Corrosion behaviors of Cr-Al-N coatings deposited by reactive magnetron sputtering
来源期刊:中国有色金属学报(英文版)2007年增刊第1期(Part ⅡB)
论文作者:多树旺 朱明 刘庭芝 李忠峻 王鹏 李美栓
文章页码:841 - 846
Key words:Cr-Al-N coatings; corrosion behavior; magnetron sputtering
Abstract: CrN and Cr-Al-N coatings were deposited by reactive magnetron sputtering on the glass substrate, and their corrosion behavior was studied. The electrochemical tests using both DC (polarization curves) and AC techniques (EIS) were carried out on Potentiostat/Galvanstat (EG&G) in 3.5% (mass fraction) NaCl solution. After immersed into NaCl solution for 1 h, the mass of the CrN coating keeps constant with the time continuing. This can be explained by the passivation of the coating. The comparison between the corrosion potential (φcorr) of the Cr-Al-N coatings with different aluminum contents reveals that the corrosion potentials of the aluminum contain coatings are nobler than that of the CrN coatings. This means that the addition of aluminum shifts the corrosion potential to more positive potential value. Among these coatings, CrN in NaCl solution exhibits the worst corrosion resistance, while the corrosion resistance of Cr0.63Al0.37N in NaCl solution is the best. The polarization data and EIS data suggest that addition of aluminum can improve the corrosion resistance of CrN coating.
基金信息:the National Natural Science Foundation of China

DUO Shu-wang(多树旺)1, 2, ZHU Ming(朱 明)2, 3, LIU Ting-zhi(刘庭芝)1,
LI Zhong-jun(李忠峻)1, WANG Peng(王 鹏)1, LI Mei-shuan(李美栓)3
1. Jiangxi Key Laboratory of Surface Engineering, Jiangxi Science and Technology Normal University, Nanchang 330013, China;
2. Department of Materials Science and Engineering, Xi’an University of Science and Technology, Xi’an 710054, China;
3. Shenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China
Received 15 July 2007; accepted 10 September 2007
Abstract: CrN and Cr-Al-N coatings were deposited by reactive magnetron sputtering on the glass substrate, and their corrosion behavior was studied. The electrochemical tests using both DC (polarization curves) and AC techniques (EIS) were carried out on Potentiostat/Galvanstat (EG&G) in 3.5% (mass fraction) NaCl solution. After immersed into NaCl solution for 1 h, the mass of the CrN coating keeps constant with the time continuing. This can be explained by the passivation of the coating. The comparison between the corrosion potential (φcorr) of the Cr-Al-N coatings with different aluminum contents reveals that the corrosion potentials of the aluminum contain coatings are nobler than that of the CrN coatings. This means that the addition of aluminum shifts the corrosion potential to more positive potential value. Among these coatings, CrN in NaCl solution exhibits the worst corrosion resistance, while the corrosion resistance of Cr0.63Al0.37N in NaCl solution is the best. The polarization data and EIS data suggest that addition of aluminum can improve the corrosion resistance of CrN coating.
Key words: Cr-Al-N coatings; corrosion behavior; magnetron sputtering
1 Introduction
Thin ceramic coatings are of interest in a number of technological fields because of their physical and mechanical properties. Among these materials, transition metal nitride coatings fabricated by physical vapor deposition (PVD) method are widely used because of their superior bonding to the substrate and excellent resistance to wear, erosion and corrosion. Generally, ternary nitride coatings show better mechanical properties and better chemical stability compared with binary nitride coatings. The modification of the properties was due to the solid solution of the third element into the binary nitride coatings. The most extensively studied ternary nitride coating is Ti-Al-N system[1-2]. It has been reported that due to the addition of aluminum, the hardness, wear resistance, corrosion resistance and oxidation resistance of the TiN increase obviously. And the degree of the improvement depends on the content of aluminum added. Other ternary nitride system coatings such as Ti-Hf-N[3], Ti-Cr-N[4], Zr-Hf-N[5], Cr-Nb-N[6], Cr-V-N[7] and Cr-Al-N[8-11], have been reported as well. Compared with Ti-Al-N system, Cr-Al-N system Cr-Al-N coatings have attracted more and more interests in recent years.
According to MAKINO and NOGI[9], CrN shows the highest solubility for AlN among the transition nitrides with B1 structure, and the critical composition for the B1/B4 phase transition in the Cr-Al-N system was calculated as containing 77% (mole fraction) AlN. Selected properties of Cr-Al-N coatings have been reported by several researchers[10-11], with the addition of aluminum, hardness, wear resistance and oxidation resistance of CrN coating have been improved markedly.
The corrosion behavior of CrN coating has been widely studied[12-13]. A very thin CrN coating would provide excellent protection to the substrate. However,the major problem in using CrN coating in aggressive environment, is the possible presence of open porosity and pinholes in the coating forming during the deposition process. Nitride coatings were usually considered to be chemical insert coatings in the corrosive environment by researchers. The practice on improving the corrosion resistance of the nitride coatings was focused on modificating the microstructure of these coatings. In most of the previous works, nitride coatings were deposited on the tools. The corrosion behavior consists the decomposition of the coating and the corrosion of the substrate through the pinholes of the coatings. Up to now, the corrosion behavior of the bulk nitride has seldom been studied. As mentioned above, addition of aluminum can improve the mechanical properties of CrN. The influence of aluminum on the corrosion behavior of CrN is not clear yet. In the present work, CrN and Cr-Al-N coatings were deposited on glass substrate and the corrosion behaviors of them were studied.
2 Experimental
Glass was chosen as the substrates in the present study. They were cut into dimensions of 15 mm× 10 mm×2 mm, and then the samples were degreased ultrasonically in acetone, cleaned by ethanol and dried in air.
Cr-Al-N coatings were fabricated on a JGP560C14 magnetron sputtering system (SKY Technology Development Co Ltd, CAS, China) by reactive magnetron co-sputtering from a chromium (purity of 99.2%) target simultaneously with an aluminum target (purity of 99%) in a mixed Ar/N2 atmosphere at 300 ℃. Both the chromium and aluminum target were operated in DC mode. The applied power to the aluminum target was kept constantly at 120 W and the four power values were chosen as 30, 60, 90 and 120 W, respectively, to apply on the chromium target to adjust the content of aluminum. Before deposition the system was evacuated to a base pressure of 2×10-4 Pa and the depositions were performed at a total pressure of 0.6 Pa. The flow rate of the reactive gas (N2, 99.999%) and the insert gas (Ar, 99.999%) was both 10 mL/min, controlled by electronic mass flow meters. Pure CrN coating was also deposited from a single chromium target under the same conditions.
XRD analysis was used to identify the crystal structure of the coatings. The XRD data were collected by a step-scanning diffractometer with Cu Kα radiation (Rigaku D/max-2400, Japan). The lattice parameters for the coatings with different aluminum contents were calculated using the data from the XRD analysis by Rietveld method[14]. The compositions of the Cr-Al-N coatings were determined by XPS analysis (ESCALABMKⅡ, UK) using Mg Kα X-ray as the source of excitation. The survey spectra were obtained over a kinetic energy range of 200-1 300 eV in steps of 1.0 eV for a pass energy (CAE) mode. Detailed data were obtained for selected ranges of photoelectron and X-ray induced Auger electron energies in steps of 0.05 eV.
The electrochemical tests using both DC (polarization curves) and AC techniques (EIS) were carried out on Potentiostat/Galvanstat (EG&G) in 3.5% NaCl solution. For all experiments a three- electrode cell was used, with an Ag/AgCl electrode used as reference electrode and a platinum counter electrode (20 mm×20 mm). The polarization curves were obtained by PAR 273 potentiostat, with a potential scanning rate of 1mV/s. The impedance measurement was carried out using a frequency response analyzer coupled with the potentiostat. A perturbation of 5mV over a bandwidth from 105 to 10-2 Hz was applied. All the experiments were controlled by a personal computer, which was also used for the acquisition, storage and plotting of data. After electrochemical tests, the surface morphologies were observed by scanning electron microscopy (SEM, Philip XL30). The mass variation of the CrN coating in 3.5% NaCl solution was in situ measured by Quartz Crystal Microbalance (QCA992, EG&G) with a sensitivity better than 10-8 g?cm-2. CrN coating was deposited on one side of the AT-cut quartz crystal disk with area of 0.2 cm2 and thickness of around 3 μm. The coated quartz crystal disk was fitted in a holder with the coated side contacting with the solution. The holder was immersed in the solution for 2 h and the change in resonant frequency (?f ) of the oscillating crystal was acquired by a soft controlled PC. The relationship between mass change per unit area (?m/A) and the change in resonant frequency of the oscillating crystal ?can be expressed as
?m/A = -5.45?f (1)
3 Results and discussion
The nitrogen to metal ratio was analyzed by XPS and it maintained a constant value of 0.98-1.00. This allows us to describe the chemical composition by a simple Cr1-xAlxN formula. The values of x obtained from the XPS analysis are listed in Table 1. Cr1-xAlxN coatings deposited at different Cr/Al power ratios (120 W/120 W, 90 W/120 W, 60 W/120 W, 30 W/120 W) are characterized as Cr0.82Al0.18N, Cr0.74Al0.26N, Cr0.63Al0.37N and Cr0.53Al0.47N, respectively. Fig.1 shows the XRD patterns of the Cr-Al-N coatings with different aluminum contents. All of the coatings crystallized into the (B1), rocksalt structure with the (111) preferred orientation. Such a preferred orientation is often observed in CrN thin films deposited at relatively low substrate temperatures. The peak positions of the CrN (111) shift to larger angle with increasing x, indicating the decrease of the lattice parameter. The phase transition from B1 structural CrN to B4 structural AlN would occur when 77% (mole fraction) Al is added into the lattice of CrN[9].
Table 1 Values of x in Cr1-xAlxN obtained from XPS and EDS analysis

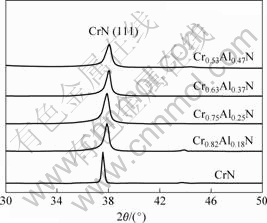
Fig.1 XRD patterns of Cr-Al-N coatings with different aluminum contents
The polarization curves of the Cr-Al-N coatings with different aluminum contents in a 3.5% NaCl solution are shown in Fig.2. The corrosion potential (φcorr), corrosion current density (Jcorr) and polarization resistance (Rp) obtained from the polarization data are listed in Table 2. The comparison between φcorr for the Cr-Al-N coatings with different aluminum contents reveals that the corrosion potentials of the aluminum contain coatings are nobler than that of CrN coatings. This means that the addition of aluminum shifts φcorr to more positive potential values. Cr0.63Al0.37N exhibits the highest Rp (12.11 kΩ?cm-2) and the lowest Jcorr (1.792 μA?cm-2) while CrN exhibits the lowest Rp (0.143 kΩ?cm-2) and the highest Jcorr (22.39 μA?cm-2). Therefore, among these coatings, CrN in NaCl solution exhibits the worst corrosion resistance, while the corrosion resistance of Cr0.63Al0.37N in NaCl solution is the best.
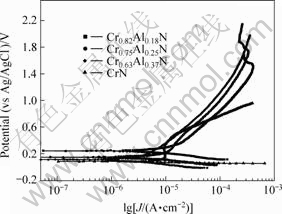
Fig.2 Polarization curves of Cr-Al-N coatings with different aluminum contents in 3.5% NaCl solution
The EIS provided useful information to describe the electrochemical behavior of complex corrosion system. Electrode impedance is a complex number and the spectra of it can normally be displayed in a Nyquist plot, where the opposite of imaginary part is plotted against real part, or in Bode plot in which the modulus and phase angle of the impedance are plotted as a function of the frequency. In the present work, the Bode mode is chosen, which is depicted in Fig.3. It can be seen that the absolute value of the impedance of CrN coating decreases obviously with increasing frequency while only slightly decline appears for the other aluminum contain coatings. The absolute value of the impedance increases with the aluminum content in the coatings, more noticeable in the high frequency limit of the spectra. This means that the addition of aluminum can improve the corrosion resistance of the CrN coating, and the
Table 2 Values of φcorr, Jcorr, Rp and C of CrN and CrAlN coatings in 3.5% NaCl solution
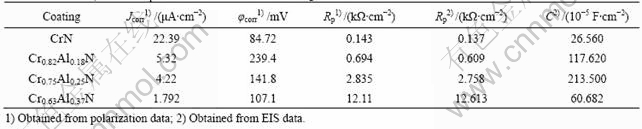
degree of the improvement increases with increasing aluminum content. These results agree well with the polarization curves. The equivalent circuit presented in Fig.4 is applied to simulate the EIS data for the coating system. The equivalent circuit consists of the following elements: a solution resistance Rs of the test electrolyte between the working electrode and the reference electrode; a capacitance C and a charge transfer resistance Rp. The values for Rp and C are listed in Table 2. The values obtained from the EIS are consistent with that obtained from the polarization data well.
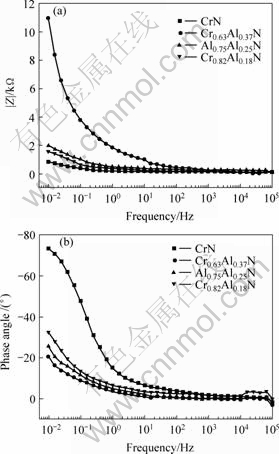
Fig.3 Bode plots of Cr-Al-N coatings with different aluminum contents in 3.5% NaCl solution: (a) Phots of impedance vs frequency; (b) Plots of phase angle vs frequency
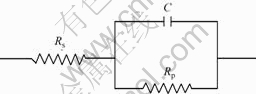
Fig. 4 Equivalent circuit for CrAlN coating systems
The polarization data and EIS data suggest that the addition of aluminum can improve the corrosion resistance of CrN coating. The influence of additional elements on the corrosion behavior of the binary nitride coatings is complicated. Contrary results were reported for Ti-Al-N system. IBRAHIM et al[15] have reported the improvement of the corrosion resistance of TiN film by addition of aluminum. However, the corrosion resistance of Ti-Al-N film was poor than that of TiN film by the researches of LI et al[16]. The contrary results can be attributed to the microstructure sensitivity of the PVD coatings. Sometimes, the defects of the coatings would counteract the positive effects of the additional elements.
The mass change of the CrN coating vs time is depicted in Fig.5. It can be seen that there exists an obvious decline in the mass during the first 0.5 h then the mass of the CrN coating nearly keeps constant.
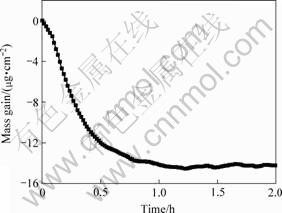
Fig.5 Mass change of CrN coating in 3.5% NaCl solution
Since the measured corrosion current is caused both by corrosion through pinholes of the substrate and by the decomposition of the coating when the coatings are coated on the easy corrosion substrates. However, in this work, the CrN and Cr-Al-N coatings are deposited on an insert substrate (glass), and the substrate had no contribution to the current density. Therefore, the measured corrosion current in the polarization curves must only result from the decomposition of the CrN coating.
The possible reaction on the surface of the CrN coating is as
4CrN+3O2+6H2O→4Cr(OH)3+2N2(g) (2)
IBRAHIM et al[15] have studied the corrosion behavior of CrN coated SS304 stainless steel. They have observed the color change of the solution indicating the decomposition of the coating and the diffusion of the corrosion products into the solution. The diffusion of the corrosion products can also explain the mass decline of CrN coating (see Fig.5). The formation of chromium hydroxide, which was detected out by XPS on the surface of the as-deposited CrN coating[15], is typical for the low temperature oxidation of chromium in a humid environment.
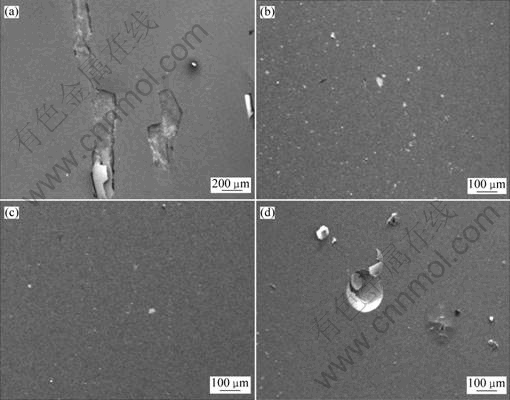
Fig.6 Surface morphologies of Cr-Al-N coatings with different aluminum contents after corrosion tests: (a) CrN; (b) Cr0.63Al0.37N; (c)Cr0.75Al0.25N; (d) Cr0.82Al0.18N
After immersed into NaCl solution for 1 h, the mass of the coating keeps constant with the time continuing. This can be explained by the passivation of the coating. Some of the corrosion products would deposit on the surface of the coating or seal the pinholes of the coating to keep back the decomposition of the coating. When the two steps come to balance no mass change appears for the coating.
The surface morphologies of the Cr-Al-N coatings with different aluminum contents after corrosion tests are shown in Fig.6. There are no obvious differentiations of the morphologies between the pre-corrosion and after-corrosion except that spallation is observed on the surface of the CrN coating.
4 Conclusions1) CrAlN coatings with different Al concentrations were deposited on glass substrates using a reactive magnetron sputtering system. The corrosion behaviors of the CrAlN coatings in a 3.5% NaCl solution were investigated by potentiodynamic tests, electrochemical impedance spectroscopy (EIS) and surface analyses.
2) After immersed into NaCl solution for 1 h, the mass of the CrN coating keeps constant with the time continuing due to the passivation of the coating.
3) The comparison between φcorr for the Cr-Al-N coatings with different aluminum contents reveals that the corrosion potentials of the aluminum contain coatings are nobler than those for CrN coatings.
4) The addition of aluminum shifts φcorr to more positive potential value. Among these coatings, CrN in NaCl solution exhibits the worst corrosion resistance, while the corrosion resistance of Cr0.63Al0.37N in NaCl solution is the best. The polarization data and EIS data suggest that addition of aluminum can improve the corrosion resistance of the CrN coating.
References[1] STEYER P, MENDIBIDE C, MILLET J P, MAZILLE H. Improvement of high temperature corrosion resistance of tool steels by nanostructured PVD coatings[J]. Mater Sci Forum, 2003, 426: 2503-2508.
[2] YANG Q, SEO D Y, ZHAO L R, ZENG X T. Erosion resistance performance of magnetron sputtering deposited TiAlN coatings[J]. Surf Coat Technol, 2004, 188/189: 168-173.
[3] FENSKE G R, KAUFHERR N AND SPROUL W D. Solid solution hardening in high rate reactively sputtered (Hf,Ti)N coatings[J]. Thin Solid Films, 1987, 153: 159-168.
[4] ZHANG G A, YAN P X, WANG P, CHEN Y M, ZHANG J Y. The structure and tribological behaviors of CrN and Cr-Ti-N coatings[J]. Applied Surface Science,2007, 253: 7353-7359.
[5] ATAR E, KAYALI E S, CIMENOGLU H. Reciprocating wear behavior of (Zr, Hf)N coatings[J]. Wear, 2004, 257: 633-639.
[6] HSIEH J H, LI C, TAN A L, POH C K, TAN N J. Study of oxidation and wear behavior of (Nb,Cr)N thin films using Raman spectroscopy[J]. Surface and Coatings Technology, 2004, 177/178: 299-305.
[7] UCHIDA M, NIHIRA N, MITSUO A, TOYODA K, KUBOTA K, AIZAWA T. Friction and wear properties of CrAlN and CrVN films deposited by cathodic arc ion plating method[J]. Surf Coat Technol, 2004, 177/178: 627-630.
[8] MO J L, ZHU M H, LEI B, LENG Y X, HUANG N. Comparison of tribological behaviours of AlCrN and TiAlN coatings—Deposited by physical vapor deposition[J]. Wear, 2007, 263: 1423-1429.
[9] MAKINO Y, NOGI K. Synthesis of pseudobinary Cr-Al-N films with B1 structure by rf-assisted magnetron sputtering method[J]. Surf Coat Technol, 1998, 98: 1008-1012.
[10] DING X Z, ZENG X T. Structural, mechanical and tribological properties of CrAlN coatings deposited by reactive unbalanced magnetron sputtering[J]. Surf Coat Technol, 2005, 200: 1372-1376.
[11] KAWATE M, HASHIMOTO A K, SUZUKI T. Oxidation resistance of Cr1-xAlxN and Ti1-xAlxN films[J]. Surf Coat Technol, 2003, 168: 163-167.
[12] CONDE A, NAVAS C, CRIST?BAL A B, HOUSDEN J, DAMBORENEA J de. Characterisation of corrosion and wear behaviour of nanoscaled e-beam PVD CrN coatings[J]. Surf Coat Technol, 2006, 201: 2690-2695.
[13] CUNHA L, ANDRITSCHKY M, REBOUTA L and PISCHOW K. Corrosion of CrN and TiAlN coatings in chloride-containing atmospheres[J]. Surf Coat Technol, 1999, 116/119: 1152-1160.
[14] WILES D B, YOUNG R A. A new computer program for Rietveld analysis of X-ray powder diffraction patterns[J]. J Appl Crystallogr, 1981, 14: 149-151.
[15] IBRAHIM M A M, KORABLOV S F, YOSHIMURA M. Corrosion of stainless steel coated with TiN, (TiAl)N and CrN in aqueous environments[J]. Corr Sci, 2002, 44: 815-828.
[16] LI Y, QU L, WANG F. The electrochemical corrosion behavior of TiN and (Ti,Al)N coatings in acid and salt solution[J]. Corr Sci, 2003, 45: 1367-1381.
Foundation item: Project (50371095) supported by the National Natural Science Foundation of China
Corresponding author: DUO Shu-wang; Tel: +86-791-3831266; Fax: +86-791-3801423; E-mail: swduo@imr.ac.cn

