Gemini双季铵盐捕收剂BDDA和EDDA对高岭石、叶腊石、伊利石浮选行为的比较及机理
来源期刊:中国有色金属学报(英文版)2013年第10期
论文作者:黄志强 钟 宏 王 帅 夏柳荫 刘广义
文章页码:3055 - 3062
关键词:铝硅酸盐矿物;Gemini阳离子表面活性剂;反浮选;吸附机理
Key words:aluminosilicate minerals; Gemini cationic surfactants; reverse flotation; adsorption mechanism
摘 要:采用了新型Gemini双季铵盐捕收剂丁烷-1,4-双十二烷基二甲基溴化铵(BDDA)和乙烷-1,2-双十二烷基二甲基溴化铵(EDDA)对比研究了其对高岭石、叶腊石、伊利石的浮选行为及作用机理。单矿物试验结果表明,在广泛的pH范围内,新型Gemini双季铵盐捕收剂BDDA和EDDA对三种铝硅酸盐矿物具有优异的捕收性能,且BDDA的捕收能力强于EDDA。红外光谱和动电位研究表明,Gemini双季铵盐捕收剂对三种铝硅酸盐矿物的作用机理为静电吸附和氢键作用。采用DFT密度泛函理论,在B3LYP/6-31G(d)水平上对捕收剂阳离子BDDA2+和EDDA2+进行量化计算,结果表明BDDA的捕收能力强于EDDA。这与单矿物浮选结果、动电位测定结果一致。
Abstract: Gemini quaternary ammonium salt surfactants, butane-α, ω-bis(dimethyl dodeculammonium bromide) (BDDA) and ethane-α, β-bis(dimethyl dodeculammonium bromide) (EDDA) were adopted to comparatively study the flotation behaviors of kaolinite, pyrophyllite and illite. It was found that three silicate minerals all exhibited good floatability with Gemini cationic surfactants as collectors over a wide pH range, while BDDA showed a stronger collecting power than EDDA. FTIR spectra and zeta potential analysis indicated that the mechanism of adsorption of Gemini collector molecules on three silicate minerals surfaces was almost identical for the electronic attraction and hydrogen bonds effect. The theoretically obtained results of density functional theory (DFT) at B3LYP/6-31G (d) level demonstrated the stronger collecting power of BDDA presented in the floatation test and zeta potential measurement.
Trans. Nonferrous Met. Soc. China 23(2013) 3055-3062
Zhi-qiang HUANG, Hong ZHONG, Shuai WANG, Liu-yin XIA, Guang-yi LIU
School of Chemistry and Chemical Engineering, Key Laboratory of Resources Chemistry of Nonferrous Metals, Ministry of Education, Central South University, Changsha 410083, China
Received 18 September 2012; accepted 5 March 2013
Abstract: Gemini quaternary ammonium salt surfactants, butane-α, ω-bis(dimethyl dodeculammonium bromide) (BDDA) and ethane-α, β-bis(dimethyl dodeculammonium bromide) (EDDA) were adopted to comparatively study the flotation behaviors of kaolinite, pyrophyllite and illite. It was found that three silicate minerals all exhibited good floatability with Gemini cationic surfactants as collectors over a wide pH range, while BDDA showed a stronger collecting power than EDDA. FTIR spectra and zeta potential analysis indicated that the mechanism of adsorption of Gemini collector molecules on three silicate minerals surfaces was almost identical for the electronic attraction and hydrogen bonds effect. The theoretically obtained results of density functional theory (DFT) at B3LYP/6-31G (d) level demonstrated the stronger collecting power of BDDA presented in the floatation test and zeta potential measurement.
Key words: aluminosilicate minerals; Gemini cationic surfactants; reverse flotation; adsorption mechanism
1 Introduction
For diaspore-bauxites in China, reverse flotation has been proved to be an efficient and economic method to obtain high grade bauxites (Al2O3-to-SiO2 mass ratio greater than 8 [1-4]. The gangue minerals in Chinese diasporic bauxites are mainly kaolinite, pyrophyllite and illite, and the purpose of the reverse flotation technique for silica removal is to separate these minerals from the diaspore. New cationic collectors have been extensively investigated in recent years to improve the floatability of kaolinite, pyrophyllite and illite, such as, alkylamines [5,6], N-alkyl-1, 3-diaminopropanes [7,8], RL [9], N-(3- aminopropyl)-dodecanamide [10], N-(2-aminoethyl)-1- naphthalene-acetamide [11], methyl-naphthaleneamine [12], γ-alkyl- propylamines [13], quaternary ammonium salts [14]. Due to our activity in the field of cationic surfactants [15-18] and, in particular, of gemini ones [19-22], we have proved that BDDA is a significantly effective collector for the reverse flotation of diasporic-bauxite ores with soluble starch as a depressant, and it is more efficient than corresponding conventional monomeric surfactant (dedecyl trimethyl ammonium bromide, DTAB) for the aluminosilicate minerals of kaolinite, illite as well as pyrophyllite. In order to search for a more efficient Gemini surfactant, it is desirable to do some comparative studies on flotation of aluminosili- cate minerals with BDDA and its homologue EDDA.
The main purpose of this study is to illustrate the flotation behaviors of kaolinite, pyrophyllite and illite with Gemini quaternary ammonium salt BDDA and EDDA as collectors. In this work, the interaction of Gemini collectors and aluminosilicates minerals surfaces is followed by infrared surface analysis, zeta potential measurement and density functional theory (DFT) calculation studies. The chemical structures of the Gemini surfactants BDDA and EDDA are shown in Fig. 1.
2 Experimental
2.1 Minerals and reagents
Hand-picked kaolinite, pyrophyllite and illite were obtained from the geological museum of China, Qingtian and Ouhai of Zhejiang province in China, respectively. They were 90% pure based on the mineralogical analysis, X-ray diffraction, and chemical analysis. The chemical analysis results are listed in Table 1.

Fig. 1 Chemical structures of Gemini surfactants BDDA (a) and EDDA (b)
Table 1 Chemical analysis results of pure minerals
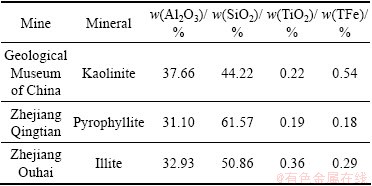
Each pure mineral was porcelain ground to a diameter smaller than 0.076 mm. The specific surface area (A) of minerals was determined using Brunauer- Emment-Teller (BET) technique. The measurements were performed by nitrogen adsorption using a NOVA-1000 surface area analyzer. The specific surface areas were calculated as 0.835 m2/g for kaolinite, 1.170 m2/g for pyrophyllite and 1.300 m2/g for illite, respectively.
Gemini quaternary ammonium salts butane-α, ω-bis (dimethyl dodecyl ammonium bromide) (BDDA) and ethane-α, β-bis(dimethyl dodeculammonium bromide) (EDDA), as the collectors, were provided by the Daochun Chemical Engineering and Technology Corporation of Henan, China. Solutions of HCl and NaOH were used to adjust the pH of the system and distilled water was used in all tests.
2.2 Micro-flotation
Flotation tests were carried out with a XFG5-35 flotation machine with 40 mL effective cel1 volume, at the impeller speed fixed at l650 r/min. Pure mineral particles (3 g) were placed in a plexiglass cell, which was then filled with distilled water. After adding the desired amount of reagents, the suspension was agitated for 3 min, and the pH was adjusted before flotation. The flotation was conducted for 6 min. The products and tails were weighed separately after filtration and drying, and the recovery was calculated.
2.3 FTIR spectrum
Diffuse reflectance infrared spectroscopy (DIR) was used to characterize the surface species on the mineral particles treated. Samples were ground to be less than 5 μm and prepared as the same as these used for the micro- flotation tests. The spectrum was obtained with DIR Nicolet accessory (Nicolet spectrometer, AVATAR360, USA), and presented without any baseline correction.
2.4 Zeta potential measurement
Zeta potentials were measured using a Brookhaven ZetaPlus zeta-potential analyzer (USA). All measurements were conducted in a 0.1 mol/L KNO3 background electrolyte solution. Samples were ground to ≤5 μm. A 0.05 g sample was placed in a 100 mL breaker, for 5 min, with 80 mL distilled water, and the pH was adjusted and measured. The results presented were the average of three independent measurements with a typical variation of ±2 mV. Repeating tests showed that the conditioning procedure was capable of producible mineral surfaces suitable for studying the effect of various treatments.
2.5 DFT calculation
Calculations on geometric structure and atomic charges of collectors were made using the Gaussian 03 and Chemoffice 2005 program. The energies were corrected by means of the full counterpoise technique. The initial molecular modeling of the Gemini group was optimized by the MM2 and PM3 methods. The obtained geometries were further optimized and calculated with DFT methods at the B3LYP/6-31G (d) level.
3 Results and discussion
3.1 Micro-flotation tests
Micro-flotation tests were conducted to show flotation behaviors of kaolinite, pyrophyllite and illite as functions of pulp pH and collector dosage, and to determine the collecting power of Gemini surfactants BDDA and EDDA. Figure 2 plots the impact of pH on the floatability of the three aluminosilicate minerals when 2×10-4 mol/L BDDA and EDDA were used. Floatability of the three aluminosilicate minerals is pH-dependent at different degrees; the recovery—pH curves show drops in recovery with increasing pulp pH, especially in high alkaline condition (pH>12). This is consistent with the previous observation that the floatability of kaolinite, pyrophyllite and illite decreases in alkaline condition when cationic amine collectors are used [8,10,16,19,20]. It can be seen that the floatability of the three aluminosilicates with BDDA and EDDA is almost the same. Over a wide pH range, the recoveries of three aluminosilicates can reach up to or be above 80%, and BDDA shows a stronger collecting power than EDDA. At pH 8, the recoveries of kaolinite, pyrophyllite and illite are 96.0%, 92.6% and 85.4% for the use of Gemini surfactants BDDA, whereas they are about 78.3%, 77.5% and 80.2% for EDDA, respectively.

Fig. 2 Flotation recoveries of kaolinite (a), pyrophyllite (b) and illite (c) as function of pulp pH with 2×10-4 mol/L Gemini collectors BDDA and EDDA
The flotation responses of the aluminosilicates minerals as a function of the concentration of Gemini collectors BDDA and EDDA are presented in Fig. 3, and the pulp pH maintains at 8. As can be observed from Fig. 3, the recoveries of the three aluminosilicates increase with increasing dosage of collectors; however, the recovery—dosage curves show much less rises in recovery with increasing dosage when it is up to 2.5×10-4 mol/L. It also indicates that the Gemini collector BDDA is more efficient than EDDA, particularly true for the flotation of kaolinite. With 1.0×10-4 mol/L BDDA, about 76.6% of kaolinite can be floated, but that is only 50.6% when EDDA is used. When the concentrations of BDDA and EDDA are up to 3.5×10-4 mol/L, the Gemini collectors both display a superior collecting power for the three silicate minerals, and the maximum recoveries of kaolinite, pyrophyllite and illite are 99.8%, 98.5% and 92.5% with BDDA, while they are 99.7%, 97.3% and 90.2% with EDDA, respectively.
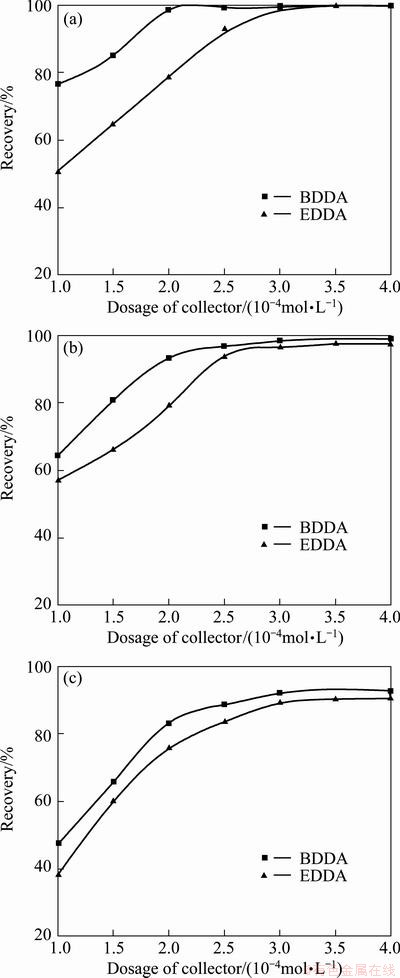
Fig. 3 Flotation recoveries of kaolinite (a), pyrophyllite (b) and illite (c) as function of collector dosage at pulp pH 8
3.2 FTIR spectral analysis
FTIR spectra of minerals and reagents were recorded to detect the adsorption type of Gemini collectors BDDA and EDDA on kaolinite, pyrophyllite and illite particles as shown in Fig. 4, and the spectra of pure kaolinite, pyrophyllite and illite observed agreed with those previously reported [21-23].
It can be seen that the spectra of aluminosilicates minerals with BDDA and EDDA are almost identical, suggesting that the two surfactants may have the same mechanism of interaction to mineral surfaces. The spectra of clay minerals treated by BDDA and EDDA both exhibit new peaks around at 2926 and 2850 cm-1, which previously attributes to the stretching bands of —CH2. It is the evidence that two surfactants BDDA and EDDA were adsorbed on the aluminosilicates minerals surfaces and the adsorption of BDDA and EDDA on three clays is dominated by physical electrostatic adsorption.
Upon adsorption of BDDA and EDDA, new bands around at 1465 cm-1 for three aluminosilicates minerals were examined. We assigned the new bands to the C—H···O twist vibration, because CHEN [24] assigned the band at 1470 cm-1 of trimethylene-1,3-bis (dodecyldimethyl ammonium bromide), abbreviated as TDDA, to the asymmetrical bending vibrations of —CH3. Due to the solution chemistry of kaolinite, it has been shown that at acidic pH, the apex oxygen atoms in the silica tetrahedral are hydrolyzed and form dangling OH groups and naked O anions on the surface to provide extra hydrogen bonding sites [15]. Therefore, the coupled inner-surface hydroxyl sites may leave naked O anions alone, which offers a chance for hydrogen bonding to BDDA and EDDA. As no other peak shift was observed, it can be concluded that the adsorption between Gemini collectors BDDA, EDDA and the three aluminosilicates is dominated by physical electrostatic adsorption and hydrogen bonding interactions.
3.3 Zeta potential analysis
Electrokinetic measurements are usually used to delineate interfacial phenomena where electrical double layer effects are of relevance to flotation. In this study, we illustrate the utility of zeta potential to investigate the mechanism of interaction between surfactant cations and mineral particles. Zeta potential of the aluminosilicates minerals in the absence and presence of Gemini collectors BDDA, EDDA with concentration of 2.0×10-4 mol/L were investigated, and the results are shown in Fig. 5.
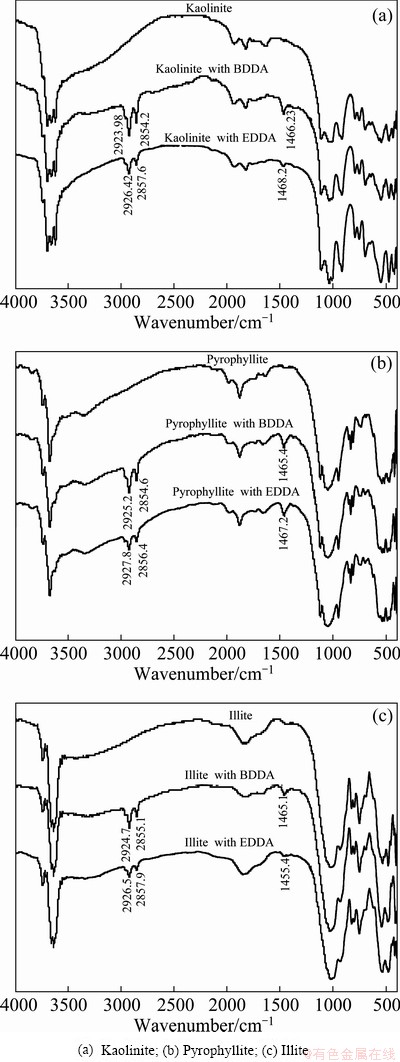
Fig. 4 FTIR spectra of aluminosilicates and aluminosilicates treated by Gemini collectors BDDA, EDDA solution

Fig. 5 Zeta potential of aluminosilicates and aluminosilicates with Gemini collectors BDDA, EDDA solution at 2.0×10-4 mol/L as function of pH
From the zeta potential results, the isoelectric points (IEPs) of kaolinite, pyrophyllite and illite are 3.4, 3.0 and 2.4, respectively, which are in accordance with those previously reported [17,19]. The ζ-potentials of kaolinite, pyrophyllite and illite show a pronounced shift towards more positive zeta potentials in the presence of BDDA or EDDA, indicating that Gemini collectors molecules positively charged were absorbed through electrostatic force onto aluminosilicates minerals and the electrostatic force was a definitely main mechanism in the flotation.
Based on the electrostatic adsorption explanation for cationic flotation of aluminosilicates, good flotation in the region where the mineral surfaces are negatively charged should be expected. However, in this work, as Fig. 2, the three minerals exhibit the best flotation response in acidic media (pH4+ by Al3+ on the surface of aluminosilicates) such as K+, Na+, Mg2+ and H+ can dissolve in aqueous solution, and the solubilities of those ions increase with increasing pH. As a result, the adsorption competition between Gemini collectors and above compensatory ions onto the aluminosilicates surface weakens the adsorption ability of BDDA2+ and EDDA2+ cations and then decreases the flotation response. In strong alkaline, the cation amount decreases sharply, so recoveries of the minerals are reduced.
3.4 Properties of Gemini collectors by DFT calculation
The first principle density functional theory (DFT) calculations were carried out on the BDDA2+ and EDDA2+ cationic group and the optimized geometries are shown in Fig. 6. Some selected atomic charges, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energies of these species are described in Table 2. The selected optimized bond distances of the BDDA2+and EDDA2+ cationic group are given in Table 3.
Table 2 indicates that the HOMO eigenvalues of the BDDA2+ and EDDA2+ cationic group are both very low, so their electron-donating power is also very weak. Their HOMO compositions are mainly constituted by px or pz-orbits of carbon atoms which have fully filled valence orbits and have no chance to offer p-orbit electrons to other atoms. The LUMO eigenvalues of the BDDA2+ and EDDA2+ are also low and the LUMO compositions are mainly composed of an s-orbit of N, C and H atoms, indicating that LUMO cannot accept feedback electrons to form π-bonds. Therefore, the Gemini cations, BDDA2+ and EDDA2+, both have difficulty forming a covalent bond with the aluminum atom on the minerals surface, and this is consistent with FTIR spectra studies that no other new peak shifts are observed except the —CH2 stretching bands of the collectors (Fig. 4). But the Mulliken charges of the cationic groups —CH2N+(CH3)2(CH2)4(CH3)2N+CH2—, and —CH2N+(CH3)2(CH2)2(CH3)2N+CH2— are 1.6864 and 1.6207, indicating that BDDA2+ and EDDA2+ cations are easily adsorbed on the negative charge sites of mineral surface through electrostatic attraction, also consistent with the above zeta potential results (Fig. 5).
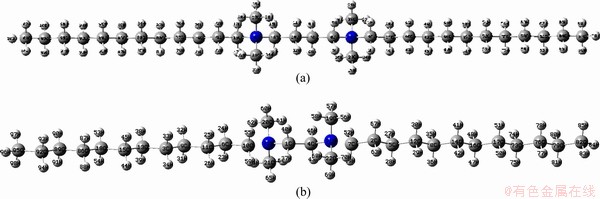
Fig. 6 Optimized geometries of BDDA2+ (a) and EDDA2+ (b) at B3LYP/6-31G (d) level
Table 2 Frontier orbital eigenvalues and some selected Mulliken charges of BDDA2+and EDDA2+ cationic groups at B3LYP/6-31G(d) level

Table 3 Selected optimized bond distances of BDDA2+and EDDA2+ cationic groups
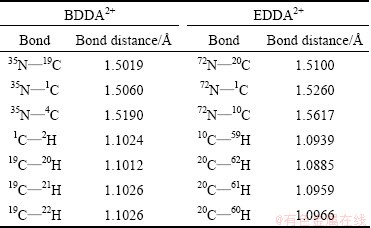
Table 3 shows that the N—C bond lengths in N—CH3 group of BDDA and EDDA collectors is the shortest, compared with N—C12H25 , N—(CH2)4 and N—(CH2)2, and it indicates that the N—C bond length in N—CH3 group is the strongest hence the —CH3 would have no chance to be replaced by other groups. The C—H bond distances in the alkyl chain in BDDA2+ and EDDA2+ cations are 1.1024  and 1.0939
and 1.0939  , while the C—H lengths in N—CH3 in BDDA2+ cation are 1.1012
, while the C—H lengths in N—CH3 in BDDA2+ cation are 1.1012  for 19C—20H and 1.1026
for 19C—20H and 1.1026  for 19C—21H and 19C—22H, and the C—H lengths in N—CH3 in EDDA2+ cation are 1.0885
for 19C—21H and 19C—22H, and the C—H lengths in N—CH3 in EDDA2+ cation are 1.0885  for 20C—62H and 1.0959
for 20C—62H and 1.0959  for 20C—61H and 1.0966
for 20C—61H and 1.0966  for 20C—60H. Compared with the C—H bond distance in the alkyl chain, one of the three bonds in N—CH3 is a slight weakening while the others show a slight strengthening. Furthermore, the two N atoms have a charge of -0.7479 in BDDA2+ cation and -0.3904 and -0.3902 in EDDA2+ cation. The H atoms in N—CH3 in BDDA2+ cation have a charge of 0.2264 for 20H, 0.2327 for 21H and 0.2554 for 22H, while the H atoms in N—CH3 in EDDA2+ cation have a charge of 0.2137 for 61H, 0.2262 for 60H and 0.2526 for 62H. Thus 22H is more active in BDDA2+ cation and 62H is more active in EDDA2+ cation. This means that one H atom in N—CH3 group in BDDA and EDDA cations is easily dissociated and the others may form the C—H···O hydrogen bond between H atoms and O atoms in Al—O and Si—O on the aluminosilicate minerals surfaces, agreeing with the FTIR spectra studies of new stretching frequency of around 1470 cm-1 for the clay minerals (Fig. 4).
for 20C—60H. Compared with the C—H bond distance in the alkyl chain, one of the three bonds in N—CH3 is a slight weakening while the others show a slight strengthening. Furthermore, the two N atoms have a charge of -0.7479 in BDDA2+ cation and -0.3904 and -0.3902 in EDDA2+ cation. The H atoms in N—CH3 in BDDA2+ cation have a charge of 0.2264 for 20H, 0.2327 for 21H and 0.2554 for 22H, while the H atoms in N—CH3 in EDDA2+ cation have a charge of 0.2137 for 61H, 0.2262 for 60H and 0.2526 for 62H. Thus 22H is more active in BDDA2+ cation and 62H is more active in EDDA2+ cation. This means that one H atom in N—CH3 group in BDDA and EDDA cations is easily dissociated and the others may form the C—H···O hydrogen bond between H atoms and O atoms in Al—O and Si—O on the aluminosilicate minerals surfaces, agreeing with the FTIR spectra studies of new stretching frequency of around 1470 cm-1 for the clay minerals (Fig. 4).
From the Mulliken charges of H atoms, —CH2N+(CH3)2(CH2)4(CH3)2N+CH2— and —CH2N+- (CH3)2(CH2)2(CH3)2N+CH2— cationic groups (Table 2), and the distances of C—H bonds in Gemini collectors (Table 3), the electrostatic attraction and the ability for forming C—H···O hydrogen bond are decreased in this order: BDDA2+>EDDA2+, also consistent with the micro-flotation tests results above (Figs. 2 and 3).
4 Conclusions
1) Gemini cationic collectors display stronger collecting power for kaolinite, pyrophyllite and illite, while BDDA shows a stronger collecting power than EDDA. At pH 8, the maximum recoveries of kaolinite, pyrophyllite and illite are reached when the dosage of Gemini collectors is up to 3.5×10-4 mol/L and they are 99.8%, 98.5% and 92.5% with BDDA, while 99.7%, 97.3% and 90.2 with EDDA, respectively.
2) Physical electrostatic attractions and hydrogen bond effects mainly account for the mechanisms of the Gemini cations adsorption on the mineral surfaces by FTIR spectra analysis, zeta potential measurement and DFT calculation. The H-bond is effective at pH
References
[1] HU Yue-hua, JIANG Hao, QIU Guan-zhou, WANG Dian-zuo. Solution chemistry of flotation separation of diasporic bauxite [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(1): 125-130. (in Chinese)
[2] LUO Zhao-jun, HU Yue-hua, WANG Yu-hua, QIU Guan-zhou. Mechanism of dispersion and aggregation in reverse flotation for bauxite [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(4): 680-683. (in Chinese)
[3] HU Yue-hua. Progress in flotation de-silica [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(3): 656-662.
[4] XU Zheng-he, PLITT V, LIU Qi. Recent advances in reverse flotation of diasporic ores—A Chinese experience [J]. Mineral Engineering, 2004, 17(9-10): 1007-1015.
[5] JIANG Hao, HU Yue-hua, QIN Wen-qing, WANG Yu-hua, WANG Dian-zuo. Mechanism of flotation for diaspore and aluminum-silicate minerals with alkyl-amine collectors [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(4): 688-692. (in Chinese)
[6] DU Ping, CAO Xue-feng, HU Yue-hua, JIANG Yu-ren, LI Hai-pu. Study of structure and property of amine collectors [J]. Light Metal, 2003(1): 27-31. (in Chinese)
[7] CAO Xue-feng, HU Yue-hua, JIANG Yu-ren, LI Hai-pu. Flotation mechanism of aluminium silicate minerals with N-dodecyl-1, 3-diaminopropane [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(4): 693-696. (in Chinese)
[8] HU Yue-hua, CAO Xue-feng, LI Hai-pu, JIANG Yu-ren, DU Ping. Synthesis of N-decyl-1,3-diaminopropanes and its flotation properties on aluminium silicate minerals [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(2): 417-420.
[9] LU Yi-ping, ZHANG Guo-fang, FENG Qi-ming, OU Le-ming. A novel collector RL for flotation of bauxite [J]. Journal of Central South University of Technology, 2002, 9(1): 21-24.
[10] ZHAO Shi-min, WANG Dian-zuo, HU Yue-hua, XU Jing. The flotation behaviour of N-(3-aminopropyl)-dodecanamide on three aluminosilicates [J]. Minerals Engineering, 2003, 16(12): 1391-1395.
[11] ZHAO Shi-min, WANG Dian-zuo, HU Yue-hua, XU Jing. Flotation of aluminosilicates using N-(2-aminoethyl)-1-naphthaleneacetamide [J]. Minerals Engineering, 2003, 16(10): 1031-1033.
[12] ZHAO Shi-min, WANG Dian-zuo, HU Yue-hua, XU Jing. Flotation alimiumsilicate minerals with methyl-nephthaleneamine [J]. Non- Metallic Mines, 2003, 26(5): 34-35, 62. (in Chinese)
[13] CAO Xue-feng, HU Yue-hua, XU Jing. Synthesis of γ-alkoxy-propylamines and their collecting properties on aluminosilicate mineral [J]. Journal of Central South University of Technology, 2004, 11(3): 280-286.
[14] CHEN Xiang-qing, HU Yue-hua, WANG Yu-hua. Mechanisms of flotation separation of diaspore and kaolinite by quaternary ammonium salt DTAL [J]. Transactions of Nonferrous Metals Society of China, 2004, 14(3): 609-612.
[15] LIU Guang-yi, ZHONG Hong, HU Yue-hua, ZHAO Sheng-gui, XIA Liu-yin. The role of cationic polyacrylamide in the reverse flotation of diasporic bauxite [J]. Minerals Engineering, 2007, 20(13): 1191-1199.
[16] ZHAO Sheng-gui, ZHONG Hong, LIU Guang-yi. Flotation effect of quaternary ammonium salts on aluminosilicate minerals [J]. Metal Mine, 2007(2): 45-47. (in Chinese)
[17] ZHONG Hong, LIU Guang-yi, XIA Liu-yin, LU Yi-ping, HU Yue-hua, ZHAO Sheng-gui, YU Xin-yang. Flotation separation of diaspore from kaolinite, pyrophyllite and illite using three cationic collectors [J]. Minerals Engineering, 2008, 21(12-14): 1055-1061.
[18] GUAN Feng, ZHONG Hong, LIU Guang-yi, ZHAO Sheng-gui, XIA Liu-yin. Flotation of aluminosilicate minerals using alkylguanidine collectors [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(1): 228-234.
[19] XIA Liu-yin, ZHONG Hong, LIU Guang-yi, HUANG Zhi-qiang, CHANG Qing-wei. Flotation separation of the aluminosilicates from diaspore by a Gemini cationic collector [J]. International Journal of Mineral Processing, 2009, 92(1): 74-83.
[20] XIA Liu-yin, ZHONG Hong, LIU Guang-yi, WANG Shuai. Utilization of soluble starch as a depressant for the reverse flotation of diaspore from kaolinite [J]. Minerals Engineering, 2009, 22(6): 560-565.
[21] XIA Liu-yin, ZHONG Hong, LIU Guang-yi, HUANG Zhi-qiang, CHANG Qing-wei, LI Xin-gang. Comparative studies on flotation of illite, pyrophyllite and kaolinite with Gemini and conventional cationic surfactants [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(2): 446-453.
[22] XIA Liu-yin. Flotation characteristics and adsorption mechanism of the aluminosilicate minerals by a class of bis-quaternary ammonium salt Gemini collector [D]. Tianjin: Tianjin University, 2009. (in Chinese)
[23] HU Yue-hua, LIU Xiao-wen, XU Zheng-he. Role of crystal structure in flotation separation of diaspore from kaolinite, pyrophyllite and illite [J]. Minerals Engineering, 2003, 16(3): 219-227.
[24] CHEN Li-fei, XIE Hua-qing, LI Yang, YU Wei. Applications of cationic Gemini surfactant in preparing multi-walled carbon nanotube contained nanofluids [J]. Colloids and Surfaces A, 2008, 330(2-3): 176-179.
黄志强,钟 宏,王 帅,夏柳荫,刘广义
中南大学 化学化工学院,有色金属资源化学教育部重点实验室,长沙 410083
摘 要:采用了新型Gemini双季铵盐捕收剂丁烷-1,4-双十二烷基二甲基溴化铵(BDDA)和乙烷-1,2-双十二烷基二甲基溴化铵(EDDA)对比研究了其对高岭石、叶腊石、伊利石的浮选行为及作用机理。单矿物试验结果表明,在广泛的pH范围内,新型Gemini双季铵盐捕收剂BDDA和EDDA对三种铝硅酸盐矿物具有优异的捕收性能,且BDDA的捕收能力强于EDDA。红外光谱和动电位研究表明,Gemini双季铵盐捕收剂对三种铝硅酸盐矿物的作用机理为静电吸附和氢键作用。采用DFT密度泛函理论,在B3LYP/6-31G(d)水平上对捕收剂阳离子BDDA2+和EDDA2+进行量化计算,结果表明BDDA的捕收能力强于EDDA。这与单矿物浮选结果、动电位测定结果一致。
关键词:铝硅酸盐矿物;Gemini阳离子表面活性剂;反浮选;吸附机理
(Edited by Hua YANG)
Foundation item: Project (2013AA064102) supported by the High-tech Research and Development Program of China; Project (51004114) supported by the National Natural Science Foundation of China; Project (2007B52) supported by the Foundation for the Author of National Excellent Doctoral Dissertation of China; Project (NCEP-08-0568) supported by the Program for New Century Excellent Talents in Chinese University
Corresponding author: Hong ZHONG; Tel/Fax: +86-731-88830654; E-mail: zhongh@mail.csu.edu.cn; Guang-yi LIU; Tel/Fax: +86-731-88830654; E-mail: guangyiliu@csu.edu.cn
DOI: 10.1016/S1003-6326(13)62833-2

