievert’s apparatus with a furnace controlled to an accuracy of ±2 K. Prior to measuring, several hydrogen absorption and desorption cycles were performed in order to activate the materials. An initial hydrogen pressure of 3 MPa was applied to induce hydrogen absorption of the alloy particles at the temperatures of 553, 573, 593 and 613 K as well as hydrogen desorption at pressure of 1×10-4 MPa at the same temperature. 300 mg sample was loaded into a cylindrical reactor for each measurement. Hydrogen desorption properties were also measured using a differential scanning calorimeter (DSC, STA449C) at heating rates of 5, 10, 15 and 20 K/min.
3 Results and discussion
3.1 Microstructure characteristics
In Fig. 1, the profiles of the as-cast and milled LaMg11Ni+xNi alloys before and after hydriding are shown. It reveals that the as-cast LaMg11Ni alloy contains a major phase La2Mg17 and a secondary phase Mg2Ni. We can see that the mechanical milling makes the diffraction peaks of the alloys merge and broaden with increasing Ni content, indicating that the crystalline structure has transformed to a nanocrystalline or amorphous structure. Furthermore, increasing Ni content in alloys makes the intensity of diffraction peaks evidently lower and its width clearly broaden; that is to say, increasing Ni content facilitates the glass forming of the alloy. As stated by ABDELLAOUI et al [14], increasing Ni content can lower the activation energy for the transformation of the crystalline to amorphous phase in the REMg12 alloys. After hydriding, there are three hydrides occurring in the as-cast alloy, including LaH3, MgH2 and Mg2NiH4. In addition, the diffraction peaks of the as-cast and milled alloys obviously broaden, which results from the lattice expansion in the process of hydrogen absorption. The as-milled LaMg11Ni+xNi (x= 100%, 200%) alloys in saturated hydrogen absorption state still display nanocrystalline and amorphous structure, indicating that the as-milled alloys have a good structure stability.

Fig. 1 XRD profiles of as-cast and milled (40 h) LaMg11Ni+xNi (x=0, 100%, 200%) alloys before (a) and after (b) hydriding
The SEM images and EDS patterns of the as-cast LaMg11Ni alloy are shown in Fig. 2. A typical cast structure can be clearly observed, and the EDS profiles display that the as-cast alloy consists of two phases La2Mg17 and Mg2Ni, which is consistent with the XRD detection.
To compare the morphological change of the milled alloy particles before and after hydrogen absorption, the surface morphologies of the powder samples are examined by SEM. Figure 3 demonstrates representative SEM micrographs of the as-milled (40 h) LaMg11Ni+ 100% Ni alloy before and after hydrogen absorption. The aggregation of the milled alloy particles is so distinct that it is difficult to distinguish Ni and alloy particle, as shown in Fig. 3(a). After hydriding, the alloy particles still remain aggregation state (see Fig. 3(b)). A lot of cracks can be clearly seen on the surfaces of the alloy particles after 5 cycles (see Fig. 3(c)), and with the increasing cycle number, the pulverization degree of the particles becomes more serious. Along with the pulverization of the alloy particles, the alloy is completely activated.
Figure 4 shows the HRTEM micrographs and ED patterns of the as-milled LaMg11Ni+xNi (x=100%, 200%) alloys. Obviously, after milled for 40 h, x=100% alloy exhibits a major nanocrystalline structure, while the x=200% alloy displays a visible nanocrystalline and amorphous structure, suggesting that increasing Ni content facilitates the glass forming of the Mg2Ni alloy during milling, which is also evidenced by TERESIAK et al [15]. The average size of the as-milled alloys measured by linear intercept method is found to be in the range of 8-12 nm. Meanwhile, some crystal defects, such as dislocation, grain boundary and twin can be clearly observed in the as-milled alloys.
3.2 Gaseous hydrogen absorption/desorption thermodynamics
To examine the gaseous hydrogen absorption/ desorption thermodynamics of as-milled LaMg11Ni+xNi alloys, the P-C-T curves of the alloys were measured at 553, 573, 593 and 613 K, as presented in Fig. 5. Based on the data in the Fig. 5, Van’t Hoff diagrams for the hydrogen absorption/desorption of the alloys can be plotted, as inserted in Fig. 5. We can see that there are good linear relations between 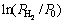 and 1/T for all the alloys. Thus the thermodynamic parameters, namely enthalpy change (△H) and entropy change (△S), can be easily calculated from Van’t Hoff equation [16]:
and 1/T for all the alloys. Thus the thermodynamic parameters, namely enthalpy change (△H) and entropy change (△S), can be easily calculated from Van’t Hoff equation [16]:
 (1)
(1)
where  is the equilibrium hydrogen gas pressure (due to the fact that the pressure plateaus have a clearly inclination, hence, here we take the 50% of the maximum hydrogen absorption/desorption capacity corresponding to pressure as equilibrium hydrogen gas pressure), P0 is the standard atmospheric pressure, T is the sample temperature, and R is the gas constant (8.314 J/(mol·K)). The obtained enthalpy and entropy changes of the as-milled alloys are listed in Table 1. It reveals that increasing Ni content slightly affects the thermodynamic properties of the as-milled alloys.
is the equilibrium hydrogen gas pressure (due to the fact that the pressure plateaus have a clearly inclination, hence, here we take the 50% of the maximum hydrogen absorption/desorption capacity corresponding to pressure as equilibrium hydrogen gas pressure), P0 is the standard atmospheric pressure, T is the sample temperature, and R is the gas constant (8.314 J/(mol·K)). The obtained enthalpy and entropy changes of the as-milled alloys are listed in Table 1. It reveals that increasing Ni content slightly affects the thermodynamic properties of the as-milled alloys.

Fig. 2 SEM image and typical EDS spectra of as-cast LaMg11Ni alloy:

Fig. 3 SEM morphologies of as-milled (40 h) LaMg11Ni+100%Ni alloy before and after hydrogen absorption:

Fig. 4 TEM micrographs and ED patterns of as-milled (40 h) LaMg11Ni+xNi alloys:
Figure 6 depicts the P-C-T curves of the as-milled LaMg11Ni+xNi alloys. Compared with the P-C-T curve of the as-cast LaMg11Ni alloys (not shown), the absorption and desorption pressure plateaus of the as-milled LaMg11Ni+xNi (x=100%, 200%) alloys are found to show a clear inclination and the hysteresis (Hf=ln(Pa/Pd) becomes larger, suggesting that the addition of Ni and mechanical milling can also affect the thermodynamic behavior of the alloys. Moreover, it can be seen that milling time has an evident effect on the maximum hydrogen storage capacities of the alloys. The milling time dependence of the maximum hydrogen storage capacities are also inserted in Fig. 6. Evidently, the hydrogen storage capacities of the alloys first augment and then decline with prolonging milling time,and the maximum value is 5.9% and 6.2% (mass fraction) for Ni content x=100% and 200%, respectively, indicating that increasing Ni content makes a positive impact on the hydrogen absorption of the as-milled alloys. This can be ascribed to the fact that Ni can decrease the stability of metal hydrides and improve the hydrogen storage capacities. The improved hydrogen absorption and desorption kinetics by increasing Ni content is believed to create relatively catalytic alloy surface for the hydrogen reactions during mechanical milling.

Fig. 5 P-C-T curves of as-milled (40 h) LaMg11Ni+xNi alloys in temperature range of 553-613 K and Van’t Hoff diagrams for hydrogen absorption/desorption of alloys:
Table 1 Enthalpy change (△H) and entropy change (△S) of as-milled alloys obtained from Van’t Hoff plots

3.3 Gaseous hydrogen absorption/desorption kinetics
The hydrogen absorption kinetics of the alloy is evaluated by hydrogen absorption saturation ratio 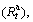 being defined as
being defined as 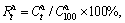 where
where 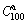 and
and  are hydrogen absorption capacities at the time of 100 min and t, respectively. Apparently, for a fixed time t, a larger saturation ratio
are hydrogen absorption capacities at the time of 100 min and t, respectively. Apparently, for a fixed time t, a larger saturation ratio 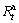 means better hydrogen absorption kinetics. In the current experiment, we find that the
means better hydrogen absorption kinetics. In the current experiment, we find that the 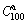 values of all the experimental alloys are more than 98% of their saturated hydrogen absorption capacity. Therefore,
values of all the experimental alloys are more than 98% of their saturated hydrogen absorption capacity. Therefore, 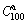 value can be reasonably considered as the saturated hydrogen absorption capacity of the alloys. Similarly, the hydrogen desorption kinetics of the alloy is symbolized by hydrogen desorption ratio
value can be reasonably considered as the saturated hydrogen absorption capacity of the alloys. Similarly, the hydrogen desorption kinetics of the alloy is symbolized by hydrogen desorption ratio 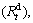 being defined as
being defined as 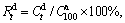 where
where 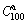 is the hydrogen absorption capacity at 100 min and
is the hydrogen absorption capacity at 100 min and 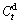 is the hydrogen desorption capacity at t min, respectively. For the better possibility of mutual comparison, we take 10 min as a benchmark for hydrogen absorption and 20 min for hydrogen absorption. Based on the above mentioned definitions, we derive the relationships between the
is the hydrogen desorption capacity at t min, respectively. For the better possibility of mutual comparison, we take 10 min as a benchmark for hydrogen absorption and 20 min for hydrogen absorption. Based on the above mentioned definitions, we derive the relationships between the  (t=10) as well as
(t=10) as well as  (t=20) values of the as-milled LaMg11Ni+xNi (x=100%, 200%) alloys and milling time, as described in Fig. 7. Evidently, with milling time prolonging, the
(t=20) values of the as-milled LaMg11Ni+xNi (x=100%, 200%) alloys and milling time, as described in Fig. 7. Evidently, with milling time prolonging, the 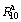 values of the as-milled alloys first increase and then decrease, while the
values of the as-milled alloys first increase and then decrease, while the  values of the alloys always increase. It means that prolonging milling time makes a beneficial contribution to the dehydriding kinetics of the alloys. More specifically, extending milling time from 5 to 60 h results in the
values of the alloys always increase. It means that prolonging milling time makes a beneficial contribution to the dehydriding kinetics of the alloys. More specifically, extending milling time from 5 to 60 h results in the  value rising from 37.8% to 48.5% for the x=100% alloy and from 41.6% to 53.4% for the x=200% alloy, respectively. It is clear that, for the same milling time, the x=200% alloy displays a much higher hydrogen absorption and desorption kinetics, indicating that increasing Ni content generates relatively catalytic alloy surface for the hydrogen reactions in the process of ball milling, as stated by ANIK et al [17].
value rising from 37.8% to 48.5% for the x=100% alloy and from 41.6% to 53.4% for the x=200% alloy, respectively. It is clear that, for the same milling time, the x=200% alloy displays a much higher hydrogen absorption and desorption kinetics, indicating that increasing Ni content generates relatively catalytic alloy surface for the hydrogen reactions in the process of ball milling, as stated by ANIK et al [17].

Fig. 6 P-C-T curves of as-milled LaMg11Ni+xNi (x=100%, 200%) alloys at 593 K and variation of hydrogen absorption capacity with milling time:

Fig. 7 Evolutions of  and
and  values of as-milled LaMg11Ni+xNi (x=100%, 200%) alloys at 593 K alloys with milling time
values of as-milled LaMg11Ni+xNi (x=100%, 200%) alloys at 593 K alloys with milling time
Figure 8 shows the evolutions of the hydrogen desorption capacities of the as-milled (40 h) LaMg11Ni+x Ni (x=100%, 200%) alloys with the reaction time in the temperature range of 553-613 K, from which we can see that the hydrogen desorption reaction is very intensive to temperature. To be specific, increasing hydrogen desorption temperature significantly promotes the hydrogen desorption rate. By comparing Fig. 8(a) with Fig. 8(b), it can be found that for all the dehydriding temperature, the x=200% alloy displays faster dehydriding kinetics than x=100% alloy, implying that increasing Ni content facilitates the improvement of the dehydriding kinetics.

Fig. 8 Hydrogen desorption kinetic curves of as-milled (40 h) LaMg11Ni+xNi (x=100%, 200%) alloys at 553, 573, 593 and 613 K:
3.4 Hydrogen desorption activation energy
Activation energy is regarded as an important parameter for evaluating the gas-solid reaction kinetics. As to gaseous hydrogen desorption, it is usually considered to be associated with total energy barrier concerning hydrogen desorption processes [18]. Hence, we can take advantage of the change in activation energy to evaluate the driving force of hydrogen desorption reaction.
Arrhenius and Johnson–Mehl–Avrami (JMA) equations were used to determine the dehydrogenation activation energy 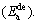 Arrhenius equation is given as follows:
Arrhenius equation is given as follows:
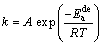 (2)
(2)
where A is a temperature independent coefficient, R is the universal gas constant (8.314 J/(mol·K)), T is the absolute temperature, and k is an effective kinetic parameter. The desorption curves have been analyzed using the JMA equation as [18-20]
 (3)
(3)
where α is the phase fraction transformed at time t which can be identified with a normalized hydrogen (ranges from 0 to 1%, mass fraction), and η is the Avrami exponent. Based on the data in Fig. 8, the logarithmic transform of Eq. (3) has been used to construct a graph of ln[-ln(1-α)] vs lnt at 553, 573, 593 and 613 K in which isothermal experimental data are found to be approximatively linear, as shown in Fig. 9. The linear fitting can be done by plotting lnk vs 1/T, which is also inserted in Fig. 9. From the slope of the Arrhenius plot of lnk vs 1/T, the activation energy can be easily calculated. The
can be easily calculated. The  values of the as-milled (40 h) LaMg11Ni+xNi (x=100%, 200%) alloys are 75.9 and 68.4 kJ/mol, respectively. Evidently, increasing Ni content can markedly decrease the hydrogen desorption activation energy of the LaMg11Ni alloys.
values of the as-milled (40 h) LaMg11Ni+xNi (x=100%, 200%) alloys are 75.9 and 68.4 kJ/mol, respectively. Evidently, increasing Ni content can markedly decrease the hydrogen desorption activation energy of the LaMg11Ni alloys.

Fig. 9 Plots of ln[-ln(1-α)] vs lnt of as-milled (40 h) LaMg11Ni+xNi (x=100%, 200%) alloys at 553, 573, 593 and 613 K and Arrhenius plots:
Another common way to calculate the activation energy is Kissinger method, and Kissinger equation is given as follows [21]:
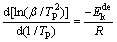 (4)
(4)
where 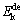 is the activation energy, β is the heating rate, TP is the absolute temperature corresponding to the maximum desorption rate in the DSC curves, and R is the ideal gas constant (8.314 J/(mol·K)). To meet the calculation requirement of Kissinger method, the hydrogen desorption reaction of the as-milled (40 h) LaMg11Ni+xNi (x=100%, 200%) alloys which have absorbed hydrogen at 573 K and 3 MPa are measured by DSC at heating rates of 5, 10, 15 and 20 K/min, as presented in Fig. 10. There is a clear endothermic peak occurring, which is in correspondence with the hydrogen desorption. Furthermore, all the alloys are found to display similar peak shapes, suggesting that each reaction is involved in the same reaction process.
is the activation energy, β is the heating rate, TP is the absolute temperature corresponding to the maximum desorption rate in the DSC curves, and R is the ideal gas constant (8.314 J/(mol·K)). To meet the calculation requirement of Kissinger method, the hydrogen desorption reaction of the as-milled (40 h) LaMg11Ni+xNi (x=100%, 200%) alloys which have absorbed hydrogen at 573 K and 3 MPa are measured by DSC at heating rates of 5, 10, 15 and 20 K/min, as presented in Fig. 10. There is a clear endothermic peak occurring, which is in correspondence with the hydrogen desorption. Furthermore, all the alloys are found to display similar peak shapes, suggesting that each reaction is involved in the same reaction process.

Fig. 10 DSC curves of as-milled (40 h) LaMg11Ni+xNi (x=100%, 200%) alloys at various heating rates and Kissinger plots:
Meanwhile, by comparing Fig. 10(a) with Fig. 10(b), we find that for each heating rate, the endothermic peak of the x=200% alloy has a drift to low temperature, indicating that the reaction rate is ameliorated as well by increasing Ni content in the desorption process. Based on the data in Fig. 10, the logarithmic transform of Eq. (4) has been used to build a graph of 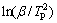 vs ln(1/TP), namely Kissinger plots, which are found to be approximatively linear, as inserted in Fig. 10. From the slope of the Kissinger plot, the activation energy
vs ln(1/TP), namely Kissinger plots, which are found to be approximatively linear, as inserted in Fig. 10. From the slope of the Kissinger plot, the activation energy 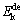 can be easily calculated. The
can be easily calculated. The 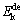 values of the as-milled (40 h) LaMg11Ni+xNi (x=100%, 200%) alloys are 70.4 and 63.0 kJ/mol, respectively.
values of the as-milled (40 h) LaMg11Ni+xNi (x=100%, 200%) alloys are 70.4 and 63.0 kJ/mol, respectively.
To reveal the effect of the milling time on the hydrogen activation energy of the alloys, Arrhenius and Kissinger methods are used to calculate the hydrogen desorption activation energies of all the as-milled LaMg11Ni+xNi (x=100%, 200%) alloys, and the variation of the  (for Arrhenius method Ede=
(for Arrhenius method Ede= for Kissinger method Ede=
for Kissinger method Ede= values of the alloys with the milling time are presented in Fig. 11. It is very evident that the activation energies calculated by the two methods both decrease with milling time prolonging, suggesting that the enhancement of the hydrogen desorption kinetics resulting from extending milling time originates from a decrease in the activation energy. Moreover, no matter which method, increasing Ni content both brings on a decrease in the hydrogen desorption activation energy, meaning that increasing Ni content obviously facilitates the amelioration of the hydrogen desorption kinetics of the alloys. By comparing the results calculated by the two methods, we can conclude that, the activation energy derived by Arrhenius method is larger than that by Kissinger method, which is likely ascribed to the change of hydrogen desorption reaction mechanism. A very similar result is also reported by BARICCO et al [22].
values of the alloys with the milling time are presented in Fig. 11. It is very evident that the activation energies calculated by the two methods both decrease with milling time prolonging, suggesting that the enhancement of the hydrogen desorption kinetics resulting from extending milling time originates from a decrease in the activation energy. Moreover, no matter which method, increasing Ni content both brings on a decrease in the hydrogen desorption activation energy, meaning that increasing Ni content obviously facilitates the amelioration of the hydrogen desorption kinetics of the alloys. By comparing the results calculated by the two methods, we can conclude that, the activation energy derived by Arrhenius method is larger than that by Kissinger method, which is likely ascribed to the change of hydrogen desorption reaction mechanism. A very similar result is also reported by BARICCO et al [22].
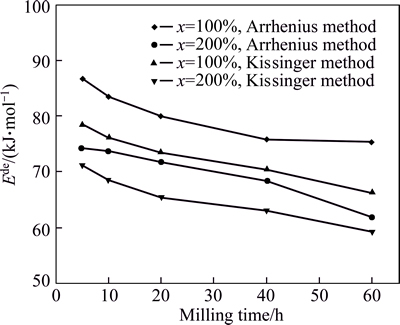
Fig. 11 Variations of hydrogen desorption activation energies of the as-milled LaMg11Ni+xNi (x=100%, 200%) alloys calculated by Arrhenius and Kissinger methods with milling time
Some elucidations can be provided for the effects of Ni content and milling time on the hydrogen absorption and desorption kinetics of the alloys. With respect to the positive contribution of the mechanical milling to the hydrogen storage kinetics, it is believed to be associated with the change of the alloy structure resulting from the ball milling. The crystalline alloy milled mechanically becomes at least partially disordered and its structure changes into nanocrystalline or amorphous. Besides, a lot of new crystallites and grain boundaries occur (see Fig. 4), which can provide numerous sites with low diffusion activation energy, thus facilitating the diffusion of hydrogen atoms in alloys [23]. Here, it is noteworthy that the ball milling for a higher time 20 h for the x=100% alloy or 10 h for the x=200% alloy will incur an undesirable decrease in the hydriding rate of the alloys, which is ascribed to the facilitated glass forming by adding Ni due to the fact that the diffusion ability of hydrogen atoms in an amorphous phase is much lower than in a nanocrystalline phase [24]. As to the positive action of increasing milling time on the hydrogen desorption kinetics, it is now well established that reducing the grain size far below the micrometer scale can dramatically improve the dehydrogenation properties of Mg-based alloys [25]. The improved hydrogen absorption and desorption kinetics by increasing Ni content is believed to create relatively catalytic alloy surface for the hydrogen reactions during mechanical milling [26].
4 Conclusions
1) The variation of Ni content incurs a very slight effect on the thermodynamic parameters (△H and △S) of the as-milled alloys, whereas it dramatically improves hydrogen absorption and desorption kinetics of the alloys, for which the decreased hydrogen desorption activation energy caused by increasing Ni content is basically responsible.
2) The gaseous hydrogen absorption capacity  and kinetics
and kinetics 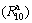 of the alloys have maximum values with varying milling time, but hydrogen desorption kinetics
of the alloys have maximum values with varying milling time, but hydrogen desorption kinetics 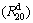 always increases with milling time prolonging, which is attributed to the facilitated glass forming by increasing Ni content and decreased hydrogen desorption activation energy by extending milling time.
always increases with milling time prolonging, which is attributed to the facilitated glass forming by increasing Ni content and decreased hydrogen desorption activation energy by extending milling time.
3) Arrhenius and Kissinger methods are used to evaluate hydrogen desorption activation energy of the as-milled alloy. The results reveal that increasing Ni content and prolonging milling time can markedly reduce the hydrogen desorption activation energy, which is responsible for the enhancement of the hydrogen desorption kinetics of the as-milled alloys.
References
[1] MORI D, HIROSE K. Recent challenges of hydrogen storage technologies for fuel cell vehicles [J]. International Journal of Hydrogen Energy, 2009, 34(10): 4569-4574.
[2] O’MALLEY K, ORDAZ G, ADAMS J, RANDOLPH K, AHN C C, STETSON N T. Applied hydrogen storage research and development: A perspective from the U.S. Department of Energy [J]. Journal of Alloys and Compounds, 2015, 645: S419-S422.
[3] ZHANG Y H, YANG T, ZHAI T T, YUAN Z M, ZHANG G F, GUO S H. Effects of stoichiometric ratio La/Mg on structures and electrochemical performances of as-cast and annealed La–Mg– Ni-based A2B7-type electrode alloys [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(6): 1968-1977.
[4] WANG Y, WANG X, LI C M. Electrochemical hydrogen storage of ball-milled MmMg12 alloy-Ni composites [J]. International Journal of Hydrogen Energy, 2010, 35(8): 3550-3554.
[5] ZHANG Y H, XU S, ZHAI T T, YANG T, YUAN Z M, ZHAO D L. Hydrogen storage kinetics of nanocrystalline and amorphous Cu–Nd-added Mg2Ni-type alloys [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(11): 3524-3533.
[6] ZHANG Y H, HAN Z G, YUAN Z M, YANG T, QI Y, ZHAO D L. Electrochemical properties of nanocrystalline and amorphous Mg–Y–Ni alloys applied to Ni-MH battery [J]. Transactions of Nonferrous Metals Society of China, 2015(11): 3736-3746.
[7] MENDOZA Z L, MEYER M, BAUM L. Complex quaternary hydrides Mg2(Fe, Co)Hy for hydrogen storage [J]. International Journal of Hydrogen Energy, 2011, 36(1): 600-605.
[8] YANG T, YUAN Z M, BU W G, JIA Z C, QI Y, ZHANG Y H. Evolution of the phase structure and hydrogen storage thermodynamics and kinetics of Mg88Y12 binary alloy [J]. International Journal of Hydrogen Energy, 2016, 41(4): 2689-2699.
[9] KUMAR L H, VISWANATHAN B, MURTHY S S. Hydrogen absorption by Mg2Ni prepared by polyol reduction [J]. Journal of Alloys and Compounds, 2008, 461(1, 2): 72-76.
[10] ZHANG Y H, ZHANG G F, LI X, HOU Z H, REN H P, ZHAO D L. Structure and hydrogen storage kinetics of asspun Mg2Ni type alloys [J]. Journal of Central South University (Science and Technology), 2012, 43(6): 2101-2107. (in Chinese)
[11] YUAN H J, AN Y, XU G H, CHEN C P. Hydriding behavior of magnesium-based hydrogen storage alloy modified by mechanical ball-milling [J]. Materials Chemistry and Physics, 2004, 83(2, 3): 340-344.
[12] ZHANG Q A, JIANG C J, LIU D D. Comparative investigations on the hydrogenation characteristics and hydrogen storage kinetics of melt-spun Mg10NiR (R=La, Nd and Sm) alloys [J]. International Journal of Hydrogen Energy, 2012, 37(14): 10709-10714.
[13] POLETAEV A A, DENYS R V, MAEHLEN J P, SOLBRG J K, TARASOV B P, YARTYS V A. Nanostructured rapidly solidified LaMg11Ni alloy: Microstructure, crystal structure and hydrogenation properties [J]. International Journal of Hydrogen Energy, 2012, 37(4): 3548-3557.
[14] ABDELLAOUI M, MOKBLI S, CUEVAS F, LATROCHE M, PERCHERON G A, ZARROUK H. Structural and electrochemical properties of amorphous rich MgxNi100-x nanomaterial obtained by mechanical alloying [J]. Journal of Alloys and Compounds, 2003, 356-357: 557-561.
[15] TERESIAK A, GEBERT A, SAVYAK M, UHLEMANN M, MICKEL C H, MATTERN N. In situ high temperature XRD studies of the thermal behaviour of the rapidly quenched Mg77Ni18Y5 alloy under hydrogen [J]. Journal of Alloys and Compounds, 2005, 398(1, 2): 156-164.
[16] ZHANG R J, LV M Q, CHEN D M, YANG K. Investigation of stability and hydrogen storage properties of LaNi5-xMx (M = Al, Mn) alloys [J]. Acta Metallurgica Sinica, 2005, 41(4): 427-432.
[17] ANIK M, KARANFIL F,  N. Development of the high performance magnesium based hydrogen storage alloy [J]. International Journal of Hydrogen Energy, 2012, 37(1): 299-308.
N. Development of the high performance magnesium based hydrogen storage alloy [J]. International Journal of Hydrogen Energy, 2012, 37(1): 299-308.
[18] SADHASIVAM T, HUDSON M S L, PANDEY S K, BHATNAGAR A, SINGH M K, GURUNATHAN K. Effects of nano size mischmetal and its oxide onimproving the hydrogen sorption behaviour of MgH2 [J]. International Journal of Hydrogen Energy, 2013, 38(18): 7353-7362.
[19] AVRAMI M. Kinetics of phase change—I. General theory [J]. Journal of Chemical Physics, 1939, 7(12): 1103-1112.
[20]  C R. Simultaneous TDS-DSC measurements in magnesium hydride [J]. Journal of Alloys and Compounds, 2003, 356-357: 348-352.
C R. Simultaneous TDS-DSC measurements in magnesium hydride [J]. Journal of Alloys and Compounds, 2003, 356-357: 348-352.
[21] KISSINGER H E, Reaction kinetics in differential thermal analysis, Analytical Chemistry, 1957, 29(11): 1702-1706.
[22] BARICCO M, RAHMAN M W, LIVRAGHI S, CASTELLERO A, ENZO S, GIAMELLO E. Effects of BaRuO3 addition on hydrogen desorption in MgH2 [J]. Journal of Alloys and Compounds, 2012, 536S: S216-S221.
[23] WU Y, HAN W, ZHOU S X, LOTOTSKY M V, SOLBERG J K, YARTYS V A. Microstructure and hydrogenation behavior of ball-milled and melt-spun Mg-10Ni-2Mm alloys [J]. Journal of Alloys and Compounds, 2008, 466(1, 2): 176-181.
[24] XIE D H, LI P, ZENG C X, SUN J W, QU X H. Effect of substitution of Nd for Mg on the hydrogen storage properties of Mg2Ni alloy [J]. Journal of Alloys and Compounds, 2009, 478(1, 2): 96-102.
[25] SONG M Y, YIM C D, KWON S N, BAE J S, HONG S H. Preparation of Mg-23.5Ni-10(Cu or La) hydrogen-storage alloys by melt spinning and crystallization heat treatment [J]. International Journal of Hydrogen Energy, 2008, 33(1): 87-92.
[26] ANIK M, Electrochemical hydrogen storage capacities of Mg2Ni and MgNi alloys synthesized by mechanical alloying [J]. Journal of Alloys and Compounds, 2010, 491(1, 2): 565-570.
(Edited by FANG Jing-hua)
Cite this article as: ZHANG Yang-huan, YUAN Ze-ming, YANG Tai, BU Wen-gang, HOU Zhong-hui, ZHAO Dong-liang. Gaseous hydrogen storage thermodynamics and kinetics of RE–Mg–Ni-based alloys prepared by mechanical milling [J]. Journal of Central South University, 2017, 24(4): 773-781. DOI: 10.1007/s11771-017-3479-8.
Foundation item: Projects(51161015, 51371094, 51471054) supported by the National Natural Science Foundation of China
Received date: 2016-01-15; Accepted date: 2016-05-10
Corresponding author: ZHANG Yang-huan, Professor, PhD; Tel: +86-10-62183115; E-mail: zhangyh59@sina.com