Preparation and sintering of nano Fe coated Si3N4 composite powders
来源期刊:中南大学学报(英文版)2009年第2期
论文作者:银锐明 范景莲 刘勋
文章页码:184 - 189
Key words:Fe; Si3N4; coating; heterogeneous precipitation; sintering
Abstract: Fe/Si3N4 composite powder was synthesized by the heterogeneous precipitation-thermal reduction process, and then pressed into flakes under a pressure of 10 MPa. Flakes were sintered by pressureless and hot-pressing at 1 600 ℃ under 0.1 MPa N2. The chemical composition, phases and microstructure of composite powder and sintered flakes were investigated by energy dispersive spectroscopy (EDS), X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The results show that the structure of composite powders is Si3N4 coated by nano Fe. The crystal phases of sintered flakes by pressureless are Fe(Si) compound, SiC and Si3N4. The crystal phases of the sintered samples by hot-pressing are Fe, Fe(Si) compound and Si3N4. It is found that crystal phases flakes obtained by pressureless and hot-pressing are very different.
基金信息:the National Natural Science Foundation of China
J. Cent. South Univ. Technol. (2009) 16: 0184-0189
DOI: 10.1007/s11771-009-0031-5 ![]()
YIN Rui-ming(银锐明)1, 2, FAN Jing-lian(范景莲)1, LIU Xun(刘 勋)1
(1. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
2. College of Packaging and Material Engineering, Hunan University of Technology, Zhuzhou 412008, China)
Abstract: Fe/Si3N4 composite powder was synthesized by the heterogeneous precipitation-thermal reduction process, and then pressed into flakes under a pressure of 10 MPa. Flakes were sintered by pressureless and hot-pressing at 1 600 ℃ under 0.1 MPa N2. The chemical composition, phases and microstructure of composite powder and sintered flakes were investigated by energy dispersive spectroscopy (EDS), X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The results show that the structure of composite powders is Si3N4 coated by nano Fe. The crystal phases of sintered flakes by pressureless are Fe(Si) compound, SiC and Si3N4. The crystal phases of the sintered samples by hot-pressing are Fe, Fe(Si) compound and Si3N4. It is found that crystal phases flakes obtained by pressureless and hot-pressing are very different.
Key words: Fe; Si3N4; coating; heterogeneous precipitation; sintering
1 Introduction
Silicon nitride (Si3N4) is regarded as one of the most promising structural materials for its light mass, high strength, good thermal shock, outstanding wear resistance and excellent high-temperature properties such as resistance to creep and to corrosion within utilization atmosphere, and good mechanical properties over a wide temperature range. However, the wide use of Si3N4 is undermined due to its catastrophic fracture [1-3].
Combining the properties of cermet materials with the superior mechanical properties of ductile solids can produce high strength materials with reasonable toughness. Therefore, this technique has attracted much attention [4-5]. Metal iron, with lower cost, excellent ductile properties, could be used as a ductile phase to improve the toughness of brittle nitride based upon its fine wetting and cohesiveness with Si3N4.
Fine particle coating, an interesting researching area, has numerous applications. In advanced technology, a composite material is employed where the characteristics of the surface are specifically required to be different from those of the core material. This is caused by the requirement on the material to exhibit a combination of various and conflicting properties. For example, a mechanical component may require both high hardness and toughness. This can be obtained by designing a composite material with a high surface toughness and, meanwhile, a hardness core [6-7].
There are many coating methods intended for metal coating, such as sol-gel [8], electroless plating [9], physical deposition [10], chemical deposition [11] and heterogeneous precipitation [12-13]. The heterogeneous precipitation-thermal reduction process has been intensively investigated recently due to its lower cost and facility to obtain well-distributed particles on the coating layers.
In this study, the heterogeneous precipitation- thermal reduction method was introduced to fabricate nano Fe coated Si3N4. Si3N4 was sintered by pressureless and hot-pressing at 1 600 ℃ under 0.1 MPa N2. The chemical composition, phases and microstructure of composite powder and sintered flakes were investigated.
2 Experimental
2.1 Rough material
Si3N4 powders (Ube SN-E10, D50 = 1.3 μm, Particles micromorphology shown in Fig.1), FeSO4?7H2O (analytical grade), Na2CO3 (analytical grade) and polyethylene glycol (PEG 400) were used; water for the preparation of all solutions was double distilled.
2.2 Sample preparation
Precursor with nano Fe coated Si3N4 was prepared by gradually adding iron sulfate solution and sodium carbonate solution into the reactor, in which Si3N4 powders and PEG 400 were suspended in aqueous solution under ultrasonic dispersion and mechanical stirring at room temperature. The precursor of nano Fe coated Si3N4 was obtained after the precipitates were filtered and washed three times with distilled water, and then dried at 80 ℃ for 12 h. Nano Fe coated Si3N4 powders were then derived from thermal reduction of the prepared precursors at 800 ℃ for 1 h under H2 atmosphere in a tubular oven. The as-prepared composite powders were non-pressurized at 1 600 ℃ under N2 (with pressure of 0.1 MPa) for 1 h and hot-pressed at 1 600 ℃ under uniaxial pressure of 20 MPa and N2(with pressure of 0.1 MPa) for 1 h. The preparation process is shown in Fig.2.

Fig.1 SEM image of Si3N4 powder
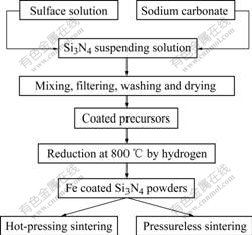
Fig.2 Flow chart of preparing nano Fe coated Si3N4
2.3 Sample detection
The phase, composition and morphology of the as-prepared precursors, resultant products derived from thermal reduction of the precursors and the morphologies and elements distributions of sintered samples were characterized by X-ray diffractometry (XRD) (D/max 2550) with Cu Ka radiation, energy dispersive spectroscopy (EDS) (EDX-GENESIS 60S), scanning electron microscopy (SEM) (JSM-6360LV) and transmission electron microscopy (TEM).
3 Results and discussion
3.1 Preparation of precursors and composite powers
According to the heterogeneous precipitation theory [14-15], regulation of several processing factors such as concentration and adding rate of reactant solutions, pH value during the precipitation processes and stirring speed of agitation can impact uniformly the coatings on Si3N4. Effects of preparative conditions on precursors for nanometer Fe coated Si3N4 are listed in Table 1. It is found that uniform coatings on Si3N4 are obtained under the optimized precipitation conditions: 15 g/L of Si3N4 powders, 0.15 mol/L of FeSO4 solution, 3 mL/min of feeding rate, 1 200 r/min of agitation speed, and pH 8.
Table 1 Effects of preparative conditions on precursors for nano Fe coated Si3N4

The XRD pattern for the precursors of nano Fe coated Si3N4 is shown in Fig.3. It can be seen that only the Fe2O3, Si3N4 and Na2SO4 phases are detected. Na2SO4 is the reaction product between Na2CO3 and FeSO4, and it can be removed by washing with distilled water. There are three reactions taking place in the precipitation process [16-17]:
FeSO4+Na2CO3=FeCO3↓+Na2SO4 (1)
FeCO3+O2+2H2O=4FeO(OH)+4CO2↑ (2)
2FeO(OH)(Roasting)=Fe2O3+H2O↑ (3)

Fig.3 XRD pattern of Fe/Si3N4 composite powder precursor
The XRD pattern for nano Fe coated Si3N4 is shown in Fig.4. It can be seen that only the Fe and Si3N4 phases are detected. There is a reaction taking place in the thermal reduction process.

Fig.4 XRD pattern of Fe/Si3N4 composite powder
Fe2O3 +3H2=2Fe+3H2O (4)
Fig.5 shows EDS linescan analysis of nano Fe coated Si3N4 single particle. Peaks for element Fe distribution on the scanline were observed. These results show that Fe is uniformly dispersed on the Si3N4 surface.
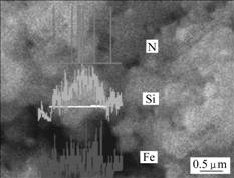
Fig.5 EDS linescan analysis of Fe/Si3N4 composite powder
Fig.6 shows the structure of single particles. It can be clearly seen the core-shell structure. The diameter and pattern of core is very similar to those of Si3N4 in Fig.1. The thickness of shell is about 20 nm. Fig.4 shows that only the Fe and Si3N4 phases are detected in the composite powders. So it is considered that structure of composite powders is nano Fe coated Si3N4.

Fig.6 TEM image of Fe/Si3N4 composite powder
3.2 Sintering of composite powders and characterization of sintered samples
3.2.1 Analysis of fractograph
The as-prepared composite powders were non-pressurized at 1 600 ℃ under N2 (with pressure of 0.1 MPa) for 1 h and hot-pressed at 1 600 ℃ under uniaxial pressure of 20 MPa and N2 (with pressure of 0.1 MPa) for 1 h. The bulk density of the hot-pressed samples was 3.3 g/cm3, and the bending strength was about 400 MPa. The bulk density of the non-pressurized samples was 2.8 g/cm3, and the bending strength was about 80 MPa. The performance of the hot-pressed samples is much better than that of the non-pressurized samples.
Fig.7 shows the XRD patterns of broken sections of Fe/Si3N4 cermets by pressureless-sintering and hot-pressing sintering. It can be seen that besides α-Si3N4, new phase β-Si3N4, Fe(Si) compounds could be identified in both samples. Fe in the hot-pressed samples could be identified, but that in the non-pressurized samples could not be identified.

Fig.7 XRD patterns of broken sections of Fe/Si3N4 cermets by pressureless-sintering (a) and by hot-pressing sintering (b)
New phase β-Si3N4 could be identified in both samples. PAVARAJAM and KIMURA [18] believed that Fe and Fe(Si) compounds can form liquid phase at the sintering temperature in the samples, and it may promote the conversion.
New phase Fe(Si) compounds could be identified in the samples due to the reaction between Fe and Si3N4. There are possible reactions taking place in the sintering process:
3/2Fe+Si3N4=3/2FeSi2+2N2(g)
?rG=612 820-299.4T (5)
9Fe+Si3N4=3Fe3Si+2N2(g)
?rG=204 208-657.396T (6)
3Fe+Si3N4=3FeSi+2N2(g)
?rG=507 628-377.96T (7)
5Fe+Si3N4=Fe5Si3+2N2(g)
?rG=496 704-340.96T (8)
As the Gibbs free energy of Reaction (6) becomes negative at 310.63 K, so it could be processed at room temperature. But in fact the reactions do not take place. According to Ref.[19], coating of amorphous SiO2 with Si3N4 produces barrier film, which inhibits reactions between Fe and Si3N4. However, Fe can damage the protective layer (SiO2) of Si3N4, leading to its crystallization and cracking with the increase of temperature [20]. Therefore, Fe(Si) compounds are produced for direct contact, leading to the reaction between Fe and Si3N4.
Fe in the hot-pressed samples could be identified, but that in the non-pressurized could not be identified. It is concluded that Si3N4 reacts with non-nitride metal, the evolved N2 gas may be removed from the metal ceramic interface through cracks, and it may also be trapped by pores under high pressure in the interaction zone. The hot-pressed samples can form some enclosed-holes in the sintering process. Figs.8 and 9 show the SEM images of the cross-section of the non-pressurized samples and the hot-pressed samples. They show open-pores in the non-pressurized samples and close-pores in the hot-pressed

Fig.8 SEM images of broken sections of Fe/Si3N4 cermets by pressureless-sintering: (a) Higher magnification; (b) Lower magnification

Fig.9 SEM images of broken section of Fe/Si3N4 cermets by hot-pressing sintering: (a) Higher magnification; (b) Lower magnification
samples. The close-pores are beneficial to trapping evolved N2 gas. In addition, extreme N2 pressures are built up in the close-pores of the hot-pressed samples by extreme external pressures. As Fe and Fe(Si) compounds are in liquid phase at 1 600 ℃, it can be thought that close-pores are in liquid at 1 600 ℃. According to PASCAL’s law [21], the force on the close-pores in the liquid is equal to the external pressure approximately, so build-up, N2 pressures is equal to 20 MPa approximately in the close-pores of the hot-pressed samples. Fig.10 shows force diagram of close-pores in the hot-pressing sintering process. The pressure build-up can control the extent of the reaction through the dissociation constant of silicon nitride:
Si3N4→3[Si]Fe+2N2 (9)
Which gives:
![]() =k (10)
=k (10)
where aSi is the decomposition of Si3N4, pN is the N2 pressure, and k is a constant.
According to Reaction (10), the higher the N2 pressure, the more difficult the decomposition of Si3N4。
HEIKINHEIMO et al [22] presented a pN2=f(t) stability diagram (Fig.10) showing regions of the solid solution and the silicides in equilibrium with Si3N4 as a function of nitrogen pressure. It gives us an estimate of the nitrogen pressure for each phase in equilibrium with Si3N4. It shows the tendency of the silicides converting to solid solution in the reaction product between Fe and Si3N4 with the increase of partial nitrogen pressure. Thus, it is thought that the reaction between Fe and Si3N4 can be controlled when nitrogen pressure is equal to 20 MPa, and Fe cannot be depleted in the reaction, but kept up in the hot-pressed examples.
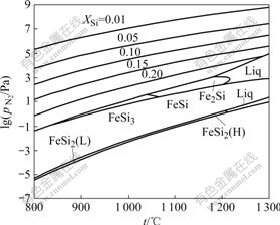
Fig.10 Stability diagram for Fe-Si-N system
The evolved N2 gas can easily remove from open-pores in the non-pressurized samples. High nitrogen pressure cannot form in the samples and Fe can be depleted during the sharp reaction between Fe and Si3N4.
3.2.2 Fracture composition analysis
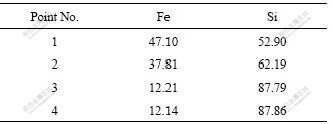
Five points (1, 2, 3, 4, 5) in Fig.8(a) and four points (1, 2, 3, 4) in Fig.9(a) were done by EDS. It shows that N-element energy spectrum is nearly zero. As N2 produced by the reaction between Fe and Si3N4 escapes or is trapped, the content of element N is very low on the face of cross-section. HEIKINHEIMO et al [21] showed the backscattered electron image of the cross-section of the diffusion couple between Fe and Si3N4 annealed at 1 150 ℃ for 16 h. Similar results were found and proved. Elements Fe and Si can be checked on every point, so element Fe was well-distributed in the cermet. Table 2 lists mass fractions of elements of Fe and Si on five points (1, 2, 3, 4, 5) in Fig.8(a), and Table 3 lists mass fractions of elements of Fe and Si on four points (1, 2, 3, 4) in Fig.9(a). There is high content of element of Fe in large particle on broken section of Fe/Si3N4 cermets by pressureless-sintering and the particles substance on broken section of Fe/Si3N4 cermets by hot-pressing sintering. It is considered that complete reaction between Fe and Si3N4 occurs and leads to grain growth.
Table 2 EDS of broken section compounds on five points in Fig.8(a) (mass fraction, %)

Table 3 EDS of broken section compounds on four points in Fig.9(a) (mass fraction, %)
4 Conclusions
(1) Nano Fe coated Si3N4 composite powders are prepared by heterogeneous precipitation-thermal reduction process.
(2) N2 gas can easily remove from open-pores in the non-pressurized samples. High nitrogen pressure cannot form in the samples and Fe can be depleted by the sharp reaction between Fe and Si3N4.
(3) Extreme N2 pressures are built up in the close-pores of the hot-pressed samples by extreme external pressures. The pressure build-up can control the extent of the reaction between Fe and Si3N4. Fe cannot be depleted and kept up in the hot-pressed examples.
References
[1] VILA M, CARRAPICHANO J M, GOMES J R, CAMARGOJR S S, ACHETE C A, SILVA R F. Ultra-high performance of DLC-coated Si3N4 rings for mechanical seals [J]. Wear, 2008, 265(5/6): 940-944.
[2] BELMONTE M, de PABLOS A, OSENDI M I, MIRANZO P. Effects of seeding and amounts of Y2O3?Al2O3 additives on grain growth in Si3N4 ceramics [J]. Materials Science and Engineering A, 2008, 475(1/2): 185-189.
[3] KRNEL K, MAGLIC A, KOSMA? T. β-SiAlON/TiN nanocomposites prepared from TiO2-coated Si3N4 powder [J]. Journal of the European Ceramic Society, 2008, 28(5): 953-957.
[4] SHI Xiao-liang, YANG Hua, SHAO Gang-qin, DUAD Xing-long, XIONG Zheng. Microwave sintering of Al2O3/WC-10Co/ZrO2/Ni cermets [J]. Journal of Central South University: Science and Technology, 2007, 38(4): 623-628. (in Chinese)
[5] YIN Rui-ming, LIU Xun, FAN Jing-lian, LING Guo-liang. Hot pressing densification process for nano Ni-Al2O3 powders [J]. Journal of Central South University: Science and Technology, 2004, 35(1): 21-25. (in Chinese)
[6] ZHANG Q C, SHEN Y G. High performance W-AlN cermet solar coatings designed by modelling calculations and deposited by DC magnetron sputtering [J]. Solar Energy Materials and Solar Cells, 2004, 81(1): 25-37.
[7] WANG Y G, ZHANG D, CHEN M. Development of coating material by in-situ reaction synthesis [J]. Key Engineering Materials, 2007, 353/358(3): 1696-1699.
[8] BRINLEY E, SEAL S, FOLKS R, BRAUNSTEIN E, KRAMER L. High efficiency SiO2-TiO2 hybrid sol-gel antireflective coating for infrared applications [J]. Journal of Vacuum Science and Technology A, 2006, 24(4): 1141-1146.
[9] ENDO H, MARUI E. Additional effect of electroless plating film (damping capacity improvement) [J]. Industrial Lubrication and Tribology, 2002, 54(6): 262-267.
[10] MALHOTRA C P, MAHAJAN R L, SAMPATH W S. High Knudsen number physical vapor deposition: Predicting deposition rates and uniformity [J]. Journal of Heat Transfer, 2007, 129(11): 1546-1553.
[11] MATHUR S, SHEN H, ALTMAYER J. Nanostructured functional ceramic coatings prepared by molecule-based chemical vapor deposition [J]. Reviews on Advanced Materials Science, 2007, 15(1): 16-23.
[12] ZHU Sheng, LI Chao, DU Jian-hua, HAN Wen-zheng. Preparation and characterization of Cu-coated nano SiC composite particles [J]. Key Engineering Materials, 2008, 373/374: 674-677.
[13] PERRARD F, DONNADIEU P, DESCHAMPS A, BARGES P. TEM study of NbC heterogeneous precipitation in ferrite [J]. Philosophical Magazine, 2006, 86(27): 4271-4284.
[14] CHANG S M, LEE M, KIM W S. Preparation of large monodispersed spherical silica particles using seed particle growth [J]. J colloid Interface Sci, 2005, 286: 536-542.
[15] NOMURA T, ALONSO M, KOUSAKA Y, TANAKA K. A model for simultaneous homogeneous and heterogeneous nucleation [J]. J Colloid Interface Sci, 1998, 203: 170-176.
[16] SHEN Xiang-qian, JING Mao-xiang, LI Wang-xing, LI Dong-hong. Fabrication of Fe, Ni and FeNi coated Al2O3 core-shell microspheres by heterogeneous precipitation [J]. Powder Technology, 2005, 160(3): 229-333.
[17] CHEN Dong, LI Dong-hong, SHEN Xiang-qian, JING Mao-xiang. The preparation of nano iron-graphite composite microspheres [J]. Mining and Metallurgical Engineering, 2006, 26(5): 75-78. (in Chinese)
[18] PAVARAJAM V, KIMURA S. Catalytic effects of metals on direct nitridation of silicon [J]. J Am Ceram Soc, 2001, 84(8): 1669-1674.
[19] ZHANG Qi-tu. Study on oxidation behavior and oxidation resistance of Si3N4 ceramics [J]. Journal of Ceramic, 2000, 21(1): 23-27. (in Chinese)
[20] SWAIN M V. Structure and properties of ceramics [M]. New York: Materials Science and Technology Press, 1998.
[21] ZHU Hong-tao. Hydraulic and pneumatic transmission [M]. Beijing: Tsinghua University Press, 2005.
[22] HEIKINHEIMO E, ISOMAKI I, KODENTSOV A A, VANLOO F J J. Chemical interaction between Fe and silicon nitride ceramic [J]. Journal of the European Ceramic Society, 1997, 17(1): 25-31.
Foundation item: Project(50804016) supported by the National Natural Science Foundation of China
Received date: 2008-07-06; Accepted date: 2008-09-29
Corresponding author: FAN Jing-lian, PhD; Tel: +86-731-8836652; E-mail: fjl@mail.csu.edu.cn
(Edited by YANG You-ping)

