
Microstructure evolution of semi-solid Mg-14Al-0.5Mn alloys during isothermal heat treatment
ZHANG Liang(张 亮), CAO Zhan-yi(曹占义), LIU Yong-bing(刘勇兵)
Key Laboratory of Automobile Materials of Ministry of Education,
Department of Materials Science and Engineering, Jilin University, Changchun 130025, China
Received 23 September 2009; accepted 30 January 2010
Abstract: A new Mg-14Al-0.5Mn alloy that exhibits a wide solidification range and sufficient fluidity for semi-solid forming was designed. And the microstructure evolution of semi-solid Mg-14Al-0.5Mn alloy during isothermal heat treatment was investigated. The mechanism of the microstructure evolution and the processing conditions for isothermal heat treatment were also discussed. The results show that the microstructures of cast alloys consist of α-Mg, β-Mg17Al12 and a small amount of Al-Mn compounds. After holding at 520 ?C for 3 min, the phases of β-Mg17Al12 and eutectic mixtures in the Mg-14Al-0.5Mn alloy melt and the microstructures of α-Mg change from developed dendrites to irregular solid particles. With increasing the isothermal time, the amount of liquid increases, and the solid particles grow large and become spherical. When the holding time lasts for 20 min or even longer, the solid and liquid phases achieve a state of dynamic equilibrium.
Key words: Mg-14Al-0.5Mn; semi-solid; isothermal heat treatment; microstructure evolution
1 Introduction
Semi-solid forming is expected to be widely applied to magnesium alloys in producing near-net shaped and light structural components that offer high performance at a favorable cost in various industries[1-3]. It provides some advantages, such as ease of handling, lower operating temperature that reduces solidification shrinkage and less thermal shock that leads to prolonged die and shot sleeve lives compared with die-casting[4-6]. Whereas, magnesium alloys currently used in semi-solid processing are restricted to a few commercial alloys such as AZ91, AM50 and AM60[7-8]. Although the feasibility of semi-solid processing has been demonstrated, its acceptance on a commercial scale has been limited. Success with these alloys has generated interest in other alloy systems and compositions[9-10].
Increasing the content of Al in magnesium alloys in a certain extent could broaden the solidification range and obtain sufficient fluidity for semi-solid forming. However, some studies on Mg-Al alloys have been reported that Mgl7A1l2 compound crystallizes in the alloys with high aluminum content and this significantly impairs the mechanical properties of the alloys, especially at low temperatures[11-13]. Here, a small quantity of Mn was added to suppress the formation of Mg17Al12 and increase the comprehensive performance of the alloys[14-15]. Based on the above ideas, a new Mg-14Al-0.5Mn alloy was developed. In this work, the semi-solid microstructure of the alloys was obtained by isothermal heat treatment with low processing costs[16]. And the microstructure evolution of the alloys during isothermal heat treatment was discussed. The results could be useful for the development of new semi-solid magnesium alloys.
2 Experimental
Material used for this study was cast Mg-14Al-0.5Mn alloy, and a practical composition (mass fraction, %) was 13.84Al, 0.48Mn, 0.02Zn, 0.006Fe, 0.02Si, and Mg balance. The Mg-14Al-0.5Mn melts were poured into a copper mold preheated at about 100 ?C to produce circular column samples of d16 mm×100 mm. The isothermal heat treatment was performed in an electric resistance furnace under a protective atmosphere of flowing gas of SF6 (1%, volume fraction) and CO2 (Balance). When the furnace was heated to 520 ?C, the circular column samples (d16 mm×10 mm) were placed into the furnace and held for 3, 5, 10, 15 and 20 min, respectively. After the isothermal treatment, the samples were taken out immediately for water quenching.
The samples were carefully ground and polished, and then etched in 4% (volume fraction) nitric acid ethanol solution. Microstructure and phase analyses were carried out by optical microscopy (OM) (PMG3) and X-ray diffractometry (XRD) (D/Max2500PC Rigaku, Japan). Detailed morphological and microchemical characterizations were conducted with scanning electron microscopy (SEM) (Model JSM-5310, Japan) equipped with an energy dispersive spectrometer (EDS) (Model Link-Isis, Britain). In this work, self-programmed software with VC++ was used for quantitative metallographic analysis of microstructure images, including the actual solid fraction and the average size of solid particles.
3 Results and discussion
3.1 Microstructure of as-cast Mg-14Al-0.5Mn alloy
Fig.1 shows the phase compositions of as-cast Mg-14Al-0.5Mn alloy by XRD analysis. It demonstrates that the alloy is mainly composed of α-Mg and β-Mg17Al12. As the content of Mn in the alloy is less than 1% (mass fraction), the diffraction peaks of manganese compounds do not appear in the XRD pattern. The optical micrograph of the as-cast alloy is shown in Fig.2.
The microstructure consists of primary α-Mg and intermetallic compounds that mainly distribute at the grain boundaries.
The typical SEM image and area analysis of elements for the as-cast Mg-14Al-0.5Mn alloy are shown in Fig.3. The SEM image indicates that the bone-like and
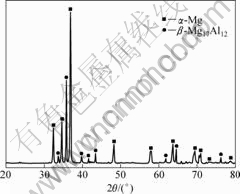
Fig.1 XRD pattern diffraction of as-cast Mg-14Al-0.5Mn alloy
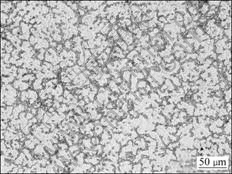
Fig.2 Microstructure of as-cast Mg-14Al-0.5Mn alloy
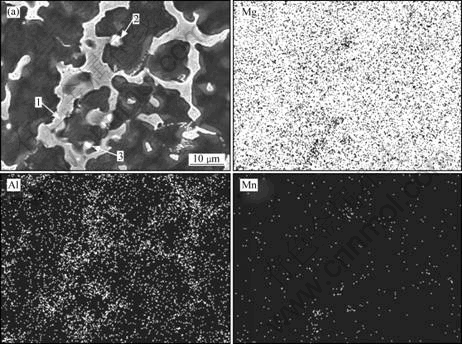
Fig.3 SEM image and area analysis of elements for as-cast Mg-14Al-0.5Mn alloy
particle-like precipitates coexist along the grain boundaries. Ordinary EDS measurements confirm the elementary contents of the precipitates. The bone-like phases are mainly β-Mg17Al12 (point 1 in Fig.3). And the particle-like phases are Al-Mn compounds as listed in Table 1 (point 2 and point 3 in Fig.3). From the analysis of SEM image and EDS measurements, it can be seen that in the Mg-Al alloys most of the Mn element exists in the form of Al-Mn compounds in the eutectic mixture, and only a small amount of Mn element is dissolved in the matrix α-Mg.
Table 1 Composition of particle-like compound in grain boundaries examined by EDS (molar fraction, %)

3.2 Microstructure evolution during isothermal treatment
The solidus and liquidus of Mg-14Al-0.5Mn alloy are about 437 ?C and 560 ?C, respectively. Studies[17] have shown that the semi-solid slurry will have good rheology and thixotropy when the solid fraction of the slurry is 20%-60%[17]. So, the isothermal temperature of 520 ?C is chosen, at which the solid fraction is about 50% in the solid-liquid equilibrium.
When the as-cast alloy is put into the furnace at 520 ?C, the β-Mg17Al12 and eutectic mixtures with low melting point begin to melt immediately. The melting of the alloy and increasing in liquid volume fraction make the microstructures of α-Mg change from developed dendrites to irregular solid particles. The size of the solid particles is not uniform, as shown in Fig.4(a). With increasing the holding time; the amount of liquid increases; the solid particles grow large and become globular; and reunion takes place between some of the particles, as illustrated in Figs.4(b), (c), (d) and (e). Fig.5 shows the variations of solid volume fraction and average size of the particles with isothermal time at 520 ?C. It can be seen from Fig.5 that the solid fraction decreases and the average size of the particles increases with increasing the isothermal time.
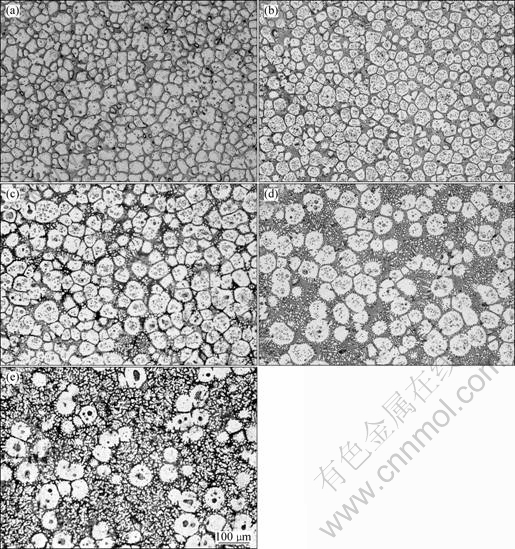
Fig.4 Microstructures of Mg-14Al-0.5Mn alloy after isothermal heat treatment at 520 ℃ for 3 min (a), 5 min (b), 10 min (c), 15 min (d) and 20 min (e)
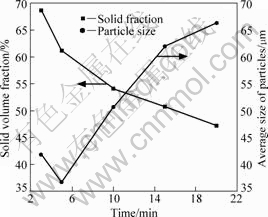
Fig.5 Variations of solid volume fraction and average size of particles with isothermal time at 520 ?C
3.3 Evolution mechanisms during isothermal treatment
Fig.6 shows the microstructure evolution of as-cast Mg-14Al-0.5Mn alloy during the isothermal treatment. In the Mg-14Al-0.5Mn alloy, as a result of increasing the Al content, the amounts of β-Mg17Al12 and eutectic mixtures increase obviously. These intermetallic compound and mixtures with low melting point distribute at the grain boundaries around the primary α-Mg, as shown in Fig.6(a). When the alloy is put into the furnace at 520 ?C, the β-Mg17Al12 and eutectic mixtures begin to melt immediately. At the bend of α-Mg dendrite arm, the melting point lowers down because of the large curvature. These areas are first melted, making the morphology of α-Mg change from dendrites to irregular solid particles, as shown in Fig.6(b). At the first few minutes, melting plays a major role on the size of the
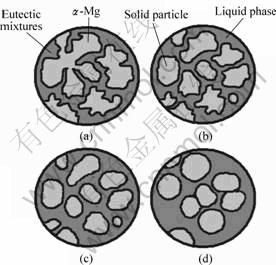
Fig.6 Illustration of microstructure evolution during isothermal treatment
solid particles, which makes the average size reduce, as illustrated in Fig.5. With increasing the isothermal time, the amount of liquid increases and the solid particles grow large and become globular. At this stage, spheroidizing and particle coarsening determine the morphology of the solid particles. Due to the effect of interface curvature, the spheroidization of the particles takes place in order to decrease the free energy. With the increase of holding time, Ostwald ripening mechanism begins to do an effect on structural coarsening[18]. The small solid particles disappear and the large particles grow larger. During the isothermal heat treatment, combination and reunion also take place among some solid particles, as shown in Figs.6(c) and (d). When the isothermal time lasts for 20 min or even more, the solid and liquid phases achieve a dynamic balance.
The semi-solid alloy billets are the basic and key for the semi-solid forming. The main requirement for alloys to be shaped in the semi-solid state is that they should exhibit a fine spheroidal or non-dendritic grain structure[5, 19-20]. From the analysis of microstructure evolution mechanisms, it can be revealed that the following processing parameters should be considered when using isothermal treatment method to prepare the semi-solid slurry.
1) Composition of the alloy. Adequate amount of the second phase with low melting point is needed, so that the semi-solid alloy will achieve an ideal solid fraction in a short isothermal time.
2) Microstructure of the as-cast alloy. The finer the size of the initial grain, the smaller the size of the semi-solid particles.
3) The isothermal time and temperature. Select high isothermal temperature between the solid-liquid range and short isothermal time to prevent the solid particles from growing up.
4 Conclusions
1) The microstructure of as-cast Mg-14Al-0.5Mn alloy consists of primary α-Mg and β-Mg17Al12 intermetallic compound that mainly distributes at the grain boundaries. Small amount of Mn element dissolves in the matrix α-Mg, and most of Mn element exists in the form of Al-Mn compounds in the eutectic mixture of α-Mg and β-Mg17Al12.
2) The microstructure evolution of semi-solid Mg-14Al-0.5Mn alloy during the isothermal treatment is as follows: β-Mg17Al12 and eutectic mixtures melt→ α-Mg dendrites melt into irregular solid particles→ spheroidizing and particle coarsening accompanied with Ostwald ripening, combination and reunion→achieving a solid-liquid dynamic balance when the isothermal time is long enough.
3) Three important parameters should be considered when using the isothermal treatment method, including composition of the alloy, microstructures of the as-cast alloy, isothermal time and temperature. Mg-14Al-0.5Mn alloy held at 520 ?C for 5-8 min can obtain good semi-solid microstructures.
References
[1] FAN Z. Semi-solid metal processing [J]. International Materials Reviews, 2002, 47(2): 49-85.
[2] CZERWINSKI F, ZIELINSKA LIPIEC A. The melting behaviour of extruded Mg-8%Al-2%Zn alloy [J]. Acta Materialia, 2003, 51(11): 3319-3332.
[3] ANTARA ING, SUZUKI K, KAYUTA T, KAMADO S, KOJIMA Y. Application of semi-solid forming to magnesium alloys with high Al and Zn contents [C]//The 2nd International Conference on Platform Science and Technology for Advanced Magnesium Alloys. Osaka, Japan: Materials Science Forum, 2003: 629-634.
[4] WANG J L, SU Y H, TSAO C Y A. Structural evolution of conventional cast dendritic and spray-cast non-dendritic structures during isothermal holding in the semi-solid state [J]. Scripta Materialia, 1997, 37(12): 2003-2007.
[5] CHEN T J, HAO Y, SUN J. Microstructural evolution of previously deformed ZA27 alloy during partial remelting [J]. Materials Science and Engineering A, 2002, 337(1/2): 73-81.
[6] NAFISI S, LASHKARI O, GHOMASHCHI R, AJERSCH F, CHARETTE A. Microstructure and rheological behavior of grain refined and modified semi-solid A356 Al-Si slurries [J]. Acta Materialia, 2006, 54(13): 3503-3511.
[7] DU X H, ZHANG E L. Microstructure and mechanical behaviour of semi-solid die-casting AZ91D magnesium alloy [J]. Materials Letters, 2007, 61(11/12): 2333-2337.
[8] TZIMAS E, ZAYALIANGOS A. Materials selection for semi-solid processing [J]. Materials and Manufacturing Processes, 1999, 14(2): 217-230.
[9] ZHANG Q Q, CAO Z Y, LIU Y B, WU J H, ZHANG Y F. Study on the microstructure evolution and rheological parameter of semi-solid Mg-10Al-4Zn alloys [J]. Materials Science and Engineering A, 2008, 478(1/2): 195-200.
[10] NAMI B, SHABESTARI S G, MIRESMAEILI S M, RAZAVI H, MIRDAMADI S H. The effect of rare earth elements on the kinetics of the isothermal coarsening of the globular solid phase in semi-solid AZ91 alloy produced via SIMA process [J]. Journal of Alloys and Compounds, 2010, 489(2): 570-575.
[11] POLMEAR I J. Recent developments in light alloys [J]. Materials Transactions, 1996(37): 12-31.
[12] BAMBERGER M, DEHM G. Trends in the development of new Mg alloys [J]. Annual Review of Materials Research, 2008, 38: 505-533.
[13] ZHANG L, CAO Z Y, LIU Y B, SU G H, CHENG L R. Effect of Al content on the microstructures and mechanical properties of Mg-Al alloys [J]. Materials Science and Engineering A, 2009, 508(1/2): 129-133.
[14] SASAKI T, TAKIGAWA Y, HIGASHI K. Effect of Mn on fracture toughness in Mg-6Al-1wt.%Zn alloy [J]. Materials Science and Engineering A, 2008, 479(1/2): 117-124.
[15] WAN D Q, WANG J C, WANG G F, CHEN X Y, LIN L, FENG Z G, YANG G C. Effect of Mn on damping capacities, mechanical properties, and corrosion behaviour of high damping Mg-3wt.%Ni based alloy [J]. Materials Science and Engineering A, 2008, 494(1/2): 139-142.
[16] ZHU Ming-fang, SU Hua-qin. A Study on producing ZA12 alloy with globular structure using semi-solid isothermal treatment [J]. Foundry, 1996(4): 1-5. (in Chinese)
[17] LIU Y Q, FAN Z. Magnesium alloy selection for semi-solid metal processing [C]//Proceeding of the 7th S2P. Tsukuba, Japan: International Conference on Semi-solid Processing of Alloys and Composites, 2002: 587-592.
[18] ZHANG L, LIU Y B, CAO Z Y, ZHANG Y F, ZHANG Q Q. Effects of isothermal process parameters on the microstructure of semi-solid AZ91D alloy produced by SIMA [J]. Journal of Materials Processing Technology. 2009, 209(2): 792-797.
[19] TZIMAS E, ZAVALIANGOS A. Evolution of near-equiaxed microstructure in the semi-solid state [J]. Materials Science and Engineering A, 2000, 289(1/2): 228-240.
[20] WANG J G, LU P, WANG H Y, LIU J F, JIANG Q C. Semi-solid microstructure evolution of the predeformed AZ91D alloy during heat treatment [J]. Journal of Alloys and Compounds, 2005, 395(1/2): 108-112.
(Edited by LI Xiang-qun)
Foundation item: Projects(2006BA104B04-1, 2006BAE04B07-3) supported by the National Science and Technology Supporting Program of China; Project(2007KZ05) supported by the Science and Technology Supporting Project of Changchun City, China; Project(2008) supported by the Open Subject of State Key Laboratory of Rare Earth Resource Utilization, China; Project supported by the “985 Project” of Jilin University, China
Corresponding author: CAO Zhan-Yi; Tel.: +86-431-85095874; Fax: +86-431-85095876; E-mail: caozy@jlu.edu.cn
DOI: 10.1016/S1003-6326(09)60286-7