从熔炉渣中氯盐浸出铅的参数优化和动力学
来源期刊:中国有色金属学报(英文版)2017年第12期
论文作者:Mohammad Hasan GOLPAYEGANI Ali Akbar ABDOLLAHZADEH
文章页码:2704 - 2714
关键词:铅;炉渣;氯盐浸出;优化;动力学
Key words:lead; melting furnace slag; chloride leaching; optimization; kinetics
摘 要:研究采用氯盐浸出法从传统熔铅炉渣中回收铅的可行性和动力学。考察工艺参数如浸出时间、NaCl浓度、FeCl3浓度、液/固比、搅拌速度、浸出温度、颗粒尺寸对铅回收率的影响。基于中心复合设计模型,利用响应面法对上述参数进行优化,得到优化工艺条件如下:浸出时间60 min、浸出温度80 °C、搅拌速度800 r/min、NaCl浓度200 g/L、FeCl3浓度 80 g/L、液固比16、颗粒尺寸小于106 μm。在此优化条件下96% Pb被回收。基于方差分析法,确定浸出温度、液固比和NaCl浓度为影响浸取过程的最有效参数。动力学研究结果表明,方铅石的氯盐浸出过程为一级反应过程。反应机理为固态反应产物的扩散和化学反应。采用Arrhenius模型计算得到从方解石中氯盐浸出铅的激活能为27.9 kJ/mol。
Abstract: The feasibility and kinetics of lead recovery from the slag of traditional lead melting furnace using chloride leaching were investigated. The effects of operating parameters such as leaching time, NaCl concentration, FeCl3 concentration, liquid/solid ratio, stirring rate, temperature, and particle size on recovery of lead were studied and the optimization was done through the response surface methodology (RSM) based on central composite design (CCD) model. The optimum conditions were achieved as follows: leaching time 60 min, 80 °C, stirring rate 800 r/min, NaCl concentration 200 g/L, FeCl3 concentration 80 g/L, liquid/solid ratio 16, and particle size less than 106 μm. More than 96% of lead was effectively recovered in optimum condition. Based on analysis of variance, the reaction temperature, liquid/solid ratio, and NaCl concentration were determined as the most effective parameters on leaching process, respectively. Kinetics study revealed that chloride leaching of galena is a first-order reaction and the diffusion through solid reaction product and chemical reaction control the mechanism. The activation energy of chloride leaching of galena was determined using Arrhenius model as 27.9 kJ/mol.
Trans. Nonferrous Met. Soc. China 27(2017) 2704-2714
Mohammad Hasan GOLPAYEGANI1, Ali Akbar ABDOLLAHZADEH1,2
1. Department of Mining Engineering, Kashan University, Kashan, Iran;
2. Department of Mining Engineering, Amirkabir University of Technology, Tehran, Iran
Received 22 October 2016; accepted 27 January 2017
Abstract: The feasibility and kinetics of lead recovery from the slag of traditional lead melting furnace using chloride leaching were investigated. The effects of operating parameters such as leaching time, NaCl concentration, FeCl3 concentration, liquid/solid ratio, stirring rate, temperature, and particle size on recovery of lead were studied and the optimization was done through the response surface methodology (RSM) based on central composite design (CCD) model. The optimum conditions were achieved as follows: leaching time 60 min, 80 °C, stirring rate 800 r/min, NaCl concentration 200 g/L, FeCl3 concentration 80 g/L, liquid/solid ratio 16, and particle size less than 106 μm. More than 96% of lead was effectively recovered in optimum condition. Based on analysis of variance, the reaction temperature, liquid/solid ratio, and NaCl concentration were determined as the most effective parameters on leaching process, respectively. Kinetics study revealed that chloride leaching of galena is a first-order reaction and the diffusion through solid reaction product and chemical reaction control the mechanism. The activation energy of chloride leaching of galena was determined using Arrhenius model as 27.9 kJ/mol.
Key words: lead; melting furnace slag; chloride leaching; optimization; kinetics
1 Introduction
Studying on the mineral industries waste is of considerable importance regarding the notable decrease of high grade mineral deposits, removal of environmental hazards of toxic metals and minimizing the industrial wastes. The conventional method of lead recovery from sulfide forms is pyrometallurgy. Although this method of recovery is economically preferred to other methods, it results in a lead dust and formation of SO2, which are environmentally harmful [1,2]. Additionally, a major part of valuable materials may be converted to melting furnace slag as lead sulfate, lead oxide, and lead sulfide and wasted in case of disobeying the suitable operating parameters [3,4]. Several studies have been carried out to consider the lead recovery from its wastes including wastes of zinc hydrometallurgy, pyrometallurgy slags, scrap metals containing lead, lead content of expired lead batteries [5-11]. There are miscellaneous methods of lead recovery from wastes including carbothermic reduction, alkaline dissolution with and without microwave, melting by sodium carbonate, dissolution in acidic or alkaline media [5].
The chloride leaching has devoted lots of studies regarding low cost of leaching agents and fast dissolution of lead chloride in the chloride ion solutions of appropriate concentration [12]. VOLSKII and AGRACHEVA [13] studied the galena leaching using ferric chloride and 98%-99% of lead could be extracted using Fe3+ solution of 100-200 g/L. About 100% lead was recovered using 28-147 g/L Fe3+ solution at 100 °C [3]. QIN et al [12] studied the feasibility of pure lead sulfate powder production through galena leaching. MOZAFFARI et al [14] investigated the leaching of lead and mercury from Lak mine lead concentrate. They recovered 99.8% of lead in FeCl3-NaCl system. ABDOLLAHI et al [15] considered the lead cementation from leaching chloride solution of lead sulfate using aluminum powder. They indicated that the chloride leaching recovery method is an economic and fast method. Regarding the importance of chloride leaching using ferric chloride, miscellaneous researchers have investigated the kinetics of this system. MURRAY [16] studied the galena solubility kinetics in 0.5 mol/L ferric chloride solution. In this research, the activation energy of reaction was 56.5 kJ/mol and the rate determining of reaction was considered the diffusion of reagents and products in the sulfur layers during the reaction. It was found that the leaching rate is strictly related to ferric chloride concentration [3]. DEMOPOULOS [17] investigated the galena solubility kinetics and obtained the activation energy of 56.6 kJ/mol, indicating that the leaching rate is controlled on the galena surface regarding the chemical reactions. Additionally, He found that this chemical control is reduced at low ferric chloride concentration. KIM et al [18] studied the granulated galena solubility in FeCl3 solution and found that the leaching rate is deeply associated with the specific area of galena particles. They also found that the leaching rate increases as the NaCl concentration is increased. They obtained the activation energy of 53 kJ/mol in the medium without NaCl while 38 kJ/mol was obtained in 3 mol/L NaCl solution. FUERSTENAU et al [19] showed that the galena leaching reaction is controlled by diffusion of ferric ions in the elemental sulfur layers at FeCl3 concentration higher than 0.2 mol/L. They obtained the activation energy of 33.7 kJ/mol. RATH et al [20] considered the granulated galena solubility in solution containing 0.1 mol/L Fe3+ and 0.5 mol/L NaCl in temperature range of 14-80 °C and indicated the electrochemical control of the reaction based on the shrinking core model. SOHN and BAEK [21] presented a combined model for the kinetics of galena leaching reaction. In this model, it was supposed that the diffusion of PbCl2 in layers of elemental sulfur produced during reaction and the reaction on the galena surface simultaneously controlled the reaction through shrinking core model. DUTRIZAC and CHEN [2] comprehensively studied the galena solubility of the FeCl3-NaCl system. They considered the relation between the PbCl2 and leaching rate to show the diffusion of PbCl2 at the porous spaces of elemental sulfur. They also found that a layer of elemental sulfur forms during the leaching and PbCl2 diffuses into the sulfur pores, and the corroded galena surface results in a nonlinear kinetic of the process. BABA and ADEKOLA [22] compared the kinetics of galena leaching in FeCl3-HCl and H2O2-HCl systems and obtained the activation energy of 26.5 kJ/mol in the system containing 0.3 mol/L FeCl3 and 8.06 mol/L HCl, while the activation energy of 40.06 kJ/mol was obtained in the presence of 8.06 mol/L of HCl and H2O2. They suggested the chemical reaction control model as the controlling mechanism of galena leaching in both studied systems.
In this work, the chloride leaching of lead from slag of lead melting furnace was studied. The parameters such as leaching time, NaCl concentration, ferric chloride concentration, liquid/solid ratio, stirring rate, leaching temperature, and particle size were considered as affecting parameters on the leaching process. These parameters were optimized through the response surface methodology (RSM) based on central composite design (CCD) model. As the thermodynamics predicts the spontaneity of the reactions, the kinetics determines the reaction rate and considers the influent parameters on yield and efficiency of reaction directly affecting the engineering and industrial planning of the process [23]. In addition to the optimization of leaching reaction, the kinetics of the system was investigated including presenting a kinetic model. The activation energy of the optimized kinetic model was determined as well.
2 Experimental
2.1 Slag sample
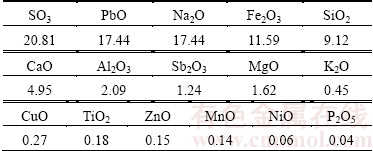
The sample was obtained from traditional furnaces from Ghaniabad melting factories in Iran. XRF analysis was used to determine the composition of samples. The results showed a 17.44% of Pb composition as PbO in the slag sample as shown in Table 1.
Table 1 Main chemical composition of sample by XRF analysis (mass fraction, %)
XRD analysis was used for mineralogy of samples. The major mineral of the Pb sample was galena (PbS). Also, a considerable ratio of Na2SO4 to Fe3O4 species was reported, as shown in Fig. 1.

Fig. 1 XRD pattern of sample
2.2 Chemical agents and instruments
Industrial FeCl3 and NaCl aqueous solutions were used as leaching agents. 1 mol/L HCl was used to adjust pH. A 1000 mL beaker, a mechanical stirrer, a digital balance, and a water bath were used to control the temperature with the precision of 0.1 °C. A digital pH meter was used to control the pH of the solution. The Pb content was measured through atomic absorption spectroscopy (AAS) using Uniqam 939 model. Also, the graphical analysis was carried out using Design-Expert software.
2.3 Chloride leaching principle
Galena is easily converted to PbCl2 solid particles in the presence of a strong FeCl3 oxidant and will be converted into 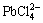 at high concentration of chloride ions. In this process, chloride ions play an important role and thus the leaching process is carried out in saturated solution of NaCl. Chloride ions convert PbCl2 to
at high concentration of chloride ions. In this process, chloride ions play an important role and thus the leaching process is carried out in saturated solution of NaCl. Chloride ions convert PbCl2 to 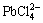 ions as ion-complexing agents [1,12]. the reactions for galena leaching in FeCl3 solution are as follows [3]:
ions as ion-complexing agents [1,12]. the reactions for galena leaching in FeCl3 solution are as follows [3]:
Pb(s)+2Fe3+(aq)+2Cl-(aq)→PbCl2(s)+2Fe3+(aq)+S0(s) (1)
NbCl(s)→Na+(aq)+Cl-(aq) (2)
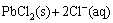 →
→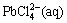 (3)
(3)
2.4 Mechanism of kinetic model determination
The first- and second-order reaction rates are often suggested for most reactions. The recovery relations for first- and second-order reactions are as follows [24,25]:
kt=-ln(1-x) for the first-order reaction (4)
kt=x/(1-x) for the second-order reaction (5)
where x is the fraction reacted, k is the kinetic constant and t is the reaction time.
Two simple idealized models called shrinking core model and progressive-conversion model are often used to explain the non-catalytic reaction relations between solid particles and the surrounding fluid. The kinetics of sulfide minerals leaching often obeys the shrinking core model [25]. According to this model, the reaction occurred through 3 steps including diffusion of leaching agent through surrounding film of the particle, diffusion of leaching agent through solid reaction product to the unreacted core surface, and reaction with the particle on its surface. The resistances of the mentioned steps are different and often the step of highest resisting strength is often named as the rate determining step or limiting of the reaction rate [25]. Equations (6)-(8) show the recovery-time relation for models including controlling film diffusion, diffusion through solid reaction product, and chemical control, respectively [25].
x=kt (6)
kt=1-3(1-x)2/3+2(1-x) (7)
kt=1-(1-x)1/3 (8)
To consider the correspondence of leaching process with control mechanisms, parameters kt, 1-3(1-x)2/3+ 2(1-x), and 1-(1-x)1/3 are plotted against reaction time and the highest correlation coefficient (R2) value is suggested as the model satisfying the leaching behavior [25].
Arrhenius model was used to determine the activation energy of reaction which is obtained from the slope (a) of lg k plotted versus 1/T as follows [23]:
lg k=lg A-E/(2.303RT) (9)
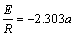 (10)
(10)
where k is the kinetic constant, A is a constant, E is the activation energy and R is the gas contant.
2.5 Experimental procedure
A given amount of FeCl3 and NaCl were poured into the reaction pot and the water was added to the system. The pH value was adjusted to be lower than 2, the reaction pot was heated and the slag sample was added to system under mechanical stirring.
The kinetic was studied at 40, 70, and 80 °C. The leaching was carried out at 3, 6, 9, 12, 17, 22, 32 and 42 min, and 5 mL of pulp was used to determine the lead content using AAS method.
2.6 Experimental design
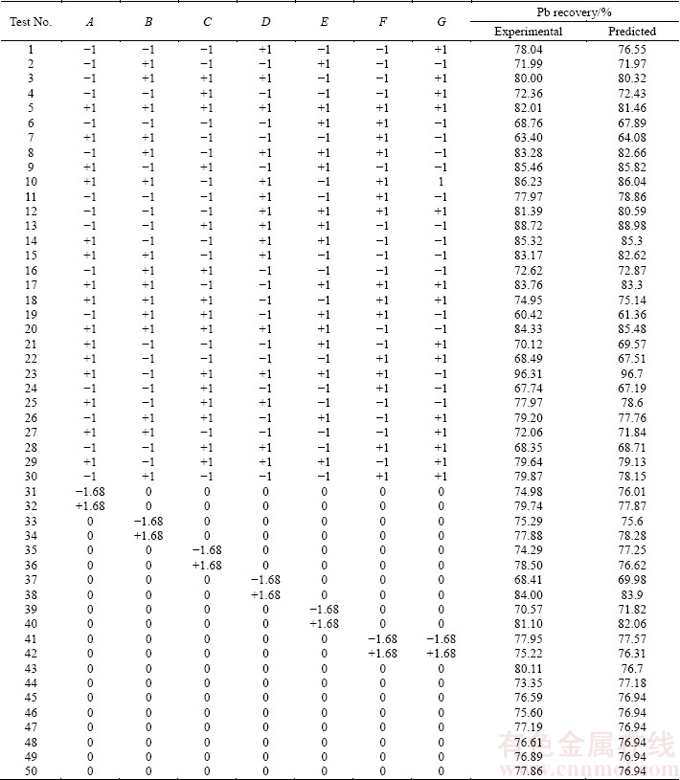
The surface response methodology is a combination of statistical and mathematical techniques, which has been widely used for modeling and analysis of the systems in which the dependent variable is affected by several parameters simultaneously. This methodology is applicable for the optimization of resulted output, considering separate effect of parameters, and analyzing the interaction between parameters [26-28]. The central composite design has been widely used as a subtype of surface response method. In this work, the central composite design was used for the experiment to study the operating parameters affecting the Pb recovery. The input includes 5 levels of each variable consisting of upper and lower axes, factorial upper, factorial lower, and central point. Based on the literature and elementary experiments, the parameters considered as experimental design input include leaching time (A), NaCl concentration (B), FeCl3 concentration (C), temperature (D), liquid/solid ratio (E), stirring rate (F), and particle size (G). The codes and variation levels of operating parameters are listed in Table 2. Through central composite design method, 50 experiments were designed, as shown in Table 3.
Table 2 Codes and variation levels of operating parameters
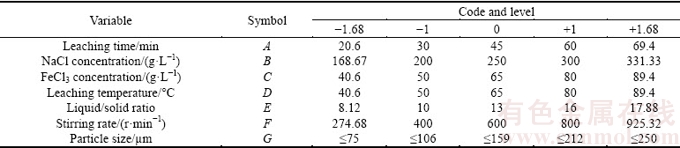
Table 3 Experiments designed by CCD method and obtained results
3 Result and discussion
3.1 Optimization and data analysis of leaching results
3.1.1 ANOVA analysis
The lead recovery as the result of the experiments was inserted to experimental design software. The results are reported in Table 3. Then, the ANOVA was used to analyze and suggest a mathematical model based on experimental leaching recovery data.
ANOVA is a statistical tool to consider the significance of data. In fact, it is used to analyze the effect of a parameter with more than two levels [29]. It considers the significance based on the ratio of variances according to Fisher ratio of variances [29,30]. In the Fisher method, the significance of a model is dependent upon F and p values. Upper level of F and lower level of p (p<0.05) indicate the significance of the model at the confidence interval of 95% [29]. The values of F and p are summarized in Table 4 for significance responses. In specified ranges, temperature, liquid/solid ratio, and NaCl concentration independently affected the leaching recovery while other parameters affected through interaction between other parameters. Finally, the leaching recovery relation with considered parameters is modeled as follows:
x=76.94+4.28D+3.15E+0.82B+2.66AF-2.44BC-2.44DG-2.42CD+1.97AE+1.6AC-1.82BE (11)
In this model, all variables are in coded values and B is NaCl concentration, D is reaction temperature, E is liquid/solid ratio, and AF, BC, DG, CD, AE, AC, BE are significance interactions on recovery rate of lead.
Since an inadequate model could lead to misleading results, the validation of model is an important part of the data analysis procedure. The adequate precision ratio indicates the precision of obtained data which should be higher than 4 [31,32]. The adequate precision ratio of data was obtained to be 25.539, indicating high precision of presented model.
The correlation coefficient (R2) was obtained to be 0.9676, showing an appropriate agreement between predicted data and actual ones. The diagram of predicted data and actual data confirms the fitted results, as shown in Fig. 2.
3.1.2 Individual effect of variables
Figure 3(a) shows the effect of temperature on Pb recovery. High temperature has the highest effect on leaching recovery since it increases the reaction rate. It’s noteworthy that increasing temperature increases the PbCl2-to-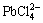 conversion reaction rate and hence facilitates the reaction. This phenomenon has been widely reported in Refs. [12,33].
conversion reaction rate and hence facilitates the reaction. This phenomenon has been widely reported in Refs. [12,33].
Figure 3(b) shows the effect of liquid/solid ratio on Pb recovery, which indicates a direct dependence. This dependence is attributed to increasing the leaching agent and hence increasing the leaching rate [2,12].
Table 4 Results of analysis of variance
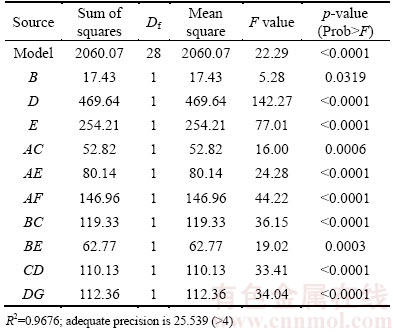
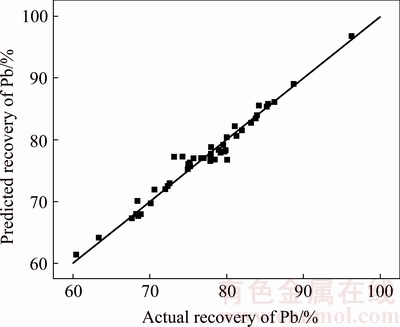
Fig. 2 Predicted recovery of Pb vs actual recovery of Pb
Figure 3(c) shows the NaCl concentration effect on Pb recovery. As can be seen, increasing NaCl concentration increases the Pb recovery. According to reactions (2) and (3), Cl- ions strongly affect the chloride leaching process. Higher amount of NaCl increases the concentration of chloride ion as complexing agents and hence PbCl2 and 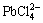 are formed easily [12,33].
are formed easily [12,33].
3.1.3 Interaction of parameters
Figure 4(a) shows the mutual effect of time and FeCl3 concentration on Pb recovery. As can be seen, at 50 g/L FeCl3, by increasing leaching time from 30 to 60 min, the Pb recovery is decreased. This phenomenon is attributed to pH effect since the pH is always under 2 in leaching studies, which prevents the hydrolysis of PbCl2 and ferric ions [17]. The pH was lower than 2 since industrial HCl was used in this work. HCl amount and consuming Fe3+ and Cl- ions as Lewis acids are the main cause of pH increase.

Fig. 3 Effect of temperature (a), liquid/slid ratio (b) and NaCl concentration (c) on Pb recovery (Other parameters are held at center level)
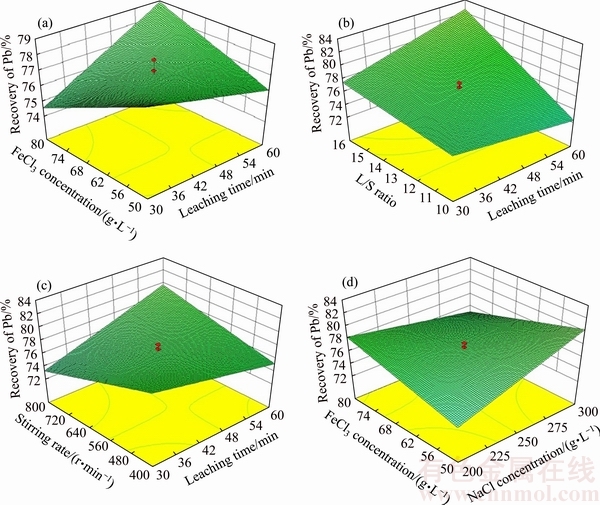
Fig. 4 Relationship among Pb recovery and leaching parameters (Other parameters are held at center level)
The experiments No. 1, 30, 37 and 44 were repeated to ensure the pH variations at 80 and 50 g/L FeCl3 in three different time, as shown in Table 5. As can be seen, pH is highly increased in lower FeCl3 concentration. Besides, pH is slightly increased in the system with lower L/S ratio. pH reaches 2.83 at low FeCl3 concentrations, which result in a decreased recovery [12]. Thus, simultaneous increase of time and FeCl3 enhances the Pb recovery in the solution.
Table 5 Effect of FeCl3 concentration on pH
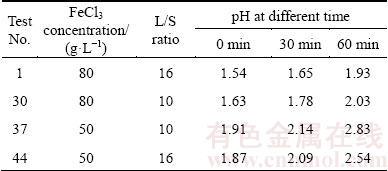
Figure 4(b) shows the effect of interaction between time and L/S ratio on Pb recovery. As can be easily seen, at higher L/S ratios, the recovery is increased with increasing leaching agent. Generally, the leaching recovery increases with reducing the pulp density since high amount of leaching agent is added to a low content of solid [34]. No noticeable change was observed at lower L/S ratios since the leaching agent was reduced. Also, slight decrease of recovery may be due to the kinetics control of the reaction regarding production of sulfur and pH increase at low leaching agent content and L/S ratios.
Figure 4(c) shows the mutual effect of time and stirring on Pb recovery. The main point in chloride leaching of PbS is the role of sulfur produced in the leaching process, which deposits on the galena surface and the PbCl2 crystals diffuse through their pores which inhibit the solubility of PbCl2 ions [1,2]. The layer around elemental sulfur plays an important role in limiting the accessibility of leaching agent to crystals trapped at the sulfur pores, and high stirring rate can overcome this limit [1]. Thus, the removal of sulfur determines the stirring role. Although the elemental sulfur has a slight effect during 1 h, it has a slight inhibitive effect. Thus, trapping PbCl2 crystals at elemental sulfur pores were used to control and reduce the recovery at low stirring rates since the layer limits the leaching agent to access the pores. At 30 min, increasing the stirring rate has not led to the increase of recovery, which is attributed to slight trapping of PbCl2 at pores of elemental sulfur at first 30 min of reaction [1].
Figure 4(d) shows the mutual effect of FeCl3 and NaCl concentrations on recovery. As illustrated, NaCl concentration results in increasing recovery at 50 g/L FeCl3 while an inverse trend is observed at 80 g/L. This may be due to the concentration of ions during leaching which limits the saturation of NaCl. At high FeCl3 concentration, the common ion effect reduces the NaCl solubility [34]. Besides, the sample includes high content of Na2SO4 which has common Na+ ion with NaCl and reduces its solubility. Thus, high part of NaCl remains insoluble, being not able to act as complexing agent. Therefore, an optimum ratio of both salts is needed.
Figure 5(a) shows the mutual effect of NaCl concentration and L/S ratio on Pb recovery. As can be seen, increasing NaCl concentration results in no noticeable change at high L/S ratios while a considerable increase has been observed at low L/S ratios. Since the FeCl3 concentration is higher at high L/S ratios, a similar justification to NaCl mutual effect can be presented in this regard. Also, the highest recovery has been obtained at higher L/S ratio regarding high content of leaching agent and also higher kinetics. Additionally, slight decrease in recovery at high NaCl concentrations is due to the same reason of NaCl / FeCl3 interaction at high L/S ratios.

Fig. 5 Effect of leaching parameters on Pb recovery
Figure 5(b) shows the mutual effect of particle size and temperature on Pb recovery. It can be seen that increasing temperature has resulted in recovery enhancement at both particle sizes while the slope of increasing is higher at particle size ≤106 μm. This phenomenon is due to larger surface area of particles and thus the solubility of PbS is increased. Temperature increase enhances the rate of endothermic reaction (3) since the Pb complexation is decreased at higher temperatures.
Figure 5(c) shows the mutual effect of FeCl3 concentration and temperature on recovery. As can be seen, increasing temperature enhances the recovery at all FeCl3 concentrations although the slope is higher at lower FeCl3 concentrations. At 50 °C, at which the reaction rate is higher compared to that at 80 °C, higher recovery has been reported at higher Fe3+ concentrations since this ion increases the reaction rate before the complete limiting of reaction due to elemental sulfur production [1,33]. At higher temperature and reaction rate, 50 g/L FeCl3 has resulted in higher recovery which is attributed to optimum ratio of FeCl3/NaCl at this concentration.
3.1.4 Optimum conditions of experiment
The maximum and minimum conditions of response can be determined with high accuracy regarding the statistic approach and optimization at designing space in surface response method [26-28]. The first differentiation of Eq. (11) which was determined with ANOVA, results in the minimum and maximum recoveries of Pb. The optimum conditions obtained by software are summarized in Fig. 6.
Based on the experimental design outputs, the optimum conditions of 60 min, 80 °C, 800 r/min, 200 g/L NaCl, 80 g/L FeCl3, L/S ratio 16, and particles ≤106 μm resulted in Pb recovery of 96.7%. The reliability tests verified the results compared to experimental results. Table 6 shows the actual and predicted results.
3.2 Kinetics of process
3.2.1 Leaching reaction order
Having optimized the parameters, the kinetics study was done based on the software outputs. Figure 7 shows the Pb recovery at 40, 70, and 80 °C. According to Eqs. (4) and (5), -ln(1-x) and x/(1-x) against time were plotted at different temperatures, as shown in Fig. 8. Comparing the correlation coefficients of these diagrams shows that the coefficient is closer to unity at the first- order reaction. Thus, it is concluded that the leaching reaction of Pb is better expressed through a first-order reaction.
3.2.2 Controlling mechanism of leaching
Based on the obtained results, the recovery diagram versus time according to Eq. (6) was plotted and shown in Fig. 9(a). As can be seen, the correlation coefficients calculated are mainly deviated from unity at different temperatures. Thus, it is concluded that the experimental results do not satisfy the controlling film diffusion model.
Based on Eq. (7) and obtained results, 1-3(1-x)2/3 + 2(1-x) values versus time at different temperatures were plotted and shown in Fig. 9(b). As can be seen, R2 calculated is close to unity, indicating that the diffusion through solid products can be taken into account as accepted rate determining step.
Based on Eq. (8), 1-(1-x)1/3 values were plotted against time at different temperatures, as shown in Fig. 9(c). Since the calculated R2 values are close to unity at different temperatures, it can be assumed that the reaction occurring on the galena surface is rate- determining step of reaction. Comparing the correlation coefficients for reaction and diffusion models through solid products, it is concluded that the R2 values of diffusion are closer to unity. Thus, it is concluded that the leaching agent diffusion through solid products is the controlling mechanism of the reaction. Clotting the sulfur pores limits the migration of leaching agent toward galena surface and thus the leaching reaction is controlled and its kinetics converts from linear to quadratic [1]. Additionally, limiting the reaction is probable in addition to elemental sulfur effect. SOHN and BAER [21] presented a combinative model for kinetics of galena leaching in which the diffusivity of PbCl2 crystals in elemental sulfur layer limits the reaction on the galena surface in shrinking core model.

Fig. 6 Optimum conditions of experiment
Table 6 Reliability results of optimum conditions (software output based on Pb recovery)

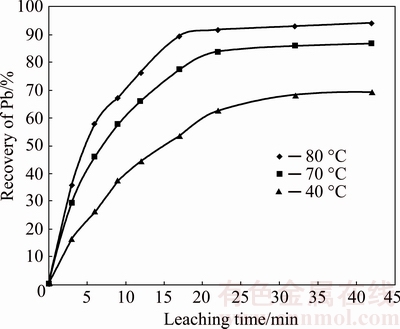
Fig. 7 Effect of leaching time on Pb recovery at different leaching temperatures
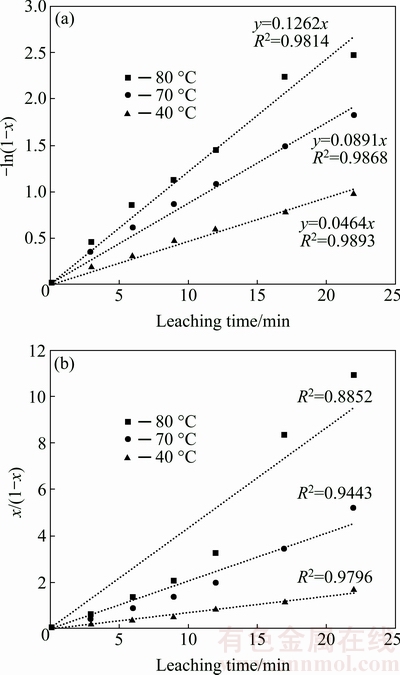
Fig. 8 Curves showing –ln(1-x) vs t (a) and x/(1-x) vs t (b)
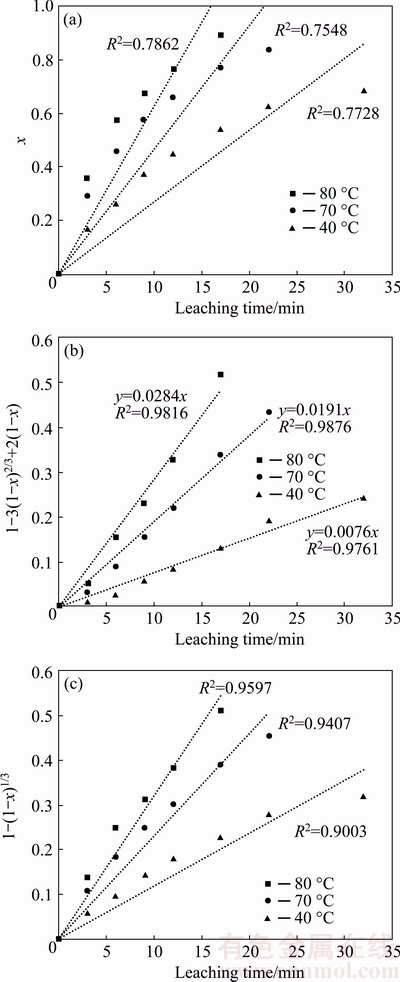
Fig. 9 Curves showing x vs t (a), 1-3(1-x)2/3+2(1-x) vs t (b) and 1-(1-x)1/3 vs t (c)
3.2.3 Determination of activation energy
Considering the kinetics model of diffusion through solid products at given kinetic rate constants at studied temperatures, lg k values were plotted against 1/T, as shown in Fig. 10. The slope of fitted curve was obtained to be 1470 and the activation energy was calculated to be 27.9 kJ/mol using Eq. (10). The activation energy range of reaction control processes via diffusion is 4.18-20.9 kJ/mol, which confirms the reaction control based on Arrhenius plot. For chemical controlled reactions which are deeply dependent upon the reaction temperature, the activation energy is higher than 41.8 kJ/mol while it is 20.9-33.4 kJ/mol for median controlled processes [23].
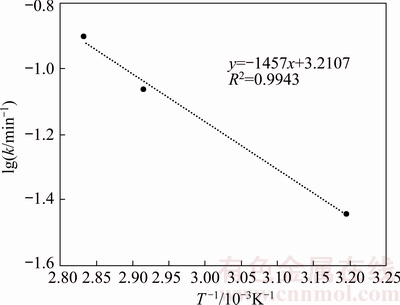
Fig. 10 Relationship between lg k and T-1
4 Conclusions
The chloride leaching was used applying ferric chloride for Pb recovery from slag of traditional lead melting furnace. The surface response method was used for experimental design and optimization of Pb leaching in which an experimental statistical model was suggested to model Pb recovery based on the studied parameters. Based on ANOVA and experimental model, temperature and liquid/solid ratio were suggested as the most effective parameters, in which increasing of them results in an enhancement in Pb recovery. Also, the stirring rate-time, NaCl concentration-FeCl3 concentration interaction, and temperature-particle size interaction are the main effective parameters on Pb recovery. Based on these interactions, increasing the time and stirring rate, optimizing the FeCl3/NaCl ratio, and simultaneous increase of temperature and decrease of particle size have the highest effect on Pb recovery. The proposed model equation using RSM has shown good agreement with the experimental data, with a correlation coefficient (R2) of 0.967. According to experimental design software output, 60 min, 80 °C, stirring rate 800 r/min, 200 g/L NaCl concentration, 80 g/L FeCl3 concentration, liquid/solid ratio 16 and particle size ≤106 μm were correspondent to the Pb recovery of 96.7%. The kinetics of leaching process was similar to first-order reaction rate and the kinetic rate was determined 0.1262 min-1 at 80 °C. Additionally, the diffusion through solid product and chemical reaction was determined as the controlling mechanism of reaction at the shrinking core model. The Arrhenius diagram was plotted for leaching reaction and activation energy of 27.9 kJ/mol was obtained. The average slope of Arrhenius indicates a thermal dependence of Pb leaching. Also, the activation energy range verifies the reaction control model.
References
[1] DUTRIZAC J E. The leaching of galena in cupric chloride media [J]. Metallurgical Transactions B, 1989, 20: 475-483.
[2] DUTRIZAC J E, CHEN T T. The effect of the elemental sulfur reaction product on the leaching of galena in ferric chloride media [J]. Metallurgical Transactions B, 1990, 21: 935-943.
[3] CHEN A A. Kinetics of leaching galena concentrates with ferric flousilicate solution [D]. Vancouver: Department of Metal and Material Engineering, University of British Colombia, 1992: 1-43.
[4] GONZALEZ-DOMINGUEZ J A, PETERS E, DREISINGER D B. The refining of lead by the Betts process [J]. Applied Electrochemistry, 1991, 21: 189-202.
[5] NAKAMURA T, TAKASU T. Fundamentals of pyrometallurgical treatment of Zinc leach residue [C]//Proceedings of International Symposium of Quality in Nonferrous Pyrometallurgy. Montreal: Canadian Institute of Mining, Metallurgy and Petroleum, 1995: 341-355.
[6] RABAH M A. Combined hydro-pyrometallurgical method for the recovery of high lead/tin/bronze alloy from industrial scrap [J]. Hydrometallurgy, 1998, 47: 281-295.
[7] ABDEL BASIR S M, RABAHM A. Hydrometallurgical recovery of metal values from brass melting slag [J]. Hydrometallurgy, 1999, 53: 31-44.
[8] BARAKAT M A. Recovery of metal values from zinc solder dross [J]. Waste Management, 1999, 19(7): 503-507.
[9] VAZARLIS H G. Hydrochloric acid-hydrogen peroxide leaching and metal recovery from a Greek zinc-lead bulk sulphide concentrate [J]. Hydrometallurgy, 1987, 19: 243-251.
[10] BEHNAJADY B, MOGHADDAM J, BEHNAJADI M A, RSHCHI F. Determination of the optimum conditions for the leaching of lead from zinc plant residues in NaCl-H2SO4-Ca(OH)2 media by the Taguchi method [J]. Industrial & Engineering Chemistry Research, 2012, 51: 3887-3894.
[11] BARAKAT M A. Recovery of lead, tin and indium from alloy wire scrap [J]. Hydrometallurgy, 1998, 49: 63-73.
[12] QIN W Q, LIU H, TANG S H, SUN W. Preparation of lead sulfate powder directly from galena concentrates [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 479-483.
[13] VOLSKII A N, AGRACHEVA R A. Hydrometallurgical procedure for treating materials containing lead sulfide: USSR patent, 112502 [P]. 1958-07-24.
[14] MOZAFFARI E, MOHSENI M, ABAIE E. Recovering lead metal from Lak mine lead concentrate by ferric chloride leaching [J]. Pure and Applied Science & Technology, 2014, 4(2): 37-43.
[15] ABDOLLAHI P, YOOZBASHIZADEH H, MORADKHANI D, BEHNIAN D. A study on cementation process of lead from brine leaching solution by aluminum powder [J]. Library Journal, 2015, 2: 1-6.
[16] MURRAY R J. The Dissolution of galena concentrate in aqueous ferric chloride solution [D]. Moscow: Department of Chemical and Materials Engineering, University of Idaho, 1972: 1-35.
[17] DEMOPOULOS G P. Ferric chloride leaching of chalcopyrite, galena and sphalerite [D]. Montreal: Department of Mining and Metallurgical Engineering, McGill University, 1978: 41-90.
[18] KIM S S, KIM I B. Ferric ch1oride leaching of galena [J]. Journal of the Korean Institute of Metals and Materials, 1980, 18: 586-592.
[19] FUERSTENAU M C, NEBO C O, ELANGO B V, HAN K N. The kinetics of leaching galena with ferric nitrate [J]. Metallurgical Transactions B, 1987, 18: 25-30.
[20] RATH P C, PARAMGURU P K, JENA P K. Kinetics of dissolution of sulphide minerals in ferric chloride solution. 1: Dissolution of galena, sphalerite and chalcopyrite [J]. Trans IMM, 1988, 97: 150-158.
[21] SOHN H Y, BAEK H D. The mixed-control kinetics of ferric chloride leaching of galena [J]. Metallurgical Transactions B, 1989, 20: 107-110.
[22] BABA A A, ADEKOLA F A. A study of dissolution kinetics of a Nigerian galena ore in hydrochloric acid [J]. Journal of Saudi Chemical Society, 2012, 16: 377-386.
[23] HABASHI F. Kinetics of metallurgical processes [M]. Quebec City: Metallurgie Extractive Quebec, 1999.
[24] GUPTA A, YAN D S. Mineral processing design and operation [M]. Amsterdam: Elsevier Science, 2006.
[25] LEVENSPIEL O. Chemical reaction engineering [M]. 3nd ed. New York: John Wiley & Sons, 1999.
[26] GHOSAL A, MANNA A. Response surface method based optimization of ytterbium fiber laser parameter during machining of Al/Al2O3-MMC [J]. Optics & Laser Technology, 2013, 46: 67-76.
[27] GUNST R F. Response surface methodology: Process and product optimization using designed experiments [J]. Technometrics, 1996, 38: 284-286.
[28] BEZERRA M A, SANTELLI R E, OLIVERRA E P, VILLAR L S. ESCALEIRA L A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry [J]. Talanta, 2008, 76: 965-977.
[29] LAZIC R. Design of experiments in chemical engineering [M]. Michigan: Wiley, 2004.
[30] MIRAZIMI S M J, RASHCHI F, SABA M. Vanadium removal from roasted LD converter slag: Optimization of parameters by response surface methodology (RSM) [J]. Separation and Purification Technology, 2013, 116: 175-183.
[31] ZHANG Z, PENG J, SRINIVASAKANNAN C, ZANG Z, ZHANG L, FERNANDEZ Y. Leaching zinc from spent catalyst: Process optimization using response surface methodology [J]. Hazardous Materials, 2010, 176: 1113-1117.
[32] KORBAHTI B K, RAUF M A. Determination of optimum operating conditions of carmine decoloration by UV/H2O2 using response surface methodology [J]. Hazardous Materials, 2009, 161: 281-286.
[33] CORREIA M J N, CARVALHO Jr. Technical note chloride leaching of Portuguese lead concentrates [J]. Minerals Engineering, 1992, 5: 245-253.
[34] HABASHI F. Textbook of hydrometallurgy [M]. 2nd ed. Quebec: Metallurgie Extractive Quebec, 1999.
Mohammad Hasan GOLPAYEGANI1, Ali Akbar ABDOLLAHZADEH1,2
1. Department of Mining Engineering, Kashan University, Kashan, Iran;
2. Department of Mining Engineering, Amirkabir University of Technology, Tehran, Iran
摘 要:研究采用氯盐浸出法从传统熔铅炉渣中回收铅的可行性和动力学。考察工艺参数如浸出时间、NaCl浓度、FeCl3浓度、液/固比、搅拌速度、浸出温度、颗粒尺寸对铅回收率的影响。基于中心复合设计模型,利用响应面法对上述参数进行优化,得到优化工艺条件如下:浸出时间60 min、浸出温度80 °C、搅拌速度800 r/min、NaCl浓度200 g/L、FeCl3浓度 80 g/L、液固比16、颗粒尺寸小于106 μm。在此优化条件下96% Pb被回收。基于方差分析法,确定浸出温度、液固比和NaCl浓度为影响浸取过程的最有效参数。动力学研究结果表明,方铅石的氯盐浸出过程为一级反应过程。反应机理为固态反应产物的扩散和化学反应。采用Arrhenius模型计算得到从方解石中氯盐浸出铅的激活能为27.9 kJ/mol。
关键词:铅;炉渣;氯盐浸出;优化;动力学
(Edited by Wei-ping CHEN)
Corresponding author: Mohammad Hasan GOLPAYEGANI; Tel: +98-44001087; Fax: +98-88045225; E-mail: mgolpayegani.mineralprocessing@gmail.com
DOI: 10.1016/S1003-6326(17)60299-1

