文章编号:1004-0609(2012)06-1616-08
Cu2+及等离子电解氧化处理对Zr-4合金腐蚀性能的影响
程英亮,伍 帆
(湖南大学 材料科学与工程学院,长沙 410082)
摘 要:采用电化学方法研究锆合金Zr-4在添加不同Cu2+浓度的0.5 mol/L NaCl溶液或0.5 mol/L Na2SO4中的腐蚀行为,探讨不同浓度Cu2+对Zr-4合金腐蚀性能的影响,同时,在10 g/L Na2SiO3·9H2O+10 g/L Na4P2O7·10H2O混合溶液中对Zr-4合金进行等离子电解氧化处理,并用极化曲线技术研究膜层的防护能力。结果表明:Cu2+能使Zr-4合金自腐蚀电位正移,降低极化曲线上钝化区的宽度,使得合金的抗孔蚀能力降低,腐蚀电流密度增加;在硫酸钠溶液中,Cu2+的添加没有使合金产生明显的孔蚀,这表明Zr-4合金的抗孔蚀能力下降是Cu2+和Cl-共同作用的结果;等离子电解氧化处理能够大幅度提高Zr-4合金的抗孔蚀能力。
关键词:Zr-4合金;Cu2+;孔蚀;等离子电解氧化
中图分类号:TG178 文献标志码:A
Effects of Cu2+ and plasma electrolytic oxidation on corrosion properties of zircaloy-4 alloy
CHENG Ying-liang, WU Fan
(College of Materials Science and Engineering, Hunan University, Changsha 410082, China)
Abstract: The corrosion behavior of zicaloy-4 alloy in 0.5 mol/L NaCl+CuCl2 solution and 0.5 mol/L Na2SO4+CuSO4 solution was studied by electrochemical methods, and the effect of the concentration of Cu2+ on the corrosion behavior of zicaloy-4 alloy was discussed, meanwhile, plasma electrolytic oxidation(PEO) was carried out on the zircaloy-4 alloy in the electrolyte of 10 g/L Na2SiO3·9H2O+10 g/L Na4P2O7·10H2O, and the protection ability of the PEO coatings was evaluated by potentiodynamic polarization curves. The results show that that Cu2+ can shift the corrosion potential of the zircaloy-4 alloy toward the positive direction and narrows the scope of the passive region on the polarization curves, as a result, the resistance of the alloy to pitting corrosion decreases and the corrosion current density increases. In the solution of Na2SO4, the addition of Cu2+ does not result in obvious pitting corrosion of the alloy, manifesting that the decrease of the pitting resistance of the zircaloy-4 is the synergetic effect of Cu2+ and Cl-. PEO treatment can greatly enhance the pitting resistance of the zircaloy-4 alloy.
Key words: zircaloy-4 alloy; Cu2+; pitting; plasma electrolytic oxidation
锆及其合金具有很高的热中子透过率、优异的延展性和强度及耐腐蚀性能,一直被用作核反应堆燃料包壳及堆芯结构材料。但在反应堆运行条件下, Zr合金包壳材料在堆内工况环境恶劣,会受到高温高压氧化、电化学、辐照以及水侧冲刷等腐蚀[1-11],这对提高燃料燃耗以及燃料元件寿命都会带来很大的影响。随着我国核电工业的快速发展, 锆合金的需求量将大幅度增长,由于锆合金服役条件十分恶劣,因此,提高其耐腐蚀性能对于提高锆合金的使用寿命和核工业安全性能具有重要意义。
锆合金的耐蚀性能通常要优于不锈钢的,然 而,在特定的工况下,尤其是在高浓度的硝酸(70%,质量分数)中,锆合金存在应力腐蚀破裂(SCC)的危 险[12],此外,在含有卤素离子的溶液中,锆及其合金也存在应力腐蚀破裂的敏感性[13]。锆是一种阀金属,与其他阀金属一样,在水溶液中可以形成一层钝化膜,这层钝化膜使得金属免受进一步的腐蚀。比如Abd EL-MOTAAL等[14]研究了锆在硝酸和硫酸中形成的阳极氧化膜的稳定性,结果发现锆在硝酸中形成的氧化膜更加稳定。此外,有研究发现[15],锆合金在卤素离子或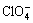 的存在下会发生点蚀, 关于锆合金在碘离子存在的条件下对氧化膜的影响也已有研究[16]。此外,对于锆合金,介质中存在的高价金属离子如 Fe3+、Cu2+会促进锆及其合金的点蚀,而且Cu2+的作用比Fe3+的作用更明显,而对于同为阀金属的钛合金,高价金属离子却有相反的作用,但详细的机理尚未见文献报道[17]。
的存在下会发生点蚀, 关于锆合金在碘离子存在的条件下对氧化膜的影响也已有研究[16]。此外,对于锆合金,介质中存在的高价金属离子如 Fe3+、Cu2+会促进锆及其合金的点蚀,而且Cu2+的作用比Fe3+的作用更明显,而对于同为阀金属的钛合金,高价金属离子却有相反的作用,但详细的机理尚未见文献报道[17]。
为了改善Zr-4合金的耐腐蚀性能,国内外采用了许多方法,其中一种就是对Zr-4合金进行表面预处理。目前一些国家主要采用的表面处理方法是高压釜中在包壳材料表面生成预生膜[18]。然而这种方法的缺点是周期长,装置复杂,对于生成效率和产品成本是一个很大的问题。人们一直希望可以找到一种替代工艺,通过更为方便、快捷、省时的方法来制备具有相似甚至更优异性能的表面氧化保护膜[19]。
等离子电解氧化技术(Plasma electrolytic oxidation,PEO),又称微弧氧化或阳极火花沉积,是一种在阀金属表面生成陶瓷氧化膜的技术。虽然PEO技术在提高镁铝合金的耐磨耐蚀性上已获得成功,但目前国内外对锆合金的PEO研究还处在起步阶段,目前仅有少数文献提到用等离子电解氧化技术来对锆合金进行表面处理来提高它的耐蚀性等综合性能[20-25]。
在此,本文作者首先研究以Cu2+为代表的高价金属离子对广泛应用于核工业的Zr-4合金(国外牌号zircaloy-4)在氯化钠或硫酸钠溶液中的腐蚀电化学行为的影响,然后在10 g/L Na2SiO3·9H2O+10 g/L Na4P2O7·10H2O 溶液中制得等离子电解氧化膜,利用极化曲线研究等离子电解氧化膜对锆合金的保护 能力。
1 实验
Zr-4合金原始材料为5 mm厚的板材,经过轧制和再结晶处理。合金的成分如下(质量分数,%):1.50 Sn,0.20 Fe,0.10 Cr,Ni<0.007,Zr余量。将板材加工成面积为1 cm2的圆形电极,电极一端与铜导线连接,用环氧树脂包封,只暴露一端为工作面。将电极的工作面用SiC水砂纸逐级打磨至2000#,然后进行抛光、水洗后经无水乙醇脱脂,蒸馏水清洗后吹干待用。
为研究Cu2+离子对Zr-4合金腐蚀行为的影响,用CuCl2和CuSO4分别配制含不同Cu2+浓度的0.5 mol/L NaC1和0.5 mol/L Na2SO4溶液,然后进行电化学测试,所有溶液均用分析纯试剂配制。实验仪器为上海辰华仪器公司生产的电化学工作站CHI660C。 实验采用三电极体系,锆合金试样为工作电极,参比电极为饱和甘汞电极,辅助电极为铂片。首先,将工作电极浸入测试溶液中,测量自腐蚀电位随时间的变化,测量时间为1 h,之后,进行动电位极化曲线的测量,从比自腐蚀电位负500 mV的电位开始,向正方向以0.5 mV/s的扫描速度进行动电位扫描,直到阳极电流明显增大时停止极化。
等离子电解氧化膜制备采用的电解液为10 g/L Na2SiO3·9H2O+10 g/L Na4P2O7·10H2O 溶液,采用分析纯试剂配制。采用直流PEO技术,电源为规格600 V,400 mA直流电源,采用恒电流PEO技术,电流密度为200 mA/cm2。试样为前述的Zr-4合金电极,在PEO处理中,试样作阳极,不锈钢板为阴极,PEO处理在1 000 mL烧杯中进行。实验过程不断搅拌,用水槽冷却电解液。实验完成后将电极取出,用蒸馏水冲洗、吹干后备用。为研究其耐腐蚀性能,在0.1 mol/L CuCl2和0.5 mol/L NaC1的混合溶液中测试极化曲线,将其和基体合金的数据进行对比,考察其腐蚀性能。试样腐蚀之后的形貌采用XTZ-E体视显微镜(上海光学仪器厂生产)观察,另外用场发射扫描电镜Jeol JSM6700F对膜层的微观结构进行观察。
2 结果与分析
2.1 Cu2+浓度对Zr-4合金在0.5 mol/L NaCl溶液中自腐蚀电位的影响
图1所示为Zr-4合金在添加不同CuCl2浓度的0.5 mol/L NaCl溶液中的自腐蚀电位随时间的变化,测试时间为1 h。从图1可以看出,Cu2+加入后,Zr-4合金的自腐蚀电位随着Cu2+浓度的增加而升高。
从图1可以看出,当溶液中加入1×10-6 mol/L Cu2+后,最初的腐蚀电位从不含Cu2+的NaCl溶液中的-0.665 V上升到-0.591 V,腐蚀电位上升74 mV。随着Cu2+浓度的进一步升高,锆合金的腐蚀电位也逐步正移。当溶液中的Cu2+浓度达到0.15 mol/L时,浸泡一段时间之后(大约500 s),锆合金的自腐蚀电位产生频率很快的波动,而Cu2+浓度更高时(0.2 mol/L),电极一旦浸入溶液就产生波动。
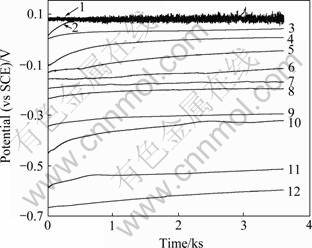
图1 Zr-4合金在不同Cu2+浓度的0.5 mol/L NaCl 溶液中腐蚀电位随时间的变化
Fig. 1 Variation of corrosion potential of zircaloy-4 alloy with time in 0.5 mol/L NaCl solution with different Cu2+ concentrations: 1—0.2 mol/L; 2—0.15 mol/L; 3—0.1 mol/L; 4—0.04 mol/L; 5—0.03 mol/L; 6—0.02 mol/L; 7—0.005 mol/L; 8—0.002 5 mol/L; 9—1×10-4 mol/L; 10—1×10-5 mol/L; 11—1×10-6 mol/L; 12—0
需要对锆合金在本实验中的表现进行解释。根据腐蚀理论,金属在实际体系中的自腐蚀电位由腐蚀过程的阴极过程和阳极过程共同决定,可以用腐蚀极化图(Evans图)来表示。锆合金在NaCl溶液中腐蚀的阴极过程为氧的去极化,而阳极过程是金属的溶解。由于锆合金属于表面有钝化膜的金属,其抗腐蚀能力取决于这层钝化膜的致密与完整性,一般来说,锆合金的钝化膜具有很强的保护性能,金属的阳极溶解受到强烈的抑制,而在溶液中形成稳定钝态的金属或合金的腐蚀过程属于阳极控制[26],因此,锆合金在NaCl溶液中的腐蚀属于阳极控制的腐蚀过程。本实验中锆合金在不同浓度Cu2+的NaCl溶液中的腐蚀过程可通过如图2的腐蚀极化图来分析说明。在图2中,阴极的起始电位是氧的平衡电位φe,O,阳极的起始电位是锆的平衡电位φe,Zr,由于体系是阳极控制的腐蚀体系,所以,阳极极化曲线φe,Zr A的斜率要大于阴极极化曲线φe,O B的斜率,阴阳极极化曲线交于S点,此时对应的电位φc,1就是锆合金在不含Cu2+的溶液中的自腐蚀电位,对应的电流Ic,1就是此时的自腐蚀电流。
当溶液中添加微量的Cu2+后,腐蚀过程发生了变化,由于加入了Cu2+,阴极极化曲线会发生变化,因为Cu2+可以还原成金属铜,起到阴极去极化剂的作 用。但由于此时添加的Cu2+浓度很低,铜的平衡电位φe,Cu较低,在图2中,Cu2+单独的阴极极化曲线为φe,Cu M,总的阴极过程为氧的还原和Cu2+的还原过程的叠加,因此,在电位φe,Cu之下,在氧分子还原的同时,Cu2+会发生还原,总的阴极极化曲线为φe,O ON。 阴阳极极化曲线交于S′点。此时对应的自腐蚀电位为φc,2,从图2看出,相比于未添加Cu2+的NaCl溶液中锆合金的腐蚀电位φc,1,电位发生正移。
根据能斯特公式
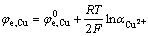 (1)
(1)
式中: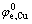 为铜的标准电极电位;R为摩尔气体常数;T为热力学温度;F为法拉第常数;
为铜的标准电极电位;R为摩尔气体常数;T为热力学温度;F为法拉第常数;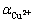 为Cu2+的活度。
为Cu2+的活度。
由式(1)可知,随着溶液中Cu2+浓度的提高,铜的平衡电极电位升高, 因此,图2中Cu2+浓度提高之后的电极电位相对于之前的电位 正移,相应的阴阳极极化曲线交于S′点也要沿
正移,相应的阴阳极极化曲线交于S′点也要沿 A正移,对应的腐蚀电位和腐蚀电流随之升高,这就解释了本实验中观察到的实验现象。
A正移,对应的腐蚀电位和腐蚀电流随之升高,这就解释了本实验中观察到的实验现象。
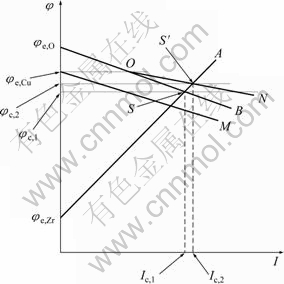
图2 Zr-4锆合金在本实验中的腐蚀过程及铜离子的影响示意图
Fig. 2 Schematic illustration of corrosion process of zircaloy-4 and effect of Cu2+
此外,当溶液中的Cu2+浓度达到一定的浓度时(0.15 mol/L及以上时),电极电位发生波动现象,这种现象曾在铝合金在NaCl溶液中腐蚀时观察到,是一种电位噪声[27-28],这种形式的电位噪声,被认为是由电极表面腐蚀微电池的腐蚀过程所引起[28]。因此,当溶液中的Cu2+浓度达到一定的程度后,锆合金表面的腐蚀微电池活动加强,其腐蚀速率也要升高。
2.2 Zr-4合金在不同Cu2+浓度的0.5 mol/L NaCl溶液中的极化曲线
图3所示为Zr-4合金在添加不同浓度的CuCl2的0.5 mol/L NaCl溶液中的极化曲线。从图3中可以看出,在0.5 mol/L NaCl溶液中加入CuCl2,可使的腐蚀电流密度明显增大,腐蚀电位明显正移。CuCl2浓度越大,变化越显著。从图4可得到极化曲线实验的一系列参数,将其列于表1中。 其中:φocp是自腐蚀电位;φb是孔蚀电位,是极化曲线上阳极电流突然增大的点对应的电位;φb-φocp是钝化区的宽度。从表1 看出,Cu2+的加入有使Zr-4合金钝化区宽度缩小的趋势,这表明Cu2+加入后锆合金发生孔蚀的倾向更大。从依据塔菲尔外推法得到的腐蚀电流来看,随着Cu2+浓度的升高,腐蚀电流相应增大。
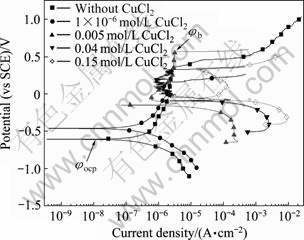
图3 Zr-4合金在添加不同浓度CuCl2的0.5 mol/L NaCl溶液中的极化曲线
Fig. 3 Polarization curves of zircaloy-4 in 0.5 mol/L NaCl solutions with different CuCl2 concentrations
2.3 Zr-4合金在不同Cu2+浓度的0.5 mol/L Na2SO4溶液中的极化曲线
从2.2节研究可以看出,添加Cu2+后,在0.5 mol/L NaCl溶液中,Zr-4合金阳极极化曲线的钝化区间缩短,表明锆合金更容易发生孔蚀。为了了解这种孔蚀性能的下降是Cu2+单独引起的还是在Cl-的综合作用下发生的,本节研究了在不含Cl-条件下Cu2+对Zr-4合金极化行为的影响。
图4所示为Zr-4合金在添加不同浓度CuSO4的0.5 mol/L Na2SO4溶液中的极化曲线。相应的极化曲 线参数列于表2中,其中自腐蚀电流由塔菲尔外推法求得。从图4看出,随Cu2+浓度的提高,Zr-4合金的自腐蚀电流增大,但是,与NaCl溶液中不同的是,图中极化曲线终止电位达到3.0 V以上,但所有的曲线都没有达到孔蚀电位,合金仍然处于钝态,实验结束后,试样表面也没有肉眼可见的蚀孔。本实验结果表明,只有在Cl-存在的条件下,Cu2+离子才加剧了Zr-4合金的孔蚀。
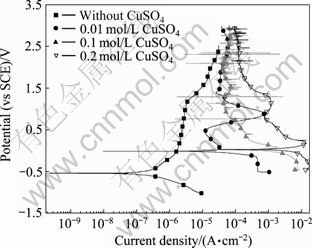
图4 Zr-4合金在添加不同浓度CuSO4的0.5 mol/L Na2SO4溶液中的极化曲线
Fig. 4 Polarization curves of zircaloy-4 in 0.5 mol/L Na2SO4 solutions with different CuSO4 concentrations
表1 Zr-4合金在添加不同浓度CuCl2的0.5 mol/L NaCl溶液中的极化曲线对应参数
Table 1 Parameters of polarization curves of zircaloy-4 in 0.5 mol/L NaCl solutions with different CuCl2 concentrations
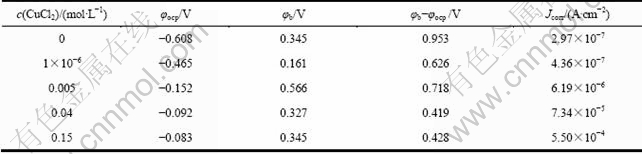
表2 Zr-4合金在添加不同浓度CuSO4的0.5 mol/L Na2SO4中的极化曲线对应参数
Table 2 Parameters of polarization curves of zircaloy-4 in 0.5 mol/L Na2SO4 solutions with different CuSO4 concentrations

2.4 PEO膜层对Zr-4合金耐蚀性的影响
在成分为10 g/L Na2SiO3·9H2O+10 g/L Na4P2O7·10H2O的电解液中,采用直流PEO技术在Zr-4合金表面制备了PEO膜层。对经过PEO处理的试样(处理时间10 min)和未经PEO处理的Zr-4合金基体在0.1 mol/L CuCl2+0.5 mol/L NaCl 溶液中进行电化学测试,图5所示为经PEO处理的试样在0.5 mol/L NaCl以及添加不同浓度的Cu2+的混合溶液中浸泡1 h的时间电位曲线(为便于比较,将Zr-4基体的实验数据也作于同一图中)。

图5 PEO处理10 min试样及Zr-4基体在添加不同浓度Cu2+的0.5 mol/L NaCl溶液中的电位—时间曲线
Fig. 5 Potential—time curves of PEO coatings for 10 min and zircaloy-4 substrate in 0.5 mol/L NaCl solutions with different Cu2+ concentrations: 1—PEO coating/0.5 mol/L NaCl; 2—PEO coating/0.5 mol/L NaCl+1×10-6 mol/L CuCl2; 3—PEO coating/0.5 mol/L NaCl+0.1 mol/L CuCl2; 4—Substrate, 0.5 mol/L NaCl
从图5看出,在Zr-4合金表面形成PEO膜层之后,其自腐蚀电位得到了大幅度的提高。但即使是经PEO处理后的试样,当溶液中添加Cu2+后,自腐蚀电位还是有所降低,而且当Cu2+浓度为0.1 mol/L时,PEO试样的电位—时间曲线产生较大的波动,预示着其表面腐蚀微电池活动的加强。
将PEO处理10 min的试样在0.5 mol/L NaCl+x mol/L CuCl2(x=0, 1×10-6, 0.005, 0.1)溶液中进行极化曲线测试,同时将Zr-4基体的数据也作于同一图中,结果如图6所示。将图6中的曲线进行拟合,得到表3的参数。
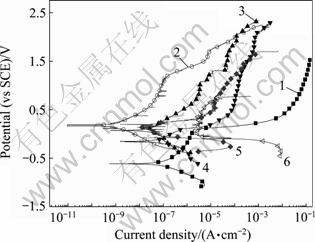
图6 经PEO处理10 min的Zr-4合金和基体合金在0.5 mol/L NaCl+不同浓度CuCl2溶液中的极化曲线
Fig. 6 Polarization curves of PEO coating for 10 min and substrate of zircaloy-4 in 0.5 mol/L NaCl solutions with different Cu2+ concentrations: 1—Zr-4 substrate/0.5 mol/L NaCl; 2—PEO coating/0.5 mol/L NaCl; 3—PEO coating/0.5 mol/L NaCl+1×10-6 mol/L CuCl2; 4—PEO coating/0.5 mol/L NaCl+ 0.005 mol/L CuCl2; 5—PEO coating/0.5 mol/L NaCl+0.1 mol/L CuCl2; 6—Zr-4 substrate/0.5 mol/L NaCl+0.1 mol/L CuCl2
从图6及表3看出,经过PEO处理的试样,很大程度上提高了合金的抗腐蚀能力,在不含Cu2+的0.5 mol/L NaCl溶液中,经PEO处理的膜层自腐蚀电流减小到9.08×10-10 A/cm2, 相比未经处理的试样耐蚀性提高了3个数量级,而且在极化曲线的测试范围内试样没有出现明显的孔蚀电位。在溶液中添加Cu2+后,经PEO处理的试样的腐蚀电流有所增加,但是相比同样条件下的未经PEO处理试样,腐蚀电流仍然小得多。当Cu2+浓度达到0.1 mol/L后,经PEO处理的膜层在1.693 V时出现了孔蚀。
图7所示为Zr-4合金基体和PEO膜层在不同的极化实验后的表面形貌。极化实验相当于腐蚀加速实验,可以用腐蚀之后的形貌来比较膜层的耐蚀程度。图7(a)所示为Zr-4基体合金在0.5 mol/L NaCl溶液中极化到图6所示的终止电位时试样的表面形貌。可以看出试样表面大部分区域已经被活性溶解所腐蚀,图7(b)所示为7(a)的放大形貌。由图7(b)可见,试样表面呈现均匀溶解的形貌。图7(c)对应于PEO膜层在0.5 mol/L NaCl中的极化曲线实验完成后的形貌,可以看出表面膜层仍然保持完整(此时的极化曲线扫描终止电位在2.2 V以上)。图7(d)所示为对应于PEO 膜层在 0.5 mol/L NaCl+0.1 mol/L CuCl2 溶液中极化曲线实验完成后的形貌。从图6可知,该曲线在1.693 V出现孔蚀电位,相应地在图7(d)中,在试样的表面观察到了一个蚀孔,其放大形貌见图7(e)。
表3 经PEO 处理10 min的膜层及Zr-4合金在添加不同浓度CuCl2的0.5 mol/L NaCl溶液中的极化曲线对应参数(对应图6)
Table 3 Parameters of polarization curves of PEO coatings for 10 min and zircaloy-4 substrate in 0.5 mol/L NaCl solutions with different CuCl2 concentrations (Fig.6)
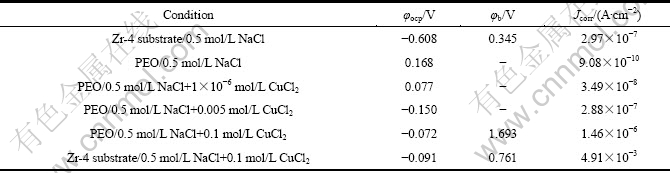
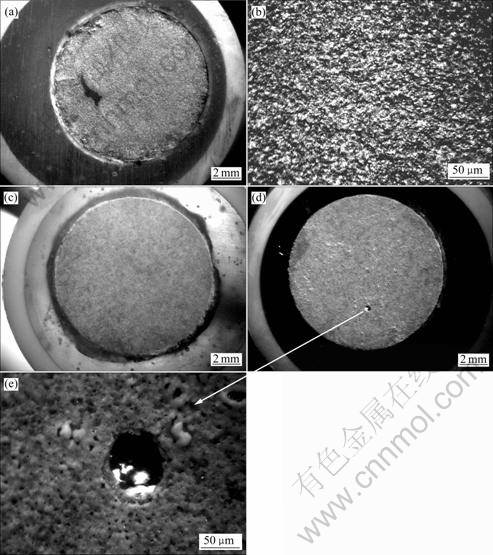
图7 Zr-4合金经及PEO 处理10 min的膜层在极化曲线实验结束后的形貌
Fig. 7 Surface morphologies of zircaloy-4 alloy and PEO coatings with 10 min after polarization curve tests: (a), (b) Morphologies with different magnifications of zircaloy-4 substrate after polarization test in 0.5 mol/L NaCl solutions; (c) Morphology of PEO coating after polarization test in 0.5 mol/L NaCl solution; (d), (e) Morphologies of coating and pit after polarization test of PEO coating in 0.5 mol/L NaCl+0.1 mol/L CuCl2 solution
从极化曲线的实验结果来看,PEO处理显著提高了Zr-4合金的耐孔蚀性能。但处理10 min的PEO试样在含有0.1 mol/L Cu2+的0.5 mol/L NaCl溶液中当电位达到1.693 V时仍然出现了蚀孔。为了考察提高膜层厚度对耐腐蚀性能的影响,我们进一步对经PEO处理60 min的试样在0.1 mol/L CuCl2+0.5 mol/L NaCl溶液中进行极化曲线测试,并和处理10 min试样的实验结果在同一图中进行对比。其结果如图8所示。从图8可以看出,处理60 min的试样腐蚀电流密度比处理10 min的试样的电流密度有较大的降低。此外,处理60 min的试样的孔蚀电位提高到2.14 V,孔蚀之后的电流相比10 min的试样小很多,且孔蚀之后还有再钝化的趋势。从此结果看,延长处理时间有利于提高膜层的耐蚀性。利用扫描电镜对处理60 min的PEO膜层进行观察,膜层的表面和截面形貌如图9所示。从图9可以得知,膜层厚度为70 μm左右,在其截面有一定数量的孔洞和裂纹。可以预期,进一步改进膜层质量,可以加强其保护性能。
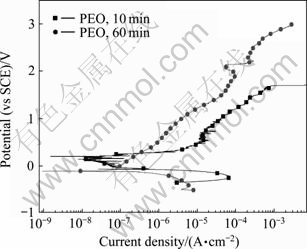
图 8 经PEO处理10 min和60 min试样在0.1 mol/L CuCl2+0.5 mol/L NaCl 溶液中的极化曲线比较
Fig. 8 Comparison of polarization curves of PEO coatings for 10 min and 60 min in 0.1 mol/L CuCl2+0.5 mol/L NaCl solution
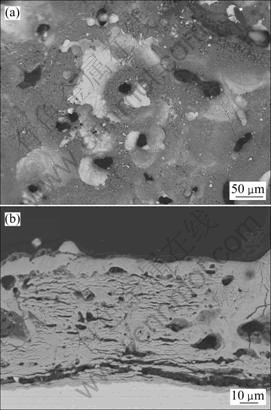
图 9 在10 g/L Na2SiO3·9H2O+10 g/L Na4P2O7·10H2O溶液中处理60 min的Zr-4合金PEO膜层的表面和截面形貌
Fig. 9 Surface(a) and cross section(b) morphologies of PEO coating formed on zircaloy-4 in 10 g/L Na2SiO3·9H2O+10 g/L Na4P2O7·10H2O solution for 60 min
3 结论
1) Cu2+浓度的提高使0.5 mol/L NaCl溶液中的Zr-4合金的腐蚀电位正移。极化曲线结果表明,添加Cu2+后,合金的抗孔蚀能力下降,腐蚀电流密度增加。
2) 在0.5 mol/L硫酸钠溶液中,Cu2+的添加未使合金产生明显的孔蚀。Zr-4合金的抗孔蚀能力下降是Cu2+和Cl-共同作用的结果。
3) 等离子电解氧化处理(PEO)能够大幅度提高Zr-4合金的抗孔蚀能力。
REFERENCES
[1] MIYAKE M, UNO M, YAMANAKA S. On the zirconium- oxygen-hydrogen ternary system[J]. Journal of Nuclear Materials, 1999, 270(1/2): 233-241.
[2] SETOYAMA D, YAMANAKA S. Phase diagram of Zr-O-H ternary system[J]. Journal of Alloys and Compounds, 2004, 370(1/2): 144-148.
[3] MOTTA A T, YILMAZBAYHAN A, DA SILVA M J G, COMSTOCK R J, WAS G S, BUSBY J T, GARTNER E, PENG Q, JEONG Y H, PARK J Y. Zirconium alloys for supercritical water reactor applications: Challenges and possibilities[J]. Journal of Nuclear Materials, 2007, 371(1/3): 61-75.
[4] YILMAZBAYHAN A, BREVAL E, MOTTA A T, COMSTOCK R J. Transmission electron microscopy examination of oxide layers formed on Zr alloys[J]. Journal of Nuclear Materials, 2006, 349(3): 265-281.
[5] YILMAZBAYHAN A, MOTTA A T, COMSTOCK R J, SABOL G P, LAI B, CAI Z. Structure of zirconium alloy oxides formed in pure water studied with synchrotron radiation and optical microscopy: Relation to corrosion rate[J]. Journal of Nuclear Materials, 2004, 324(1): 6-22.
[6] RAJ B, MUDALI U K. Materials development and corrosion problems in nuclear fuel reprocessing plants[J]. Progress in Nuclear Energy, 2006, 48(4): 283-313.
[7] QIN W, NAM C, LI H L, SZPUNAR J A. Tetragonal phase stability in ZrO2 film formed on zirconium alloys and its effects on corrosion resistance[J]. Acta Materialia, 2007, 55(5): 1695-1701.
[8] 白新德. 核材料化学[M]. 北京: 化学工业出版社, 2005: 305-319.
BAI Xin-de. Chemistry of nuclear materials[M]. Beijing: Chemical Industry Press, 2005: 305-349.
[9] 白新德. 材料腐蚀与控制[M]. 北京: 清华大学出版社, 2007: 404-416.
BAI Xin-de. Corrosion and control of materials[M]. Beijing: Tsinghua University Press, 2007: 404-416.
[10] 黄 强. 锆合金耐腐蚀性能研究综述[J]. 核动力工程, 1996, 17(3): 262-267.
HUANG Qiang. Summarization on performance study of corrosion resistance of zirconium alloys[J]. Nuclear Power Engineering, 1996, 17(3): 262-267.
[11] 周邦新, 郑斯奎, 汪顺新. Zr-2合金中应力和应变诱发氢化锆析出过程的电子显微镜原位研究[J]. 金属学报, 1989, 25(3): 190-195.
ZHOU Bang-xin, ZHENG Si-kui, WANG Shun-xin, In situ electron microscopy study on precipitation of zirconium hydrides induced by strain and stress in zircaloy-2[J]. Acta Metallurgica Sinica, 1989, 25(3): 190-195.
[12] HAGANO H, KAJIMURA H. The stress corrosion cracking performance of pure zirconium and zirconium alloys in highly oxidizing nitric acid[J]. Corrosion Science, 1996, 38(5): 781-791.
[13] FARINA S B , DUFFO G S, GALVELE J R. Stress corrosion cracking of zirconium and zircaloy-4 in halide aqueous solutions[J]. Corrosion Science, 2003, 45(11): 2497-2512.
[14] ABD EL-MOTAAL S M, HILAL N H, BADAWY W A. Stability of zirconium passive films in nitric and sulphuric acid solutions[J]. Electrochimica Acta, 1994, 39(17): 2611-2617.
[15] HORNKJOL S. Pitting corrosion of zirconium and hafnium[J]. Electrochimica Acta, 1988, 33(2): 289-292.
[16] SCHREBLER R, GOMEZ H, CORDOVA R, GARDIAZABAL I. The influence of iodine in the electroformation of ZrO2 films on zircaloy-4 electrodes in acid solutions[J]. Corrosion Science, 1989, 29(11/12): 1257-1263.
[17] PETIT J A, CHATAINIER G, DABOSI F. Inhibitors for the corrosion of reactive metals: Titanium and zirconium and their alloys in acid media[J]. Corrosion Science, 1981, 21(4): 279-299.
[18] 赵文金, 苗 志, 蒋有荣, 蒋宏曼, 周邦新. 预生氧化膜处理对锆-4包壳疖腐蚀的影响[J]. 稀有金属, 2000, 24(2): 150-153.
ZHAO Wen-jin, MIAO Zhi, JIANG You-rong, JIANG Hong-man, ZHOU Bang-xin. Effect of pre-oxidizing treatment on nodular corrosion of Zr-4 tube[J]. Chinese Journal of Rare Metals, 2000, 24(2): 150-153.
[19] 李 娟, 白新德, 张岱岚. Zr-4合金的阳极氧化膜与高压釜预生膜对比研究[J]. 稀有金属材料与工程, 2006, 35(6): 1002-1005.
LI Juan, BAI Xin-de, ZHANG Dai-lan. Study on the anodic oxide film and autoclaved oxide film of zircaloy-4[J]. Rare Metal Materials and Engineering, 2006, 35(6): 1002-1005.
[20] MATYKINA E, ARRABAL R, SKELDON P, THOMPSON G E, WANG P, WOOD P. Plasma electrolytic oxidation of a zirconium alloy under AC conditions[J]. Surface & Coatings Technology, 2010, 204(14): 2142-2151.
[21] XUE W B, ZHU Q Z, JIN Q , HUA M. Characterization of ceramic coatings fabricated on zirconium alloy by plasma electrolytic oxidation in silicate electrolyte[J]. Materials Chemistry and Physics, 2010, 120(2/3): 656-660.
[22] NYKYFORCHYN H M, AGARWALA V S, KLAPKIV M D, POSUVAILO V M. Simultaneous reduction of wear and corrosion of titanium, magnesium and zirconium alloys by plasma electrolyte oxidation treatment[J]. Advanced Materials Research, 2008, 38: 27-35.
[23] YAN Y, HAN Y, HUANG J. Formation of Al2O3-ZrO2 composite coating on zirconium by micro-arc oxidation[J]. Scripta Materialia, 2008, 59(2): 203-206.
[24] YAN Y, HAN Y. Structure and bioactivity of micro-arc oxidized zirconia films[J]. Surface & Coatings Technology, 2007, 201(9/11): 5692-5695.
[25] CHENG Y, MATYKINA E, SKELDON P, THOMPSON G. Characterization of plasma electrolytic oxidation coatings on zircaloy-4 formed in different electrolytes with AC current regime[J]. Electrochimica Acta, 2011, 56(24): 8467-8476.
[26] 魏宝明. 金属腐蚀理论及应用[M]. 北京: 化学工业出版社, 1984: 81.
WEI Bao-ming. The theory of metal corrosion and its application[M]. Beijing: Chemical Industry Press, 1984: 81.
[27] CHENG Ying-liang, ZHANG Zhao, CAO Fa-he, LI Jing-feng, ZHANG Jiang-qing, WANG Jiang-ming, CAO Chu-nan. Corrosion of LY12 aluminum alloy in sodium chloride solution[J]. Transactions of Nonferrous Metals Society of China, 2003, 13(3): 617-621.
[28] CHENG Y L, CHEN Z H, WU H L, WANG H M. The corrosion behaviour of the aluminum alloy 7075/SiCp metal matrix composite prepared by spray deposition[J]. Materials and Corrosion, 2007, 58(4): 280-284.
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51071066);湖南大学“中央高校基本科研业务费”资助项目(531107040029);湖南大学“青年教师成长计划”资助项目(531107040029)
收稿日期:2011-07-18;修订日期:2011-09-28
通信作者:程英亮,副教授,博士,电话:13036798588;E-mail: deepblacksea@163.com