超轻Mg-Li合金的腐蚀行为与表面处理
来源期刊:中国有色金属学报(英文版)2017年第7期
论文作者:孙月花 王日初 彭超群 冯艳 杨明
文章页码:1455 - 1475
关键词:Mg-Li合金;比强度;成形性;电磁屏蔽;腐蚀行为;表面处理;杂化涂层
Key words:Mg-Li alloy; specific strength; formability; electromagnetic shielding; corrosion behavior; surface treatment; hybrid coating
摘 要:Mg-Li合金作为超轻金属结构材料,由于其具有较高的比强度、较好的成形性和良好的电磁屏蔽性能,在航空航天以及军事领域具有良好的发展前景。综述Mg-Li合金的研究进展并指出其面临的三大问题。针对Mg-Li合金较差的耐蚀性,重点总结合金的腐蚀行为,并详细地介绍包括电镀、化学镀、等离子喷涂、熔盐置换、化学转化膜、阳极氧化、微弧氧化、有机涂层以及有机-无机杂化涂层等表面处理技术。最后,探讨Mg-Li合金腐蚀与防护的发展方向。
Abstract: Mg-Li alloy, as a superlight metallic engineering material, shows great potential in the fields of aerospace and military due to its high specific strength, better formability, and excellent electromagnetic shielding performance. The research process of Mg-Li alloys is reviewed and three main problems are pointed out. Aimed at the poor corrosion resistance of Mg-Li alloys, the corrosion behavior is mainly summarized. The surface treatment technologies, including electroplating, electroless plating, plasma spraying, molten salt replacement, conversion coating, anodizing, micro-arc oxidation, organic coating, and organic-inorganic hybrid coating, are introduced in detail. Finally, the future development of corrosion and protection of Mg-Li alloys is discussed.
Trans. Nonferrous Met. Soc. China 27(2017) 1455-1475
Yue-hua SUN, Ri-chu WANG, Chao-qun PENG, Yan FENG, Ming YANG
School of Materials Science and Engineering, Central South University, Changsha 410083, China
Received 9 March 2017; accepted 25 May 2017
Abstract: Mg-Li alloy, as a superlight metallic engineering material, shows great potential in the fields of aerospace and military due to its high specific strength, better formability, and excellent electromagnetic shielding performance. The research process of Mg-Li alloys is reviewed and three main problems are pointed out. Aimed at the poor corrosion resistance of Mg-Li alloys, the corrosion behavior is mainly summarized. The surface treatment technologies, including electroplating, electroless plating, plasma spraying, molten salt replacement, conversion coating, anodizing, micro-arc oxidation, organic coating, and organic-inorganic hybrid coating, are introduced in detail. Finally, the future development of corrosion and protection of Mg-Li alloys is discussed.
Key words: Mg-Li alloy; specific strength; formability; electromagnetic shielding; corrosion behavior; surface treatment; hybrid coating
1 Introduction
Mg-Li alloys are the lightest metal structure materials, which have become the promising materials in the fields of aerospace, military, and 3C industry for the outstanding advantages, such as high specific strength and stiffness, good electromagnetic shielding performance, excellent damping, and weak mechanical anisotropy [1,2]. Table 1 lists the properties of some alloys and plastics. Compared with other materials, the density of Mg-Li alloys (1.25-1.65 g/cm3) is close to that of plastics, and only about 1/2 and 3/4 that of Al and Mg alloys, respectively [3]. Therefore, the application of Mg-Li alloys in the aerospace field can reduce the mass of spacecraft by 20%-30%, which greatly improves the flight capability and lowers the flight cost. The specific strength of Mg-Li alloys can be comparable to that of Al alloys, and the specific stiffness is the greatest among these materials listed in Table 1. Moreover, the addition of Li can decrease the critical resolved shear stress and the c/a ratio in Mg alloys by changing the crystal structure, which can activate more slip systems and improve formability [4]. When Li content is below 5.7%, Mg-Li alloy is composed of α-Mg phase with HCP structure, a solid solution of Li in Mg. The alloy with Li content higher than 10.3% is composed of β-Li phase with BCC structure, which is a solid solution of Mg in Li. When Li content is higher than 5.7% and lower than 10.3%, the alloy is composed of α-Mg and β-Li phases [4-6]. Recently, with the deepening researches on Mg-Li alloys, the application fields extend continuously and have been involved in biomedicine, electrochemical power, and other civilian fields [7-9].
Although Mg-Li alloys have the above superiorities over other alloys, there still exist three main problems, namely challenging preparation method, low strength, and inferior corrosion resistance, limiting the rapid development of Mg-Li alloys [10-12]. It is well known that Li is more active than Mg, the addition of Li into Mg matrix causes the corrosion resistance of Mg-Li alloys worse than conventional Mg alloys [13-15]. Moreover, improper process might lead to burning even blasting during the process of preparation, heat treatment, and deformation. Therefore, alloying with Li brings a certain difficulty for the preparation and processing of Mg-Li alloys. Besides conventional preparation method such as melting under the protection of flux (75% LiCl and 25% LiF) [6,16] and vacuum induction melting [17,18], a wide range of methods have been proposed recently to deal with these drawbacks of Mg-Li alloys [19-22]. For instance, molten salt electrolysis is utilized to fabricate Mg-Li alloys by using salts as raw materials [23-26]. This method saves the preparation process of single metal (such as Mg, Li, Al), obtains the elements which cannot be directly added into alloy in the form of elementary substrate, and achieves the homogenization of alloy components. But the strict control of electrolytic process parameters and the separation of alloy products and salts are still the technical difficulties.
Table 1 Properties of some alloys and plastics

Great efforts have also been expended to enhance the strength of Mg-Li alloys generally by alloying, heat treatment, and plastic deformation [27-31]. Recently, it has been reported that the addition of rare-earth (RE) elements into Mg-Li-Zn alloys prompts the formation of complicate Mg-Zn-RE ternary phases, such as icosahedral quasicrystal phase (I-phase) [32,33]. I-phase can effectively enhance the strength at room and elevated temperature by inhibiting the microstructural coarsening and suppressing the microstructural evolution of matrix [34-36]. Generally, the strength increases at the expense of elongation. But the long period stacking ordered (LPSO) structure introduced into Mg-Li alloys can improve the strength and elongation at the same time [37]. In addition, severe plastic deformation (such as large strain rolling, equal channel angular pressing, and accumulative rolling bonding) has been used to improve the strength of Mg-Li alloys [38-42]. KANG et al [38] obtained Mg-10.73Li-4.49Al-0.52Y alloy with a high tensile strength of 328 MPa by large strain rolling, which far surpassed that produced by conventional extrusion and rolling.
However, the inferior corrosion resistance of Mg-Li alloys is the major cause of limiting practical application, and it is not very obvious to improve the anti-corrosion property by increasing alloy purity and alloying with alloy elements. Therefore, the researches on corrosion and protection of Mg-Li alloys become highly valuable. In this work, the emphasis will be placed only on corrosion behavior and surface treatment technologies of Mg-Li alloys.
2 Corrosion behavior of Mg-Li alloys
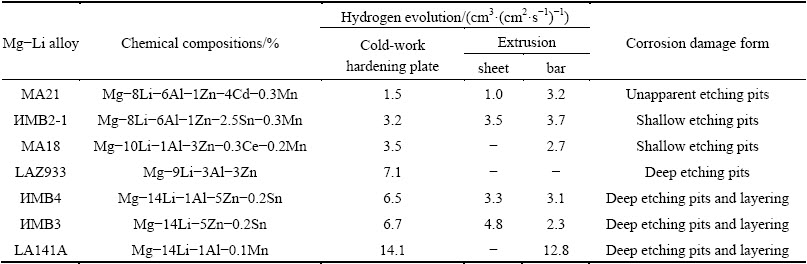
Mg and its alloys are prone to severe corrosion in humid air, sulfur atmosphere, and seawater due to their active chemical properties and the porous oxide film. Moreover, Li is more active than Mg, alloying with Li can further deteriorate the corrosion of Mg matrix. Therefore, the research on the corrosion and protection of Mg-Li alloys is of significance to the practical applications. In general, the corrosion behavior of Mg-Li alloys depends on several factors, including composition and microstructure of alloys, physical and chemical properties of oxide film, and ambient environment [43,44]. As one of alloying elements, Li content has a significant effect on the corrosion resistance of Mg-Li alloys. Table 2 gives the chemical components and corrosion susceptibility of typical Mg-Li alloys. The corrosion resistance of typical Mg-Li alloys is as follows: MA21 > ИMB2-1 > MA18 > LAZ933 > ИMB4 > ИMB3 > LA141A [45]. That is, the corrosion resistance of (α+β)-phase Mg-Li alloys is better than that of β-phase Mg-Li alloys.
2.1 Corrosion behavior of α-phase Mg-Li alloys
When Li content is below 5.7%, Mg-Li alloys are composed of α-Mg phase with HCP crystal structure. There are lots of researches on α-phase Mg-Li alloys [46-48], but the researches on corrosion behavior mainly focus on Mg-Li-Ca alloys. Mg-Li alloys alloying with Ca have a prospect in the field of biomedicine, such as orthopedic implants and vascular stents [7,49]. Compared with other biodegradable Mg alloys (e.g., Mg-Ca, Mg-Zn-Ca, and Mg-Al-Ca alloys), Mg-Li alloys exhibit an improved formability at room temperature by activating non-basal slip planes. The research of TIMMER and SANDS [50] indicated that Li was almost completely discharged by the kidney, since it was not bound to human blood plasma. Hence, the addition of Li into Mg cannot cause toxicity issues. Ca element is a main inorganic component of human bone and it also plays a key role in transmission of neural signal. The addition of Ca endows Mg alloys with similar characteristic to human bone, and improves the corrosion resistance of Mg-Li alloys due to the formation of CaCO3 surface film.
Table 2 Corrosion behavior of Mg-Li alloys in 3% NaCl solution [45]
NENE et al [51] investigated the corrosion behavior of Mg-4Li-1Ca (LC41) alloy with different states in Kokubo’s simulated body fluid (SBF) [52] at pH 7.6, and found the corrosion potential (φcorr) values to be -2.22, -2.00, and -2.18 V and the corrosion current density (Jcorr) values to be 1.02×10-3, 4.5×10-4, and 3.54×10-5 A/cm2 for homogenized (H), as-rolled (AR), and rolled + annealed conditions (RA), respectively. The RA condition showed superior biocorrosion resistance which attributed to its homogeneous microstructure. It was composed of fine equiaxed grains, a relatively low fraction of twins, and uniformly distributed eutectic texture along grain boundary. Also, they proposed the corrosion mechanism of LC41 alloy in SBF as follows.
Anodic reactions:
Li→Li++e (1)
Mg→Mg2++2e (2)
Cathodic reactions:
2H2O+2e→2OH-+H2 (3)
Total reactions:
2Li+2H2O→2LiOH+H2 (4)
Mg+2H2O→Mg(OH)2+H2 (5)
Further reactions:
2Li++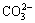 →Li2CO3 (6)
→Li2CO3 (6)
Mg2++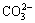 →MgCO3 (7)
→MgCO3 (7)
Ca2++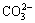 →CaCO3 (8)
→CaCO3 (8)
Besides, the reaction of hydroxyapatite (HA) formation occurs, which highly depends on the pH value of solution.
In Mg-Li-Ca alloys, the addition of Ca induces the formation of Mg2Ca owing to its low solid solubility in Mg matrix. There is a huge potential difference between Mg2Ca and Mg matrix, leading to the micro-galvanic corrosion. In general, the intermetallic compounds existing in Mg alloys are considered as cathodes [53]. However, the role of Mg2Ca in the local corrosion of Mg alloys is controversial. It has been reported that the potential of α-Mg (-2.37 V (vs SHE)) is relatively more negative than that of Mg2Ca (-1.54 V (vs SCE)) [54,55]. Thus, α-Mg assumes the role of anode and Mg2Ca assumes the role of cathode. However, several researches revealed that Mg2Ca was a more efficient anode than α-Mg owing to the more negative potential of Mg2Ca phase (OCP of Mg matrix is -1.65 V (vs SCE), OCP of Mg2Ca is -1.75 V (vs SCE) in 0.1 mol/L NaCl solution) [53,56]. Therefore, the role of Mg2Ca phase in local corrosion can be determined according to dissolution of Mg2Ca during corrosion process. Figure 1 shows the sketch map of galvanic corrosion between the Mg matrix (considered as anode) and the intermetallic Mg2Ca (considered as cathode) in SBF. At the initial stage, the corrosion pits appear in the Mg matrix around the Mg2Ca particles. Subsequently, the compact corrosion products form and are composed of Mg(OH)2 and MgCO3, which hinder the further corrosion of Mg alloys.
2.2 Corrosion behavior of (α+β)-phase Mg-Li alloys
Mg-Li alloys are composed of α-Mg phase and β-Li phase when Li content ranges from 5.7% to 10.3%. The (α+β)-phase Mg-Li alloys attract a lot of researchers’ attention owing to their good strength, elongation, and corrosion resistance. SONG et al [57] investigated the corrosion behavior of Mg-8Li alloy under ambient atmosphere, and found that the oxide film on alloy surface included four layers: the top layer contained Mg(OH)2 and Li2O; the second layer consisted of Mg(OH)2, Li2O and MgO; the third layer was composed of Mg(OH)2, MgO, LiOH, Li2O and Mg; and the bottom layer contained MgO, Li2O, Li and Mg. The outer oxide layer enriched with Li oxide was loose and only offered limited protection. Then, SONG et al [58] studied the corrosion behavior of Mg-8Li alloy in NaCl solution and found that Mg-8Li alloy was more liable to be oxidized than pure Mg due to the fast hydrogen evaluation reaction. The corrosion morphologies of Mg-8Li alloy after immersion in 0.1 mol/L NaCl solution are shown in Fig. 2. The typical local corrosion feature initiates from the boundary of α-Mg phase and β-Li phase, and then filiform corrosion occurs for longer immersion. Figure 3 indicates the sketch map of filiform corrosion of Mg-Li alloys. The back end of filament tip which is adjacent to filament tail is more inactive than the front of filament tip. It provides the hydrogen evolution reaction (cathode) with sites, thus the back end of filament tip is alkaline owing to the generation of hydroxyl ions. The dissolved metal ions (Mg2+ and Li+) can react with hydroxyl ions, leading to the formation of Mg(OH)2 and LiOH, respectively. The corrosion products result in the back end of filament tip being inert to turn into filament tail. While the anodic dissolving reaction occurs at the front of filament tip, which drives the filiform corrosion propagate frontward. This phenomenon is also found in AZ31 [59,60], AZ91 [61] and other Mg alloys [62,63] because of Mg anodic dissolving reaction. The addition of Li only promotes the cathodic hydrogen evolution reaction.

Fig. 1 Sketch map of corrosion pits occurring at α-Mg matrix adjacent to intermetallic compound Mg2Ca [44]
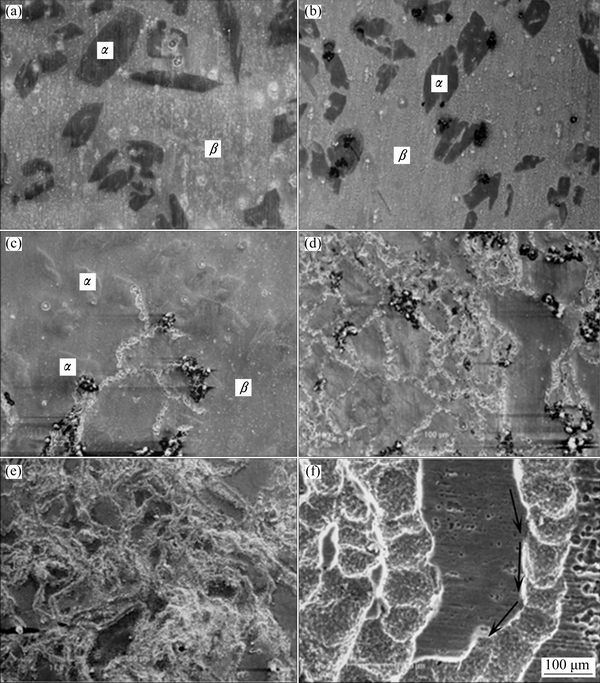
Fig. 2 Corrosion morphologies of Mg-8Li alloy after immersion in 0.1 mol/L NaCl solution for 2 h (a), 6 h (b), 9 h (c), 24 h (d), 48 h (e), and high magnification morphology (f) of Fig. 2(e) after removing corrosion products [58]
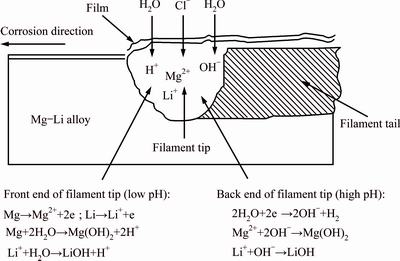
Fig. 3 Sketch map of filiform corrosion of Mg-Li alloys [58]
Alloying is a relatively effective and simple method to improve the mechanical property and corrosion resistance of Mg-Li alloys. The addition of Al into Mg-Li alloys can enhance the over-potential of hydrogen evolution, while prompting the formation of compact and stable surface film. RE elements are generally believed to have a beneficial effect on the corrosion resistance of Mg-Li alloys. They can react with oxygen to form discontinuous passivation film, reducing the stress corrosion and resulting in denser surface film. MANIVANNAN et al [64] indicated that the addition of 1.0% Ce could achieve the best refining effect and increase the corrosion resistance of Mg-8Li-3Al alloy by precipitating fine Al2Ce phase which distributed uniformly at the grain boundaries. GU et al [65] reported that Y addition resulted in the formation of Al2Y particles which could inhibit the galvanic corrosion between α-Mg phase and β-Li phase and reduce the corrosion rate in β-Li. When the Y content increased to 1.5%, the corrosion resistance of Mg-8Li-3Al-2Zn alloy was superior and the surface film became denser and more protective. Ca addition in Mg-Li alloys can also improve the corrosion resistance, attributing to the formation of denser calcium carbonate surface film. ZENG et al [66] studied the corrosion behavior of Mg-9.29Li-0.88Ca alloy and revealed that the comprehensive properties were prompted by extrusion. The form of corrosion converted from pitting corrosion for the cast alloy to uniform corrosion for the extruded alloy. The oxide film formed on the alloy consisted of four layers: the outer layer was the mixture of Li2O, LiOH and Li2CO3; the second layer contained LiOH, Li2O2, Li2CO3, MgCO3 and LiH; the third layer included Li2O2, Li2O, MgO and CaO; and the bottom layer consisted of the oxides at grain boundaries and in α-Mg, β-Li phases. Carbonate was not found at Mg-8Li alloys [57] ascribed to the distinction in exposure time, because the formation of carbonate might take a longer time.
2.3 Corrosion behavior of β-phase Mg-Li alloys
When Li content is higher than 10.3%, the crystal structure of Mg-Li alloys completely turns into β-Li phase. The corrosion behavior of β-phase Mg-Li alloys commands more attention because their excellent plasticity. The researches of GAO et al [67] and ZHANG et al [68] revealed that the increasing chloride ion concentration accelerated corrosion of Mg-Li alloys, while the increase in hydroxyl ions slowed down the corrosion rate. MORISHIGE et al [69] observed the exfoliation corrosion behavior in cold-rolled Mg-14Li-1Al alloy, but not in annealed alloy. This difference was due to the fact that severe cold rolling flatted the microstructure and gained thinner grains in cold-rolled specimens. However, in the annealed specimen, the grains tended to be equiaxial and the residual tensile stress was relaxed by annealing.
Recently, XU et al [70] reported that Mg-10.95Li- 3.29Al-0.19Zr-0.59Y exhibited a combined improvement in strength, ductility, and corrosion resistance, compared with other Mg alloys. The superior corrosion resistance was attributed to a uniform microstructure and a passive uniform Li2CO3 film. Figure 4 indicates the surface oxide film formed on HCP Mg and Mg-Li alloys after exposure to air. The oxide film on conventional Mg alloys mainly containing MgO and Mg(OH)2 is porous and cannot form complete coverage to protect Mg matrix from attack (Fig. 4(a)). In (α+β)-phase Mg-Li alloys, the magnesium oxide is attacked preferentially, while the Li2CO3 formed on β-Li can effectively withstand the oxidation in a certain degree (Fig. 4(b)). However, the β-phase Mg-Li alloys can react with air to develop a uniform and stable Li2CO3 film (PBR is 1.35) on the surface, so the matrix surface is completely covered with thick and compact oxide film (Fig. 4(c)). Therefore, XU et al [43,70] believed that the stable Li2CO3 film could be formed on the surface of β-phase Mg-Li alloys after exposure to air, leading to the superior corrosion resistance. But it is well known that the CO2 concentration in air is rare, so the formation of thick Li2CO3 film need long time or an atmosphere rich in CO2 gas.

Fig. 4 Surface layers developed on conventional Mg and Mg-Li alloys after exposure to ambient atmosphere
3 Surface treatment of Mg-Li alloys
The worse corrosion resistance of Mg-Li alloys limits their practical applications, and the galvanic corrosion and the loose surface oxide film are the main reasons. In general, the corrosion resistance of Mg-Li alloys can be improved by enhancing the alloy purity or reducing the content of harmful elements (such as Fe, Ni, Cu, and Co), alloying with some favorable elements, and plastic deformation. However, the most simple and effective method for protecting Mg-Li alloys from attack is surface modification. The coating becomes a barrier between the Mg-Li alloy matrix and the external environment to inhibit corrosion. Thus, the coating need meet several requirements including uniform thickness, good adhesion, and self-healing capability. In recent years, many surface treatment technologies for Mg-Li alloys have been developed, such as anodizing [71], micro-arc oxidation [72], electroplating [73], chemical conversion coating [74,75], and organic coating [76].
3.1 Electroplating
Electroplating is one of common methods for protecting Mg-Li alloy by electroplating other insert metals on its surface. In the previous electroplating process, the coatings containing Ni, Cr, and Cu are often elected for Mg alloys. However, several toxic ions, such as Cr6+ and cyanide, in plating bath are harmful to human health and environment. Therefore, some eco-friendly electroplating methods emerge to deposit Cr/Cu, Ni/Cu, and Cu layer on Mg alloys [77-79]. HUANG et al [80] electroplated a uniform Cu layer on Mg-9Li-1Zn (LZ91) alloy surface by anodic etching followed by electroplating in an alkaline Cu plating bath. Then a protective Cr/Cu coating was formed by electroplating the Cu-coated LA91 alloy in an acidic Cu- and Cr-plating baths. The hardness, wear resistance, and corrosion resistance of LA91 alloy with Cr/Cu coating were expressly improved. YIN et al [73] obtained a dense and uniform Cu coating with nodular structure on Mg-5Li-3Al alloy by electroplating. Figure 5 shows the surface morphology and electrochemical behavior of Cu-coated LA53 alloys. The Cu coating on LA53 alloy not only enhanced the strength, adhesion property, and corrosion resistance, but also could play a good decorative role.
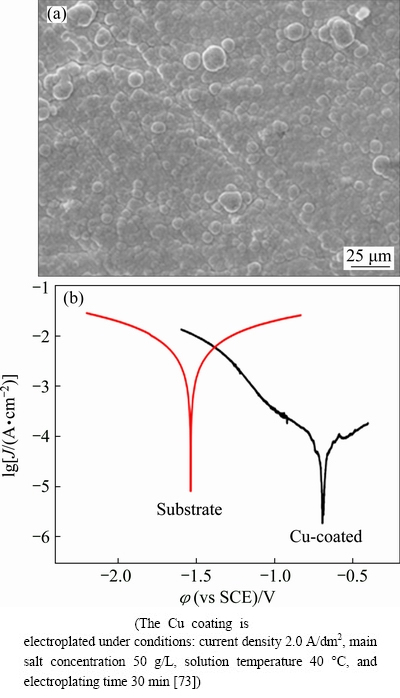
Fig. 5 Surface morphology of LA53 alloy with Cu coating (a) and polarization curves of LA53 substrate and LA53 alloy with Cu coating in 3.5% NaCl solution (b)
3.2 Electroless plating
Electroless plating is also named as auto-catalytic plating, in which the metal ions in plating solution are reduced to metal state and deposit on component surface, assisted with proper reducing agent in the absence of impressed current. During electroless Ni-plating, the reactions are as follows:
 +H2O→
+H2O→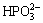 +2H+H+ (9)
+2H+H+ (9)
 →
→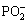 +2H (10)
+2H (10)
Ni2++2H→Ni+2H+ (11)
The reduction reaction is achieved by electron exchange between Ni2+ and hydrogen atom, and the reduced metal Ni immediately deposits on the Mg-Li alloy surface. In addition,  can also be reduced to phosphorus by hydrogen atom, or the oxidation- reduction reaction of
can also be reduced to phosphorus by hydrogen atom, or the oxidation- reduction reaction of  takes place under catalytic heating condition to deposit phosphorous.
takes place under catalytic heating condition to deposit phosphorous.
 +H→H2O+OH-+P (12)
+H→H2O+OH-+P (12)
 →
→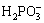 +H2O+2OH-+2P (13)
+H2O+2OH-+2P (13)
The hydrogen gas can be produced by hydrolysis of  and by incorporation of hydrogen atoms.
and by incorporation of hydrogen atoms.
 +H2O→
+H2O→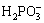 +H2 (14)
+H2 (14)
2H→H2 (15)
As a result, both Ni and P deposit and form Ni-P coating on Mg-Li alloy. All above mentioned chemical reactions happen simultaneously, but the reaction rate depends on plating solution, temperature, and pH value.
The deposition of Ni and P is related to the concentration of  and H+, and the relationships are as follows [45,81]:
and H+, and the relationships are as follows [45,81]:
 (16)
(16)
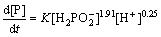 (17)
(17)
where d[Ni]/dt is the deposition rate of metal Ni, d[P]/dt is the deposition rate of P, 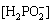 is the concentration of
is the concentration of  , [H+] is the concentration of hydrogen ion, K is a constant relating to the pH value, and β is a constant relating to the concentration of hydrogen ion. It can be indicated that the deposition of Ni is alkaline-catalyzed, while the deposition of P is acid-catalyzed. Therefore, reducing the concentration of hydrogen ion can enhance the deposition rate of Ni and degrade the deposition rate of P.
, [H+] is the concentration of hydrogen ion, K is a constant relating to the pH value, and β is a constant relating to the concentration of hydrogen ion. It can be indicated that the deposition of Ni is alkaline-catalyzed, while the deposition of P is acid-catalyzed. Therefore, reducing the concentration of hydrogen ion can enhance the deposition rate of Ni and degrade the deposition rate of P.
LUO et al [82] obtained Ni-P coating with a thickness of 20 μm on Mg-Li alloy by using two-step method. That was, a thinner film (4 μm) firstly formed on the pretreated Mg-Li alloy sheet by electroless plating with NiCO3·2Ni(OH)2·4H2O as main salt, and then the antiseptic coating (16 μm) was obtained by electroless plating with NiSO4·6H2O as main salt. This method can avoid the severe corrosion of Mg-Li alloy in an acidic plating bath and make full use of the high efficiency of acidic plating. ZOU et al [83] used Ce(NO3)3-KMnO4 solution for pretreatment, and considered that the Ni-P coating with ultrasonic assistance was more compact and well adhered to Mg-Li substrate, compared with that without ultrasonic assistance. The electrochemical and mechanical properties of Ni-P coating on Mg-Li alloys are listed in Table 3. It can be obviously observed that the Ni-P coating significantly improves the corrosion resistance and hardness of Mg-Li alloy. Compared with electroplating, electroless plating has more mature and simpler process, and the advantages of energy conservation and environmental protection. And most of all the prepared coatings exhibit more uniform structure with few cracks.
Table 3 Electroless Ni-P plating process and properties of Mg-Li alloys with and without coating

3.3 Plasma spraying
Plasma spraying is a new technology of material aggrandizement and surface character changing. In the process of plasma spraying, the materials (such as ceramic [85-87], metal [88,89], and alloy [90]) are heated to a molten state or half-molten state by using plasma arc as heat source, and then sprayed to the pretreated substrate surface in a high speed to form a firm coating. The hard coating like ceramic coating on Mg-Li alloy is brittle and easily develops hairline cracks during impact loading, although it has anti-scratch capability. However, the ductile metallic or alloy coating has resistance against cracking during deformation. Aluminum as the optimal choice for Mg-Li alloy attracts researchers’ attention owing to its good capability of plastic deformation and dense oxide film with anti-corrosion property. TSUJIKAWA et al [91,92] produced a protective pure aluminum surface layer on Mg-Li alloy via plasma thermal spraying, and considerable stain was eliminated by cold rolling of the sprayed plate slightly to avoid the separation of substrate and aluminum layer. Unfortunately, plasma thermal spraying can cause severe oxidation and considerable stain of Mg-Li alloys. However, plasma cold spraying can avoid oxidation of alloy surface and obtain dense nanostructure coating with strong surface adhesion, which is a new development of plasma spraying.
3.4 Molten salt replacement
Metallic coatings on Mg alloys have been investigated widely owing to their good ductility, superior electrical conductivity, and good welding property. Molten salt replacement is another method for producing metallic coatings besides electroplating, electroless plating, and plasma spraying. This method has several advantages including simple process, easy to control the thickness of coating, and suitable for complex-shaped samples. Above all, the sample need not contact with any solution throughout the process, which is conductive to protection of Mg-Li alloy. NIU et al [93] prepared aluminum coating on Mg-Li alloy via molten salt replacement and its specific process was as follows: the molten salt system (AlCl3-NaCl with a mole ratio of 1:1) was pretreated to remove water, and then the Mg-Li sample was buried in the AlCl3-NaCl salt to avoid the contact between the sample and the external environment, the crucible was placed in a furnace to heat to the designed temperature and the temperature was held for the designed time. The results revealed that the optimal process was 350 °C for 8 h and the aluminum in coating reacted with Mg and Li in the matrix to form compounds (e.g., AlLi and Mg17Al12) to provide an effective protection for Mg-Li alloy.
3.5 Chemical conversion coating
Chemical conversion coating is produced by electrochemical or chemical reaction to form a superficial layer of phosphates, stannates, molybdates, and other compounds which are bonded to the substrate surface. The conventional conversion coating is based on chromium compounds which are highly toxic to human body and harmful to environment. At present, the investigation on chemical conversion coating of Mg-Li alloy is mainly devoted to eco-friendly conversion coatings to replace toxic chromate conversion coating.
3.5.1 Phosphate conversion coating
Phosphate conversion coatings, according to conversion solution, can be classified as Ca, Mn, and Zn phosphate conversion coatings. In the case of Ca phosphate conversion coating, the conversion bath contains Ca(NO3)2 and NH4H2PO4, and the main components of conversion film include CaHPO4·2H2O, Ca3(PO4)2, and Mg3(PO4)2. SONG et al [94] prepared the Ca phosphate conversion coating on Mg-8.8Li alloy with deposition solution containing 25 g/L Ca(NO3)2 and 25 g/L NH4H2PO4 at pH 3 and 40 °C. The surface morphology of coating after deposition for 5 min, as shown in Fig. 6(a), was composed of a large number of leaf-like particles to exhibit lamellar structure, and its surface was more uniform and smooth. Figures 6(b-d) indicate the surface morphologies of conversion coating after being immersed in NaOH, NaCl, and Na2SO4 solutions for 24 h, respectively. The conversion film was inert in NaOH solution, while was susceptible to corrosion in NaCl and Na2SO4 solutions, and the acidic Na2SO4 solution showed more severe attack to film [74]. In the system of Mn phosphate conversion coating, KMNO4 and Na3PO4 or KH2PO4 are generally used as the main salt. ZHANG et al [95] obtained a uniform Mn phosphate conversion coating on Mg-10Li-1Zn alloy by treating in 40 g/L KMNO4 and 50 g/L KH2PO4 mixture solution at 55 °C for 20 min. Compared with traditional Cr conversion coating, this coating exhibited more effective corrosion resistance to resist the invasion form chloride ions. There are a large number of reports on Zn phosphate conversion coating bonded to conventional Mg alloys [96-98], but few about Mg-Li alloys. Zn(NO3)2 or ZnNO2 is common main salt in the Zn phosphate conversion coating. The calcium modified Zn phosphate conversion coating (Zn-Ca-P coating) was used to protect biomedical Mg-Li-Ca alloy [99]. The composition of conversion solution was composed of 4 g/L Zn(NO3)2, 4 g/L ZnNO2, 20 g/L Na2HPO4, 1 g/L Ca(NO3)2, and 1 g/L NaF. The alloy surface was covered with insoluble Ca3(PO4)2 and Zn3(PO4)2·4H2O at 50 °C, while the film prepared at 55 °C containing CaZn2(PO4)·4H2O was denser and more compact, and exhibited the best corrosion resistance than that prepared at the bath temperature of 40-50 °C.
Fig. 6 Surface morphologies of Ca phosphate conversion film before (a) and after being immersed in NaOH (b), NaCl (c), and Na2SO4 (d) solutions for 24 h [74,94]
Although phosphate conversion coating exhibits a rapid growth rate and improves the corrosion resistance of substrate, it can only be used for short-term corrosion protection of Mg-Li alloys due to complex pretreatment process, high cost of electrolyte, and short validity period. In addition, the conversion solution containing phosphate or manganate could pollute water and soil.
3.5.2 Stannate conversion coating
In stannate conversion coating, there are two types of conversion solution. One is conversion bath containing Na2SnO3 as main salt and auxiliary additives (e.g., NaOH, Na4P2O7, and CH3COONa·3H2O), and the main component of conversion film is MgSnO3·3H2O. The other is conversion bath composed of Na2SnO3 and KH2PO4, and the film components contain MgSnO3, SnO, and Mg3(PO4)2. YANG et al [100] investigated the stannate conversion coating on Mg-8Li alloy by simple immersion method. The composition of stannate bath was 0.2 mol/L Na2SnO3·3H2O, 0.1 mol/L Na4P2O7·10H2O, 0.125 mol/L NaOH, and 0.08 mol/L CH3COONa·3H2O. The film treated for 60 min was more dense and uniform, and the coating was mainly composed of hemispherical particles MgSnO3·3H2O.
3.5.3 Vanadate conversion coating
Vanadate conversion coating has been reported to improve the corrosion resistance of Mg alloys, such as AZ31 [101] and AZ91 [102]. The conversion coating exhibits an excellent corrosion resistance and a self-healing ability. Recently, the vanadate conversion coating has been applied to Mg-Li alloy [103], and the conversion solution contained NH4VO3 and K3(Fe(CN)6. The surface morphologies of Mg-14Li-1Al-0.1Ce alloy before and after being immersed under optimal condition are shown in Fig. 7. Uniform conversion coating with regularly distributed pores was formed on the Mg-Li alloy surface, and the compositions of conversion film were Mg(OH)2, V2O5, and Li2O. Moreover, the formed coating made the corrosion current density of Mg-Li-Al-Ce alloy decrease one order of magnitude and the corrosion potential positively move by 85 mV.
3.5.4 Molybdate conversion coating
Molybdate conversion coating can be used for protecting conventional Mg alloys [104-106], but also for Mg-Li alloys [107]. The main process of molybdate and molybdate/permanganate conversion coatings contains polishing, alkaline degreasing, acid pickling, and immersing in molybdate solution. Figure 8 shows the surface morphologies of molybdate coating and molybdate/permanganate coating prepared at 50 °C for 10 min. Here, the molybdate solution is 14 g/L (NH4)6Mo7O24·4H2O solution at pH 3, while the molybdate/permanganate solution is composed of 14 g/L (NH4)6Mo7O24·4H2O and 3.5 g/L KMnO4 at pH 3. It can be observed that the molybdate conversion coating nearly covers all over the alloy surface, and there are some cracks with a width of 1 μm caused by the release of hydrogen during conversion treatment. These cracks can result in the invasion from salt solution. While the molybdate/permanganate conversion coating has compact double layer with cracks, which indicates that the addition of KMnO4 is good for protecting Mg-Li alloys. In addition, the compositions of molybdate conversion coating include MgO, MoO2, and (MoO3)x(P2O5)y. While the compositions of molybdate/ permanganate conversion coating contain MgO, Mn2O3, MnO2, MoO2, MoO3, Mn3(PO4)2, and (MoO3)x(P2O5)y, and this film has better corrosion resistance than molybdate conversion coating.
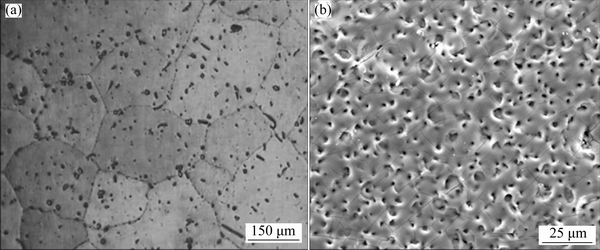
Fig. 7 Surface morphologies of Mg-14Li-1Al-0.1Ce alloy before (a) and after (b) immersion in 30 g/L NH4VO3 and 3.75 g/L K3(Fe(CN)6) solution for 10 min at 80 °C [103]
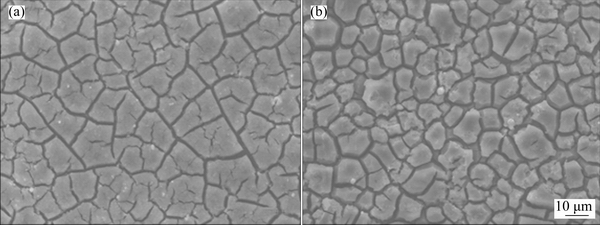
Fig. 8 Surface morphologies of molybdate coating (a) and molybdate/permanganate (b) conversion coating [107]
3.5.5 Rare-earth conversion coating
Rare-earth conversion coating applied to Mg-Li alloys mainly contains lanthanum-based and cerium-based conversion coating. In case of lanthanum-based conversion coating, the conversion bath mainly contains La(NO3)3, and the composition of coating is lanthanum compounds. Figure 9 indicates the surface morphologies of lanthanum conversion coating, cerium conversion coating, and lanthanum-cerium conversion coating on Mg-Li alloys. YANG et al [108] prepared lanthanum conversion coating with a uniform aciculate structure by treating in 5 g/L La(NO3)3 solution for 20 min at pH 5 and 25 °C. The coating was mainly composed of La(OH)3 and provided corrosion protection to Mg-8.8Li alloy. SONG et al [109] considered that the lanthanum conversion coating (Fig. 9(a)) formed in La(NO3)3 solution by microwave radiation method at 40 °C was more uniform and compact than that synthesized at room temperature. In cerium-based conversion coating, Ce(NO3)3 is selected as main salt, and the compositions of coating are mainly composed of Ce(OH)4, Ce2O3, Ce(OH)3, and CeO2. GAO et al [110] obtained a fiber-like coating (Fig. 9(b)) with a thickness of 12 μm by immersing in 0.05 mol/L Ce(NO3)3 solution at 35 °C for 20 min. In the research of YANG et al [111], the conversion bath contained Ce(NO3)3 and La(NO3)3 as main salt, as well as KMnO4 as catalyst. The lanthanum-cerium conversion coating exhibited a uniform and cracked morphology (Fig. 9(c)), and the coating was composed of La2O3, CeO2, Mn2O3, and MnO2.
3.5.6 Phytic acid conversion coating
Phytic acid (PA, C6H18O24P6) is a metal multi-tooth chelate and its molecular structure is shown in Fig. 10(a). Phytic acid consists of 6 phosphate carboxyl groups, 12 hydroxyl groups, and 24 oxygen atoms [112,113]. In addition, only one of phosphate carboxyl groups is in a position and the other is in e position, and there are 4 phosphate carboxyl groups among them in the same plane. Phytic acid is a polybasic mezzo forte acid, and it can be prone to ionization in aqueous solution. While the active metals (such as Mg and Li) are easy to lose electrons to form metal ions. The oxygen atoms in phosphate carboxyl groups as coordination atoms can chelate with the metal ions to form complex, and the hydrogen ions obtain electrons to form hydrogen gas. The insoluble metal complexes deposit and the phytic acid conversion coating forms on the surface of alloy. GAO et al [75] prepared a uniform phytic acid conversion film with white flower-like morphology (Fig. 10(b)) on Mg-Li alloy by immersing in 20 g/L phytic acid at pH 6 and 35 °C for 10 min. This coating increased the corrosion potential, reduced the hydrogen evolution rate and corrosion current density.

Fig. 9 Surface morphologies of lanthanum conversion coating (a), cerium conversion coating (b), and lanthanum-cerium conversion coating (c) on Mg-Li alloy [109-111]

Fig. 10 Molecular structure of phytic acid (a) and surface morphology of phytic acid conversion coating on Mg-Li alloy (b) [75]
On the whole, chemical conversion coating exhibits strong surface adhesion. But its corrosion resistance is far worse than that of coatings formed via electroplating and electroless plating. This is due to the coating with many cracks and pores which provide channels for external corrosion medium. Among these chemical conversion coatings, phytic acid conversion coating possesses higher corrosion resistance and good adhesion strength with organic coating, which can be used as a pretreatment of organic coating.
3.6 Anodizing
Anodizing is the most widely used surface treatment technology for Mg alloys. The anodic oxide film forms on the substrate surface in proper electrolyte solution by chemical reaction between cathode (stainless steel or Pt electrode) and anode (Mg or Mg alloys). Anodic oxide film has many advantages, such as its in-situ growth on the substrate, superior binding strength to the substrate, good electrical insulation, and excellent optical performance. Moreover, coloring and sealing process can be performed on anodic oxide film ascribed to its porous structure, and the anodic oxide film can also provide a fine basement for other coatings. There are many factors that influence the quality of anodic oxide film, including the composition and concentration of electrolyte, the temperature and pH value of solution, the type of voltage and current, and the anodizing time.
The electrolyte for anodizing of Mg-Li alloys can be classified into two categories: chromate system and silicate system. In chromate system, the electrolyte mainly contains K2Cr2O7 and (NH4)2SO4 or H2SO4, and the oxide film is black. SHARMA et al [71,114] obtained a black anodizing coating conducted in K2Cr2O7 and (NH4)2SO4 mixture solution by a galvanic anodizing method, and the processing steps are shown in Fig. 11. The oxide film is formed by chemical reaction between hexavalent chromium and Mg-Li alloys. The hexavalent chromium is reduced to the trivalent state by alloy metals which are oxidized. When the alloy is immersed in the electrolyte solution and connected to the cathode electrode, the dissolution of alloy occurs and the current flows from the alloy to the cathode which cause a pH rise at the solid-liquid interface. As a result, a thin complex chromium metal gel forms on alloy surface. This soft gel becomes hardened after heat treatment to provide a barrier between the substrate alloy and external environment. LI et al [115] achieved the galvanic black anodizing of Mg-Li alloy by conducting in 25 g/L K2Cr2O7 + 25 g/L H2SO4 solution and indicated that the oxide film obtained at room temperature with pH 4.5 and 5.5 had better corrosion resistance.
However, the chromate systems mentioned above (e. g., K2Cr2O7 + (NH4)2SO4 and K2Cr2O7 + H2SO4) cannot meet the requirements of green and environmental protection, due to the presence of toxic haxavalent chromium. Recently, many researchers dedicated to the study of eco-friendly silicate system, namely Na2SiO3 and NaOH or KOH as main salt, Na2B4O7, C6H5O7Na3·2H2O, or Na3PO4 as additive. CHANG et al [116] performed anodizing in an alkaline silicate solution and showed that the main compositions of anodic oxide film were MgO, Mg(OH)2 and LiOH. The anodic oxide films with amino acid as additive were more compact, while the corrosion resistance of oxide film with aminoacetic acid as additive was optimal. ONO et al [117] selected NaAlO2 as additive and indicated that the addition of aluminum ions in electrolyte made the anodic film denser and more uniform.
3.7 Micro-arc oxidation
Micro-arc oxidation (MAO) is also known as plasma electrolytic oxidation (PEO), which is a promising surface treatment of Mg-Li alloys. MAO springs from anodizing and has been applied on surface treatment of many valve metals, such as Al, Mg, Ti, Zr, and Ta [118-120]. It combines conventional anodizing with a high-voltage spark/arc discharge, and prompts the in-situ formation of ceramic oxide film relying on instantaneous high temperature and pressure. There are several factors that influence the quality of micro-arc oxide film, including operating voltage, current density, frequency, and reaction time. Generally, the thickness of film is thicker and more surface defects appear with an increasing terminal voltage, the number of surface holes increases as frequency increases, and the growth rate of film is faster and surface is rougher when current density increases.

Fig. 11 Process flow diagram of black anodizing of Mg-Li alloy [114]
At present, the electrolytes for MAO mainly include acidic/alkaline silicate, phosphate, aluminate, molybdate, tungstate, fluoride, and their hybrid system. The alkaline electrolyte system is more eco-friendly to meet the requirements of green development. XU et al [72] obtained the ceramic coatings composed of MgO and Mg2SiO4 on Mg-5Li alloy by MAO in the alkaline Na2SiO3 system. Compared with the substrate, the pitting and general corrosion resistance of coatings improved greatly and the optimal coating was formed with 9 g/L Na2SiO3. LI et al [121,122] fabricated the MAO coating on Mg-Li alloy in alkaline Na2SiO3-C6H18O24P6 solution. The compositions of coating were MgO, Li2O, and Mg2SiO4, and there existed many micropores on the surface of coating with a diameter of 3-20 μm. Subsequently, the PEO coatings on Mg-Li alloys were prepared in aluminate/silicate, molybdate/silicate, and silicate/tungstate composite electrolytes [123,124]. The coating formed in aluminate/silicate electrolyte was composed of MgO, Mg2SiO4, and MgAl2O4. Whereas the coating achieved in molybdate/silicate electrolyte, which was composed of MgO, MoO3, Mg2SiO4, and MgMoO4, exhibited a more uniform surface and a better corrosion resistance. The addition of tungstate improved the anti-corrosion property of PEO coating ascribed to the thermodynamically stable WO3 and Mg2SiO4 phase and the microstructure change of coating.
For MAO of Mg-Li alloys, the spark/arc discharge causes a high temperature atmosphere, leading to the strong oxidation and the dissolution of Li. Therefore, the formative MAO film on Mg-Li alloy surface will exhibit even poorer structure with many pores and cracks than that of conventional Mg alloys. The proper additives can improve the quality of coating, and there exist many researches on MAO coating of Mg-Li alloys formed in electrolyte with additives. SHI et al [125] formed MAO coating on Mg-5Li alloy in Na2SiO3-Na3PO4 electrolyte with additives (Na2B4O7 and EDTA). The addition of additives had no effect on crystal phase of coating which was composed of MgO and Mg2SiO4. The doping of Na2B4O7 made the coating much more compact and thicker, and was in favor of pitting corrosion resistance of coating. While the addition of EDTA made the coating much thinner and more even, and improved general corrosion resistance of coating. SONG et al [126] obtained Ti film after adding K2TiF6 into alkaline polyphosphate electrolyte, and it exhibited a unique hybrid structure which was composed of a dense inner layer, a sealed/semi-sealed porous mediate layer, and a dense outer layer. Meanwhile, Ti film showed a more compact structure and better corrosion resistance than P film formed without K2TiF6 (Figs. 12(a) and (b)). The sols as additive, such as silica sol [127] and titania sol [128,129], are also introduced in electrolyte to improve the quality of coating. The introduction of silica sol into the alkaline silicate electrolyte can change the compositions of coatings, namely crystalline SiO2 appears on coating instead of MgO phase. The addition of silica sol can obtain more uniform PEO coating with a superior anti-corrosion property and less structure imperfections. MA et al [128] fabricated white and blue coatings via PEO method in phosphate electrolyte with and without titania sol, respectively. The blue ceramic coating composed of MgO, TiO2, and Ti2O3 was smoother and more compact than that of white coating only containing MgO. The blue coating had a great prospect for protecting soft Mg-Li alloy due to its higher microhardness and lower surface roughness. Then, the titania sol was introduced into silicate electrolyte to form PEO coating for Mg-Li alloy [129] (Figs. 12(c) and (d)). The compositions of coating prepared without titania sol were MgO, Mg2SiO4, and ZrO2, and this coating presented better wear property. Whereas the coating prepared with titania sol was composed of MgO, ZrO2, and TiO2, and this coating exhibited better anti-friction performance.
Compared with conventional anodizing, the micro-arc oxide film with smaller pores and lower porosity is greatly improved in bonding strength, corrosion resistance, and wear resistance. However, micro-arc oxidation also has some drawbacks, such as high power consumption, porous film, easy oxidation oxidized at high voltage. Therefore, the quality and corrosion resistance of micro-arc oxide film need to be improved by adjusting electrolyte composition and electrical parameter, adding composite additive, and using proper sealing process.
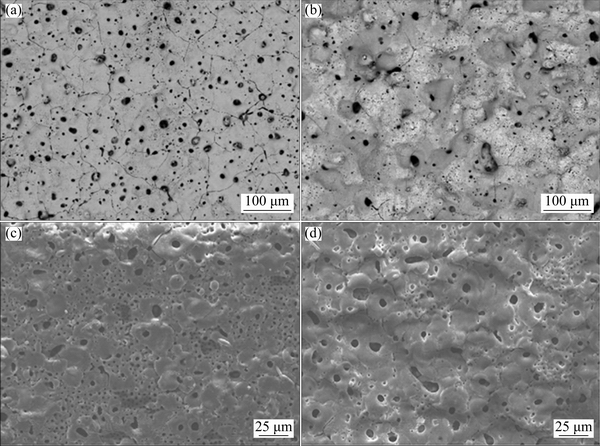
Fig. 12 Surface morphologies of MAO coatings on Mg-8.5Li-1Al alloy in alkaline polyphosphate electrolyte (5 g/L Na5P3O10, 1 g/L (NaPO3)6, 3 g/L NaOH, some organic additives and distilled water) without (a) and with (b) 10 g/L K2TiF6, and in alkaline silicate electrolyte (10 g/L Na2SiO3, 3 g/L NaOH, 0.6 g/L K2ZrF6, and 10 ml/L triethanolamine solutions) without (c) and with (d) 4% titania sol [126,129]
3.8 Organic coating and organic-inorganic hybrid coating
Organic coating is widely used in various industries to protect substrate from attack of the external environment. Epoxy coating is the most common organic coating owing to its excellent bonding strength on the substrate surface, good physical and chemical properties, and small deformation shrinkage. It has been reported that the epoxy coating containing polyaniline (PANI) offers higher corrosion resistance than conventional epoxy coating on Mg-Li alloys, and the coating with 2% PANI takes the best effect [76,130]. The addition of PANI can change the chemical structure of corrosion components and decrease the corrosion growth probability of Mg-Li alloys. Organic coating exhibits poor mechanical properties and tends to fall off. Hence, it can only be used for short-term protective treatment, or coated on other conversion coating to form composite coating.
Organic-inorganic hybrid coating is a homogeneous multiphase coating formed by combining organic and inorganic in nanometer scale. The inorganic can enhance strength, bonding strength, wear resistance, aging resistance, and complexity and stability of structure. The organic can increase structural diversity and change coating properties by changing ligand type. Compared with conventional organic coating, organic-inorganic hybrid coating has better anti-corrosion property and anti-UV radiation performance attributed to the nanometer effect and compound effect between organic phase and inorganic phase. In organic-inorganic hybrid coating, the organic components can be epoxy, polyurethane, polyaniline, and acrylic resin, while the inorganic components can be oxide (such as SiO2, ZnO, TiO2, MnO2) and metal (such as Ti) nanoparticles. CHEN et al [131] considered that the epoxy coating containing PANI-SiO2 composites offered higher corrosion protection in comparison to bare epoxy coating on Mg-Li alloy. ZHANG et al [132] protected Mg-Li alloy via combining a chemical conversion coating (PA based or Ce based) and an epoxy/SiO2 hybrid coating. The optimal SiO2 concentration for PA based and Ce based hybrid coating was 3%, and the PA-based epoxy/SiO2 hybrid coating had better corrosion resistance than Ce-based epoxy/SiO2 hybrid coating.
In recent years, researchers have made great effort to obtain non-toxic anti-corrosive organic coating with good ion-exchangeable performance. Furthermore, the anti-corrosive coating with self-healing property is a vital pursuit of surface modification. Modified pigment in organic coatings exhibits an interesting protective property for self-healing performance [133,134]. WANG et al [135] fabricated epoxy coating containing Ce-MCM-22 zeolites on Mg-Li alloy, and its corrosion resistance was better than the blank epoxy coating and the epoxy coating containing MCM-22 zeolites. During the coating forming process, MCM-22 zeolites could be as reservoirs of active Ce3+ to avoid the spontaneous leakage of Ce3+. Active Ce3+ ions were released from MCM-22 zeolites and precipitated to the scratched areas of Mg-Li alloy by using ion-exchange property of zeolites. Thus, the epoxy coating containing Ce-MCM-22 zeolites exhibited a self-healing property and provided effective long-term protection for Mg-Li alloy. YU et al [136] designed a nano-sized molybdate pillared hydrotalcite (HT-MoO42-)/in-situ created ZnO composite (HTMZ), combining the excellent inhibited performance of molybdate and polarization property of ZnO nanoparticles. Compared with the HT-MoO42- primer coating, the addition of ZnO in HTMZ composite could increase the density of coating and reduce the transport paths of oxygen and electrolyte. The epoxy coating containing HTMZ composite displayed a higher anti-corrosion property than those coatings only containing ZnO or HT-MoO42-. The anti-corrosion mechanism was proposed as follows: MoO42- ions released from HTMZ in NaCl solution and chloride ions were adsorbed, and then ZnO nanoparticles attracted negative-charged molydbate anions to prompt the formation of barrier on the substrate surface. In this study, the release of molydbate anions from composite was controlled by ion-exchange rather than chemical solubility.
3.9 Other coatings
3.9.1 DLC coating
Diamond-like carbon (DLC) is a class of amorphous carbon materials that display some typical properties of diamond. DLC coating is usually deposited on other materials due to its low friction, high hardness, chemical inertness, and excellent wear resistance [137]. It has been reported that there are two pretreatment methods to improve the adhesion of DLC coating on the substrate surface. One is depositing a thin metallic film (such as Si and Cr) as an interlayer between DLC film and substrate by using the ion beam sputter method [138]. The other is mechanical pretreatment by a peening process using nonmetal medium (such as SiC and graphite), prior to coating with the DLC coating [139].
YAMAUCHI et al [140] investigated the effectiveness of DLC coating with two different pretreatment methods (Si interlayer and SiC peening) in enhancing the corrosion and wear resistance of Mg-14Li-1Al-0.1Mn alloy. The thickness of Si interlayer deposited via the ion sputter method was 0.3 μm, whereas the SiC medium layer was about 60 μm. The results indicated that the SiC peening process enabled a better adhesion deposition of DLC coating, and this coating exhibited a relatively low friction coefficient and superior wear resistance. Unfortunately, both DLC coatings had no ability to withstand the corrosion effect of the acidic/alkaline artificial perspirations.
3.9.2 ZSM-5 zeolite coating
Zeolites have been considered as excellent “building blocks” for constructing hierarchical porous materials and as component for functional coatings [141]. Zeolite coating has a good prospect to be an eco-friendly coating due to its non-toxicity. ZSM-5 zeolite is an aluminosilicate zeolite with high silicon to aluminum ratio, which is well known for its chemical stability, mechanical stability, thermal stability, solvent resistance, and diverse fine-tunable zeolite characteristic [142,143]. ZSM-5 zeolite cannot lose its crystallinity up to 1100 °C and it can stably exist in an acidic/alkaline environment. Therefore, ZSM-5 zeolite coating can offer a corrosion protection for many metals and alloys.
SONG et al [144,145] prepared ZSM-5 zeolites by in-situ hydrothermal crystallization method with tetrapropylammonium bromide (TPABr), tetrapropyl- ammonium hydroxide (TPAOH), and n-butylamine (NBA) as macromolecular templates, respectively. Figure 13 depicts the surface morphologies of ZSM zeolite coating by hot-pressing with various templates used to block the pores. The coatings with a thickness of 110 μm prepared using TPABr and TPAOH as templates had an ellipsoid structure and sheet covering on the substrate surface. While the compact and polycrystalline coating formed with NBA as template showed a structure of regular hexagon and had a complete and even coverage over the surface of Mg-Li alloy. Above all, the ZSM-5 zeolite coating showed good corrosion resistance and could be applied to protect other active metals.
3.9.3 Superhydrophobic coating
Many natural materials, such as lotus leaf, exhibit hydrophobic and self-cleaning properties. The water falling onto the surface of lotus leaf automatically aggregates into water droplets and takes away the dirt particles by rolling droplets, so that the lotus leaf is always self-cleaning, which is the so-called lotus effect. The self-cleaning property of lotus leaf is caused by the papilla of micro/nanoscale hierarchical structures and hydrophobic wax material on surface. LIU et al [146] designed a hydrophobic coating on Mg-Li alloy via two-step method on the basis of lotus effect. The clean surface of Mg-Li alloy was firstly etched in 0.1 mol/L HCl solution, then immersed in 1.0% ethanol solution of fluoroalkylsilane (CF3(CF2)7CH2CH2Si(OCH3)3, FAS) for 12 h, and subsequently heated at 100 °C for 2 h. Figure 14 shows that the microscale papillaes randomly distributed on the surface which is similar to lotus leaf. The single papilla presented a beautiful peony-like morphology with a diameter of 2-3 μm, which was composed of many disorderly packed nanoscale slices (like the peony petals, Fig. 14(b)). The space between these slices with a thickness of 30-60 nm was 50-400 nm. While the no-protuberant section was rough and irregular (Fig. 14(c)). The thickness of nanoslices was 30-50 nm and the space between them was 40-300 nm. The formed coating on Mg-Li alloy presented a micro/nanoscale hierarchical structure which displayed long-term superior corrosion resistance and superhydrophobic property.
3.9.4 Composite coating
In general, a single protective method has many defects and cannot provide adequate protection for the substrate. Hence, the composite coating combining two or more protection methods gradually attracts the researchers' attention. CHEN et al [147] produced a protective Ni/Cu/Ni-P triple-layered coating on Mg-Li alloy by combining electroless plating (Ni-P coating) and electroplating (Ni and Cu coating). The outmost layer was Ni layer with a thickness of 10 μm, the middle layer was Cu layer with a thickness of 20 μm, and the innermost layer was Ni-P layer with a thickness of 5 μm. This composite coating exhibited a quite low current density nearly 25 times and 4 times less than the Ni-P and Cu/Ni-P coatings, respectively. LI et al [148] fabricated a novel and eco-friendly PEO/sol-gel composite coating on Mg-Li alloy by using plasma electrolytic oxidation followed by sol-gel technique. The sol-gel treatment could fill the numerous micropores and microcracks distributing on the surface of PEO coating. SUN et al [149] combined plasma electrolytic oxidation and chemical conversion process to obtain duplex plasma electrolytic oxidation/molybdate conversion (PEO/MoC) coating on substrate surface. There were numerous uniform-sized spherical microparticles distributing uniformly on PEO/MoC coating with a decrease in diameter and number of micropores, in comparison with single PEO coating. After molybdate conversion treatment, the NaMgF3 and MoO3 phases presented in composite coating. The electrochemical results revealed that the anti-corrosion behavior increased in order of alloy substrate, PEO coating, and PEO/MoC coating.

Fig. 13 Surface morphologies of ZSM-5 zeolite coatings prepared with TPABr (a), TPAOH (b), and NBA (c) as template on Mg-Li alloy [144,145]

Fig. 14 Surface morphology of superhydrophobic coating on Mg-Li alloy (a), HRSEM image of single microsized protrudion at point A (b) and HRSEM image of unprotrudent section at point B (c) [146]
4 Prospects
Mg-Li alloy is one of the lightest structural materials, which shows great potential in aerospace, military, and 3C industry. But the inferior corrosion resistance constrains its practical applications. In recent years, although the researches on Mg-Li alloy make some progresses in corrosion and protection, there exist some questions needing further exploration.
1) The preparation of corrosion-resistant Mg-Li alloys by improving melting technology, alloying, and plastic deformation. I-phase introduced by alloying with a larger Zn/RE ratio can improve the corrosion resistance and strength of Mg-Li alloys [150]. In addition, the uniform microstructure and a passive Li2CO3 film provide effective protection for β-phase Mg-Li alloys [70].
2) The exploration of self-healing, eco-friendly, and effective surface treatment technologies for Mg-Li alloys. The new environment-friendly conversion solution, electroless plating solution, and micro-arc oxidation composite electrolyte are emphases to investigate. In addition, a single surface treatment method only provides limited protection for Mg-Li alloys, so that the effective composite coating is becoming a research point.
3) The deep and systematical study on film-forming mechanism and anticorrosion mechanism of surface coating on Mg-Li alloys. For instance, the electrochemistry, thermodynamics, and energy transfer mechanism in formation process of film, and anticorrosion mechanisms of chemical conversion coating, micro-arc oxide film, and organic-inorganic hybrid coating.
References
[1] ZHU T, CUI C, ZHANG T, WU R, BETSOFEN S, LENG Z, ZHANG J, ZHANG M. Influence of the combined addition of Y and Nd on the microstructure and mechanical properties of Mg-Li alloy [J]. Materials and Design, 2014, 57(1): 245-249.
[2] KARAMI M, MAHMUDI R. Orientation-dependent microstructure and shear flow behavior of extruded Mg-Li-Zn alloys [J]. Materials Science and Engineering A, 2015, 636: 493-501.
[3] XU D K, WANG B J, LI C Q, ZU T T, HAN E H. Effect of icosahedral phase on the thermal stability and ageing response of a duplex structured Mg-Li alloy [J]. Materials and Design, 2015, 69: 124-129.
[4] XU T C, PENG X D, QIN J, CHEN Y F, YANG Y, WEI G B. Dynamic recrystallization behavior of Mg-Li-Al-Nd duplex alloy during hot compression [J]. Journal of Alloys and Compounds, 2015, 639: 79-88.
[5] KIM Y H, KIM J H, YU H S, CHOI J W, SON H T. Microstructure and mechanical properties of Mg-xLi-3Al-1Sn-0.4Mn alloys (x=5, 8 and 11 wt%) [J]. Journal of Alloys and Compounds, 2014, 583: 15-20.
[6] DONG H, WANG L, LIU K, WANG L, JIANG B, PAN F. Microstructure and deformation behaviors of two Mg-Li dual-phase alloys with an increasing tensile speed [J]. Materials and Design, 2016, 90: 157-164.
[7] ZHOU W R, ZHENG Y F, LEEFLANG M A, ZHOU J. Mechanical property, biocorrosion and in vitro biocompatibility evaluations of Mg-Li-(Al)-(RE) alloys for future cardiovascular stent application [J]. Acta Biomaterialia, 2013, 9(10): 8488.
[8] WANG N, WANG R, FENG Y, XIONG W, ZHANG J, DENG M. Discharge and corrosion behaviour of Mg-Li-Al-Ce-Y-Zn alloy as the anode for Mg-air battery [J]. Corrosion Science, 2016, 112: 13-24.
[9] MA Y, LI N, LI D, ZHANG M, HUANG X. Performance of Mg-14Li-1Al-0.1Ce as anode for Mg-air battery [J]. Journal of Power Sources, 2011, 196(4): 2346-2350.
[10] WU R, YAN Y, WANG G, WURR L E, HAN W, ZHANG Z, ZHANG M. Recent progress in magnesium-lithium alloys [J]. International Materials Reviews, 2015, 60(2): 65-100.
[11] CUI C, WU L, WU R, ZHANG J, ZHANG M. Influence of yttrium on microstructure and mechanical properties of as-cast Mg-5Li-3Al-2Zn alloy [J]. Journal of Alloys and Compounds, 2011, 509(37): 9045-9049.
[12] WU R Z, QU Z K, ZHANG M L. Reviews on the influences of alloying elements on the microstructure and mechanical properties of Mg-Li base alloys [J]. Reviews on Advanced Materials Science, 2010, 24(1): 35-43.
[13] XU D K, HAN E H. Effect of quasicrystalline phase on improving the corrosion resistance of a duplex structured Mg-Li alloy [J]. Scripta Materialia, 2014, 71(3): 21-24.
[14] SONG Y, SHAN D, CHEN R, HAN E. Effect of second phases on the corrosion behaviour of wrought Mg-Zn-Y-Zr alloy [J]. Corrosion Science, 2010, 52(5): 1830-1837.
[15] ZHANG C, HUANG X, ZHANG M, GAO L, WU R. Electrochemical characterization of the corrosion of a Mg-Li alloy [J]. Materials Letters, 2008, 62(14): 2177-2180.
[16] CAO F, XIA F, HOU H, DING H, LI Z. Effects of high-density pulse current on mechanical properties and microstructure in a rolled Mg-9.3Li-1.79Al-1.61Zn alloy [J]. Materials Science and Engineering A, 2015, 637: 89-97.
[17] CAO D, WU L, SUN Y, WANG G, LV Y. Electrochemical behavior of Mg-Li, Mg-Li-Al and Mg-Li-Al-Ce in sodium chloride solution [J]. Journal of Power Sources, 2008, 177(2): 624-630.
[18] LI J, QU Z, WU R, ZHANG M. Effects of Cu addition on the microstructure and hardness of Mg-5Li-3Al-2Zn alloy [J]. Materials Science and Engineering A, 2010, 527(10-11): 2780-2783.
[19] REGENER D, TKACHENKO V. Strength characteristics of Mg-Li alloys [J]. Strength of Materials, 2009, 41(3): 294-302.
[20] MATSUDA A, WAN C C, YANG J M, KAO W H. Rapid solidification processing of a Mg-Li-Si-Ag alloy [J]. Metallurgical and Materials Transactions A, 1996, 27(5): 1363-1370.
[21] YANG J M. Rapid solidification processing of a magnesium alloy [J]. Metal Powder Report, 1997, 51(1): 35-35.
[22] HAN W, TIAN Y, ZHANG M, YE K, ZHAO Q, WEI S. Preparing different phases of Mg-Li-Sm alloys by molten salt electrolysis in LiCl-KCl-MgCl2-SmCl3 melts [J]. Journal of Rare Earths, 2010, 28(2): 227-231.
[23] ZHANG M L, YAN Y D, HOU Z Y, FAN L A, CHEN Z, TANG D X. An electrochemical method for the preparation of Mg-Li alloys at low temperature molten salt system [J]. Journal of Alloys and Compounds, 2007, 440(1-2): 362-366.
[24] LIN M C, UAN J Y. Preparation of bcc Mg-Li-Al-Zn alloy by electrolysis in molten salt LiCl-KCl and the alloy’s electrochemical performance as anode material for magnesium batteries [J]. Electrochemistry, 2009, 77(8): 604-607.
[25] YE K, ZHANG M L, CHEN Y, HAN W, YAN Y D, WEI S, CHEN L J. Study on the preparation of Mg-Li-Mn alloys by electrochemical codeposition from LiCl-KCl-MgCl2-MnCl2 molten salt [J]. Journal of Applied Electrochemistry, 2010, 40(7): 1387-1393.
[26] HAN W, CHEN Q, SUN Y, JIANG T, ZHANG M. Electrodeposition of Mg-Li-Al-La alloys on inert cathode in molten LiCl-KCl eutectic salt [J]. Metallurgical and Materials Transactions B, 2011, 42(6): 1367-1375.
[27] PENG Q Z, ZHOU H T, ZHONG F H, DING H B, ZHOU X, LIU R R, XIE T, PENG Y. Effects of homogenization treatment on the microstructure and mechanical properties of Mg-8Li-3Al-Y alloy [J]. Materials and Design, 2015, 66(10): 566-574.
[28] DONG H, XU S, WANG L, KAMADO S, WANG L. Microstructures and mechanical properties of as-cast and hot-rolled Mg-8.43Li-0.353Ymm (Y-riched mischmetch) alloy [J]. Metallurgical and Materials Transactions A, 2012, 43(2): 709-715.
[29] DONG H, PAN F, JIANG B, LI R, HUANG X. Mechanical properties and deformation behaviors of hexagonal Mg-Li alloys [J]. Materials and Design, 2015, 65(65): 42-49.
[30] WEI G, MAHMOODKHANI Y, PENG X, HADADZADEH A, XU T, LIU J, XIE W, WELLS M. Microstructure evolution and simulation study of a duplex Mg-Li alloy during double change channel angular pressing [J]. Materials and Design, 2015. 90: 266-275.
[31] LUO G X, WU G Q, WANG S J, LI R H, HUANG Z. Effects of YAl2 particulates on microstructure and mechanical properties of β-Mg-Li alloy [J]. Journal of Materials Science, 2006, 41(17): 5556-5558.
[32] ZHANG Y, ZHANG J, WU G, LIU W, ZHANG L, DING W. Microstructure and tensile properties of as-extruded Mg-Li-Zn-Gd alloys reinforced with icosahedral quasicrystal phase [J]. Materials and Design, 2015, 66: 162-168.
[33] XU D K, LIU L, XU Y, HAN E. The strengthening effect of icosahedral phase on as-extruded Mg-Li alloys[J]. Scripta Materialia, 2007, 57(3): 285-288.
[34] BAE D H, KIM S H, KIM D H, KIM W T. Deformation behavior of Mg-Zn-Y alloys reinforced by icosahedral quasicrystalline particles [J]. Acta Materialia, 2002, 50(9): 2343-2356.
[35] BAE D H, KIM Y, KIM I J. Thermally stable quasicrystalline phase in a superplastic Mg-Zn-Y-Zr alloy [J]. Materials Letters, 2006, 60(17-18): 2190-2193.
[36] XU D K, HAN E H, LIU L, XU Y B. Influence of higher Zn/Y ratio on the microstructure and mechanical properties of Mg-Zn-Y-Zr alloys [J]. Metallurgical and Materials Transactions A, 2009, 40(7): 1727-1740.
[37] ZHANG J, ZHANG L, LENG Z, LIU S, WU R, ZHANG M. Experimental study on strengthening of Mg-Li alloy by introducing long-period stacking ordered structure[J]. Scripta Materialia, 2013, 68(9): 675-678.
[38] KANG Z X, LIN K, ZHANG J Y. Characterisation of Mg-Li alloy processed by solution treatment and large strain rolling [J]. Materials Science and Technology, 2016, 32(5): 498-506.
[39] WANG T, ZHENG H, WU R, YANG J, MA X, ZHANG M. Preparation of fine-grained and high-strength Mg-8Li-3Al-1Zn alloy by accumulative roll bonding [J]. Advanced Engineering Materials, 2015, 18(2): 304-311.
[40] LIU T, WU S D, LI S X, LI P J. Microstructure evolution of Mg-14%Li-1% Al alloy during the process of equal channel angular pressing [J]. Materials Science and Engineering A, 2007, 460-461(14): 499-503.
[41] KARAMI M, MAHMUDI R. The microstructural, textural, and mechanical properties of extruded and equal channel angularly pressed Mg-Li-Zn alloys [J]. Metallurgical and Materials Transactions A, 2013, 44(8): 3934-3946.
[42] SRINIVASARAO S, ZHIYAEV A P,  I,
I,  M T. Stabilization of metastable phases in Mg-Li alloys by high-pressure torsion[J]. Scripta Materialia, 2013, 68(8): 583-586.
M T. Stabilization of metastable phases in Mg-Li alloys by high-pressure torsion[J]. Scripta Materialia, 2013, 68(8): 583-586.
[43] HOU L, RAVEGGI M, CHEN X B, XU W, LAWS K J, WEI Y, FERRY M, BIRBILIS N. Investigating the passivity and dissolution of a corrosion resistant Mg-33at.%Li alloy in aqueous chloride using online ICP-MS [J]. Journal of the Electrochemical Society, 2016, 163(6): C324-C329.
[44] ZENG R, QI W, ZHANG F, CUI H, ZHENG Y. In vitro corrosion of Mg-1.21Li-1.12Ca-1Y alloy [J]. Progress in Natural Science: Materials International, 2014, 24(5): 492-499.
[45] ZHANG M L, ELKIN F M. Ultralight magnesium-lithium alloy [M]. 1st ed. Beijing: Science Press, 2010. (in Chinese)
[46] WU R, ZHANG M. Microstructure, mechanical properties and aging behavior of Mg-5Li-3Al-2Zn-xAg [J]. Materials Science and Engineering A, 2009, 520(1): 36-39.
[47] LI J, QU Z, WU R, ZHANG M, ZHANG J. Microstructure, mechanical properties and aging behaviors of as-extruded Mg-5Li-3Al-2Zn-1.5Cu alloy [J]. Materials Science and Engineering A, 2011, 528(10-11): 3915-3920.
[48] XIANG Q, WU R Z, ZHANG M L. Influence of Sn on microstructure and mechanical properties of Mg-5Li-3Al-2Zn alloys [J]. Journal of Alloys and Compounds, 2009, 477: 832-835.
[49] YUAN X, YU D, GAO L, GAO H. Effect of phosphate-buffered solution corrosion on the ratcheting fatigue behavior of a duplex Mg-Li-Al alloy [J]. Journal of Materials Engineering and Performance, 2016, 25(5): 1802-1810.
[50] TIMMER R T, SANDS J M. Lithium intoxication [J]. Journal of the American Society of Nephrology Jasn, 1999, 10(3): 666-674.
[51] NENE S S, KASHYAP B P, PRABHU N, ESTRIN Y, AL-SAMMAN T. Biocorrosion and biodegradation behavior of ultralight Mg-4Li-1Ca (LC41) alloy in simulated body fluid for degradable implant applications [J]. Journal of Materials Science, 2015, 50(8): 3041-3050.
[52] SANKARANARAYANAN S, JAYALAKSHMI S, GUPTA M. Effect of individual and combined addition of micro/nano-sized metallic elements on the microstructure and mechanical properties of pure Mg [J]. Materials and Design, 2012, 37: 274-284.
[53] KIRKLAND N T, BIRBILIS N, WALKER J, WOODFIELD T, DIAS G J, STAIGER M P. In-vitro dissolution of magnesium- calcium binary alloys: clarifying the unique role of calcium additions in bioresorbable magnesium implant alloys [J]. Journal of Biomedical Materials Research Part B: Applied Biomaterials B, 2010, 95(1): 91-100.
[54] LEE Y H, KIM H S, KIM J Y, JUNG M, PARK Y S, LEE J S, CHOI S H, HER N H, LEE J H, HYUNG N I, LEE C H, YANG S G, HARN C H. A new selection method for pepper transformation: Callus-mediated shoot formation [J]. Plant Cell Reports, 2004, 23(1): 50-58.
[55] KIM W C, KIM J G, LEE J Y, SEOK H K. Influence of Ca on the corrosion properties of magnesium for biomaterials [J]. Materials Letters, 2008, 62(25): 4146-4148.
[56]  A D, KIRKLAND N T, BUCHHEIT R G, BIRBILIS N. Electrochemical properties of intermetallic phases and common impurity elements in magnesium alloys [J]. Electrochemical and Solid-Sate Letters, 2011, 14(2): C5-C7.
A D, KIRKLAND N T, BUCHHEIT R G, BIRBILIS N. Electrochemical properties of intermetallic phases and common impurity elements in magnesium alloys [J]. Electrochemical and Solid-Sate Letters, 2011, 14(2): C5-C7.
[57] SONG Y, SHAN D, CHEN R, HAN E. Investigation of surface oxide film on magnesium lithium alloy [J]. Journal of Alloys and Compounds, 2009, 484(1-2): 585-590.
[58] SONG Y, SHAN D, CHEN R, HAN E. Corrosion characterization of Mg-8Li alloy in NaCl solution [J]. Corrosion Science, 2009, 51(5): 1087-1094.
[59] CANO Z P, KISH J R, MCDERMID J R. On the evolution of cathodic activity during corrosion of magnesium alloy AZ31B in a dilute NaCl solution [J]. Journal of The Electrochemical Society, 2016, 163(3): C62-C68.
[60] CHEN J, SONG Y, SHAN D, HAN E. Modifications of the hydrotalcite film on AZ31 Mg alloy by phytic acid: The effects on morphology, composition and corrosion resistance [J]. Corrosion Science, 2013, 74: 130-138.
[61] LUNDER O, LEIN J E, HESJEVIK S M, AUNE T K,  K. Corrosion morphologies on magnesium alloy AZ 91 [J]. Werkstoffe und Korrosion, 1994, 45(6): 331-340.
K. Corrosion morphologies on magnesium alloy AZ 91 [J]. Werkstoffe und Korrosion, 1994, 45(6): 331-340.
[62] SONG Y W, SHAN D Y, CHEN R S, HAN E H. Corrosion resistance of Mg-8.8Li alloy compared with AZ91 [J]. Corrosion Engineering Science and Technology, 2011, 46(6): 719-723.
[63] CANO Z P, MCDERMID J R, KISH J R. Cathodic activity of corrosion filaments formed on Mg alloy AM30 [J]. Journal of The Electrochemical Society, 2015, 162(14): C732-C740.
[64] MANIVANNAN S, DINESH P, MAHEMAA R, MARIYAPILLAI N, BABU S P K, SUNDARRAJAN S. Corrosion behavior of as-cast Mg-8Li-3Al+xCe alloy in 3.5wt% NaCl solution [J]. International Journal of Minerals, Metallurgy and Materials, 2016, 23(10): 1196-1203.
[65] GU M, WEI G, ZHAO J, LIU W, WU G. Influence of yttrium addition on the corrosion behaviour of as-cast Mg-8Li-3Al-2Zn alloy [J]. Materials Science and Technology, 2016, 33(7): 1-6.
[66] ZENG R C, SUN L, ZHENG Y F, CUI H Z, HAN E H. Corrosion and characterisation of dual phase Mg-Li-Ca alloy in Hank’s solution: The influence of microstructural features [J]. Corrosion Science, 2014, 79(79): 69-82.
[67] GAO L, ZHANG C, ZHANG M, HUANG X, SHENG N. The corrosion of a novel Mg-11Li-3Al-0.5RE alloy in alkaline NaCl solution [J]. Journal of Alloys and Compounds, 2009, 468(1-2): 285-289.
[68] ZHANG C, HUANG X, ZHANG M, GAO L, WU R. Electrochemical characterization of the corrosion of a Mg-Li alloy [J]. Materials Letters, 2008, 62(14): 2177-2180.
[69] MORISHIGE T, DOI H, GOTO T, NAKAMURA E, TAKENAKA T. Exfoliation corrosion behavior of cold-rolled Mg-14 mass% Li-1 mass% Al alloy in NaCl solution [J]. Materials Transactions, 2013, 54(9): 1863-1866.
[70] XU W, BIRBILIS N, SHA G, WANG Y, DANIELS J E, XIAO Y, FERRY M. A high-specific-strength and corrosion-resistant magnesium alloy [J]. Nature Materials, 2015, 14(12): 1229.
[71] SHARMA A K, RANI R U, MALEK A, ACHARYA K S N, MUDDU M, KUMAR S. Black anodizing of a magnesium-lithium alloy [J]. Metal Finishing, 1996, 94(4): 16, 18, 20-22, 24, 27.
[72] XU Y, LI K, YAO Z, JIANG Z, ZHANG M. Micro-arc oxidation coating on Mg-Li alloys [J]. Rare Metals, 2009, 28(2): 160-163.
[73] YIN T, WU R, LENG Z, DU G, GUO X, ZHANG M, ZHANG J. The process of electroplating with Cu on the surface of Mg-Li alloy [J]. Surface & Coatings Technology, 2013, 225: 119-125.
[74] SONG Y, SHAN D, CHEN R, ZHANG F, HAN E. A novel phosphate conversion film on Mg-8.8Li alloy [J]. Surface & Coatings Technology, 2009, 203(9): 1107-1113.
[75] GAO L, ZHANG C, ZHANG M, HUANG X, JIANG X. Phytic acid conversion coating on Mg-Li alloy [J]. Journal of Alloys and Compounds, 2009, 485(1-2): 789-793.
[76] SHAO Y, HUANG H, ZHANG T, MENG G, WANG F. Corrosion protection of Mg-5Li alloy with epoxy coatings containing polyaniline [J]. Corrosion Science, 2009, 51(12): 2906-2915.
[77] HUANG C A, WANG T H, WEIRICH T, NEUBERT V. A pretreatment with galvanostatic etching for copper electrodeposition on pure magnesium and magnesium alloys in an alkaline copper-sulfate bath [J]. Electrochemical Acta, 2008, 53(24): 7235-7241.
[78] HUANG C A, WANG T H, WEIRICH T, NEUBERT V. Electrodeposition of a protective copper/nickel deposit on the magnesium alloy (AZ31) [J]. Corrosion Science, 2008, 50(5): 1385-1390.
[79] HUANG C A, LIN C K, YEH Y H. Corrosion behavior of Cr/Cu-coated Mg alloy (AZ91D) in 0.1 M H2SO4 with different concentrations of NaCl [J]. Corrosion Science, 2010, 52(4): 1326-1332.
[80] HUANG C A, LIN C K, YEH Y H. The corrosion and wear resistances of magnesium alloy (LZ91) electroplated with copper and followed by 1 μm-thick chromium deposits [J]. Thin Solid Films, 2011, 519(15): 4774-4780.
[81] XU C, CHEN L, YU L, ZHANG J, ZHANG Z, WANG J. Effect of pickling processes on the microstructure and properties of electroless Ni-P coating on Mg-7.5Li-2Zn-1Y alloy [J]. Progress in Natural Science: Materials International, 2014, 24(6): 655-662.
[82] LUO Hong-jie, SONG Bin-na, LIU Yi-han, YAO Guang-chun. Electroless Ni-P plating on Mg-Li alloy by two-step method [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(10): 2225-2230.
[83] ZOU Y, ZHANG Z, LIU S, CHEN D, WANG G, WANG Y, ZHANG M, CHEN Y. Ultrasonic-assisted electroless Ni-P plating on dual phase Mg-Li alloy [J]. Journal of The Electrochemical Society, 2015, 162(1): C64-C70.
[84] YANG L, LI J, ZHENG Y, JIANG W, ZHANG M. Electroless Ni-P plating with molybdate pretreatment on Mg-8Li alloy [J]. Journal of Alloys and Compounds, 2009, 467(1): 562-566.
[85] CAO X Q, VASSEN R, SCHWARTZ S, JUNGEN W, TIETZ F,  D. Spray-drying of ceramics for plasma-spray coating [J]. Journal of the European Ceramic Society, 2000, 20(14-15): 2433-2439.
D. Spray-drying of ceramics for plasma-spray coating [J]. Journal of the European Ceramic Society, 2000, 20(14-15): 2433-2439.
[86] BHATIA T, OZTURK A, XIE L, JORDAN E H, CETEGEN B M, GELL M, MA X, PADTURE N P. Mechanisms of ceramic coating deposition in solution-precursor plasma spray [J]. Journal of Materials Research, 2002, 17(9): 2363-2672.
[87] CHEN H, ZHANG Y, DING C. Tribological properties of nanostructured zirconia coatings deposited by plasma spraying [J]. Wear, 2002, 253(7-8): 885-893.
[88] GAO Y, XU X, YAN Z, XIN G. High hardness alumina coatings prepared by low power plasma spraying [J]. Surface and Coatings Technology, 2002, 154(2-3): 189-193.
[89] YIN Z, TAO S, ZHOU X, DING C. Microstructure and mechanical properties of Al2O3-Al composite coatings deposited by plasma spraying [J]. Applied Surface Science, 2008, 254(6): 1636-1643.
[90] ZHAO L, LUGSCHEIDER E. Reactive plasma spraying of TiAl6V4 alloy [J]. Wear, 2002, 253(11-12): 1214-1218.
[91] OKI S, TSUJIKAWA M, MORISHIGE T, KAMITA M. Thin protective aluminum layer on Mg-Li alloy by plasma spraying and cold rolling [J]. Plasma Processes and Polymers, 2009, 6(S1): s954-s957.
[92] TSUJIKAWA M, ADACHI S, ABE Y, OKI S, NAKATA K, LAMITA M. Corrosion protection of Mg-Li alloy by plasma thermal spraying of aluminum [J]. Plasma Processes and Polymers, 2010, 4(S1): s593-s596.
[93] NIU Z Y, QU Z K, MA T T, JING X Y. Preparation of Al coating on Mg-Li alloy with molten salt replacement [J]. Applied Mechanics & Materials, 2014, 644: 4798-4801.
[94] SONG Y, SHAN D, CHEN R, ZHANG F, HAN E. Formation mechanism of phosphate conversion film on Mg-8.8Li alloy [J]. Corrosion Science, 2009, 51(1): 62-69.
[95] ZHANG H, YAO G, WANG S, LIU Y, LUO H. A chrome-free conversion coating for magnesium-lithium alloy by a phosphate- permanganate solution [J]. Surface & Coatings Technology, 2008, 202(9): 1825-1830.
[96] PHUONG N V, MOON S. Comparative corrosion study of zinc phosphate and magnesium phosphate conversion coatings on AZ31 Mg alloy [J]. Materials Letters, 2014, 122(5): 341-344.
[97] PHUONG N V, MOON S, CHANG D, LEE K H. Effect of microstructure on the zinc phosphate conversion coatings on magnesium alloy AZ91 [J]. Applied Surface Science, 2013, 264: 70-78.
[98] LI Q, XU S, HU J, ZHANG S, ZHONG X, YANG X. The effects to the structure and electrochemical behavior of zinc phosphate conversion coatings with ethanolamine on magnesium alloy AZ91D [J]. Electrochemical Acta, 2010, 55(3): 887-894.
[99] ZENG Rong-chang, SUN Xin-xin, SONG Ying-wei, ZHANG Fen, LI Shuo-qi, CUI Hong-zhi, HAN En-hou. Influence of solution temperature on corrosion resistance of Zn-Ca phosphate conversion coating on biomedical Mg-Li-Ca alloys [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(11): 3293-3299.
[100] YANG L, ZHANG M, LI J, YU X, NIU Z. Stannate conversion coatings on Mg-8Li alloy [J]. Journal of Alloys and Compounds, 2009, 471(1): 197-200.
[101] HORT N, MATHAUDHU S N, NEELAMEGGHAM N R, ALDERMAN M. Formation of vanadate conversion coating on AZ31 magnesium alloy [C]//HORT N, MATHAUDHU S N, NEELAMEGGHAM N R, ALDERMAN M. Magnesium Technology. Hoboken: John Wiley & Sons, Inc, 2013: 1501-1506.
[102] NIU L, CHANG S H, TONG X, LI G, SHI Z. Analysis of characteristics of vanadate conversion coating on the surface of magnesium alloy [J]. Journal of Alloys and Compounds, 2014, 617: 214-218.
[103] MA Y, LI N, LI D, ZHANG M, HUANG X. Characteristics and corrosion studies of vanadate conversion coating formed on Mg- 14 wt%Li-1 wt%Al-0.1 wt%Ce alloy [J]. Applied Surface Science, 2012, 261(22): 59-67.
[104] YONG Z, ZHU J, QIU C, LIU Y. Molybdate/phosphate composite conversion coating on magnesium alloy surface for corrosion protection [J]. Applied Surface Science, 2008, 255(5): 1672-1680.
[105] HU J, LI Q, ZHONG X, ZHANG L, CHEN B. Composite anticorrosion coatings for AZ91D magnesium alloy with molybdate conversion coating and silicon sol-gel coatings [J]. Progress in Organic Coatings, 2009, 66(3): 199-205.
[106] ELSENTRIECY H H, AZUMI K. Electroless Ni-P deposition on AZ91 D magnesium alloy prepared by molybdate chemical conversion coatings [J]. Journal of The Electrochemical Society, 2009, 156(2): D70-D77.
[107] WANG G, ZHANG M, WU R. Molybdate and molybdate/ permanganate conversion coatings on Mg-8.5Li alloy [J]. Applied Surface Science, 2012, 258(7): 2648-2654.
[108] YANG L, LI J, YU X, ZHANG M, HUANG X. Lanthanum-based conversion coating on Mg-8Li alloy [J]. Applied Surface Science, 2008, 255(5): 2338-2341.
[109] SONG D, JING X, WANG J, LU S, YANG P, WANG Y, ZHANG M. Microwave-assisted synthesis of lanthanum conversion coating on Mg-Li alloy and its corrosion resistance [J]. Corrosion Science, 2011, 53(11): 3651-3656.
[110] GAO Li-li, ZHANG Chun-hong, ZHANG Mi-lin. Cerium chemical conversion coating on a novel Mg-Li alloy [J]. Journal of Wuhan University of Technology–Materials Science Edition, 2010, 25(1): 112-117.
[111] YANG X, WANG G, DONG G, GONG F, ZHANG M. Rare earth conversion coating on Mg-8.5Li alloys [J]. Journal of Alloys and Compounds, 2009, 487(1-2): 64-68.
[112] YANG L, LIU H, HU N. Assembly of electroactive layer-by-layer films of myoglobin and small-molecular phytic acid [J]. Electrochemistry Communications, 2007, 9(5): 1057-1061.
[113] CUI X, LI Q, LI Y, WANG F, JIN G, DING M. Microstructure and corrosion resistance of phytic acid conversion coatings for magnesium alloy [J]. Applied Surface Science, 2008, 255(5): 2098-2103.
[114] SHARMA A K, RANI R U, BHOJARAJ H, NARAYANAMURTHY H. Galvanic black anodizing on Mg-Li alloys [J]. Journal of Applied Electrochemistry, 1993, 23(5): 500-507.
[115] LI J F, ZHENG Z Q, LI S C, REN W D, ZHANG Z. Preparation and galvanic anodizing of a Mg-Li alloy [J]. Materials Science and Engineering A, 2006, 433(1): 233-240.
[116] CHANG L M, WANG P, LIU W. Effect of amino acids on the structure and corrosion resistance of Mg-Li alloy anodic oxide film [J]. Advanced Materials Research, 2010, 146-147: 785-788.
[117] ONO S, SUZUKI Y, ASOH H, HANZAWA N, HYAKUTAKE M. Microstructure of anodic films grown on magnesium-lithium- yttrium ultra light alloy [J]. Materials Science Forum, 2003, 426-432(1): 581-586.
[118] WEN L, WANG Y, ZHOU Y, GUO L, QUYANG J H. Microstructure and corrosion resistance of modified 2024 Al alloy using surface mechanical attrition treatment combined with microarc oxidation process [J]. Corrosion Science, 2011, 53(1): 473-480.
[119] LIANG J, SRINIVASAN P B, BLAWERT C,  M, DIETZEL W. Electrochemical corrosion behaviour of plasma electrolytic oxidation coatings on AM50 magnesium alloy formed in silicate and phosphate based electrolytes [J]. Electrochimica Acta, 2009, 54(14): 3842-3850.
M, DIETZEL W. Electrochemical corrosion behaviour of plasma electrolytic oxidation coatings on AM50 magnesium alloy formed in silicate and phosphate based electrolytes [J]. Electrochimica Acta, 2009, 54(14): 3842-3850.
[120] YAO Z, JIANG Z, WANG F. Study on corrosion resistance and roughness of micro-plasma oxidation ceramic coatings on Ti alloy by EIS technique [J]. Electrochimica Acta, 2007, 52(13): 4539-4546.
[121] LI J G, LV Y, WANG H W, ZHU Z J, WEI Z J, MENG X C. Corrosion characterization of microarc oxidation coatings formed on Mg-7Li alloy [J]. Materials and Corrosion, 2013, 64(5): 426-432.
[122] LI Z, YUAN Y, JING X. Effect of current density on the structure, composition and corrosion resistance of plasma electrolytic oxidation coatings on Mg-Li alloy [J]. Journal of Alloys and Compounds, 2012, 541(22): 380-391.
[123] LI Z J, YUAN Y, JING X Y. Comparison of plasma electrolytic oxidation coatings on Mg-Li alloy formed in molybdate/silicate and aluminate/silicate composite electrolytes [J]. Materials and Corrosion, 2014, 65(5): 493-501.
[124] LI Z, YUAN Y, SUN P, JING X. Ceramic coatings of LA141 alloy formed by plasma electrolytic oxidation for corrosion protection [J]. ACS Applied Materials & Interfaces, 2011, 3(9): 3682-3690.
[125] SHI L, XU Y, LI K, YAO Z, WU S. Effect of additives on structure and corrosion resistance of ceramic coatings on Mg-Li alloy by micro-arc oxidation [J]. Current Applied Physics, 2010, 10(3): 719-723.
[126] SONG L, KOU Y, SONG Y, SHAN D, ZHU G, HAN E. Fabrication and characterization of micro-arc oxidation (MAO) coatings on Mg-Li alloy in alkaline polyphosphate electrolytes without and with the addition of K2TiF6 [J]. Materials and Corrosion, 2011, 62(12): 1124-1132.
[127] LIU J, LU Y, JING X, YUAN Y, ZHANG M. Characterization of plasma electrolytic oxidation coatings formed on Mg-Li alloy in an alkaline silicate electrolyte containing silica sol [J]. Materials and Corrosion, 2015, 60(11): 865-870.
[128] MA C, LU Y, SUN P, YUAN Y, JING X, ZHANG M. Characterization of plasma electrolytic oxidation coatings formed on Mg-Li alloy in an alkaline polyphosphate electrolyte [J]. Surface & Coatings Technology, 2011, 206(2-3): 287-294.
[129] MA C, ZHANG M, YUAN Y, JING X, BAI X. Tribological behavior of plasma electrolytic oxidation coatings on the surface of Mg-8Li-1Al alloy [J]. Tribology International, 2012, 47: 62-68.
[130] CHEN X F, LUO C, ZHANG Z H, ZHAO M. Preparation and anticorrosion properties of polyaniline-containing coating on Mg-Li alloy [J]. Anti-corrosion Methods and Materials, 2012, 59(6): 291-298.
[131] CHEN X, SHEN K, ZHANG J. Preparation and anticorrosion properties of polyaniline-SiO2-containing coating on Mg-Li alloy [J]. Pigment & Resin Technology, 2010, 39(6): 322-326.
[132] ZHANG C H, LI R M, GAO L L, ZHANG M L. Corrosion protection of Mg-11Li-3Al-0.5RE alloy using hybrid epoxy/silica conversion coatings [J]. Corrosion Engineering, Science and Technology, 2013, 48(4): 276-281.
[133]  F, FISCHER H, VARLEY R, ZWAAG S V D. Moisture induced crack filling in barrier coatings containing montmorillonite as an expandable phase [J]. Surface & Coatings Technology, 2008, 202(14): 3346-3353.
F, FISCHER H, VARLEY R, ZWAAG S V D. Moisture induced crack filling in barrier coatings containing montmorillonite as an expandable phase [J]. Surface & Coatings Technology, 2008, 202(14): 3346-3353.
[134] YU Y H, YEH J M, LIOU S J, CHANG Y P. Organo-soluble polyimide (TBAPP-OPDA)/clay nanocomposite materials with advanced anticorrosive properties prepared from solution dispersion technique [J]. Acta Materialia, 2004, 52(2): 475-486.
[135] WANG Y, ZHU Y, LI C, SONG D, ZHANG T, ZHENG X, YAN Y, ZHANG M, WANG J, SHCHUKIN D G. Smart epoxy coating containing Ce-MCM-22 zeolites for corrosion protection of Mg-Li alloy [J]. Applied Surface Science, 2016, 369: 384-389.
[136] YU X, WANG J, ZHANG M, YANG L, LI J, YANNG P, CAO D. Synthesis, characterization and anticorrosion performance of molybdate pillared hydrotalcite/in situ created ZnO composite as pigment for Mg-Li alloy protection [J]. Surface & Coatings Technology, 2008, 203(3-4): 250-255.
[137] CHOI J, NAKAO S, KIM J, IKEYAMA M, KATO T. Corrosion protection of DLC coatings on magnesium alloy [J]. Diamond & Related Materials, 2007, 16(4-7): 1361-1364.
[138] YAMAUCHI N, DEMIZU K, UEDE N, CUONG N K, SONE T, HIROSE Y. Friction and wear of DLC films on magnesium alloy [J]. Surface & Coatings Technology, 2005, 193(1-3): 277-282.
[139] YAMAUCHI N, DEMIZU K, UEDE N, SONE T, TSUJIKAWA M, HIROSE Y. Effect of peening as pretreatment for DLC coatings on magnesium alloy [J]. Thin Solid Films, 2006, 506(506): 378-383.
[140] YAMAUCHI N, UEDE N, OKAMOTO A, SONE T, TSUJIKAWA M, OKI S. DLC coating on Mg-Li alloy [J]. Surface & Coatings Technology, 2007, 201(9-11): 4913-4918.
[141] SHAN W, ZHANG Y, YANG W, KE C, GAO Z, YE Y, TANG Y. Electrophoretic deposition of nanosized zeolites in non-aqueous medium and its application in fabricating thin zeolite membranes [J]. Microporous and Mesoporous Materials, 2004, 69(1): 35-42.
[142] CHENG Y, LI J S, WANG L J, SUN X Y, LIU X D. Synthesis and characterization of Ce-ZSM-5 zeolite membranes [J]. Separation and Purification Technology, 2006, 51: 210-218.
[143] KOKOTAILO G T, LAWTON S L, OLSON D H, MEIER W M. Structure of synthetic zeolite ZSM-5 [J]. Nature, 1978, 272(5652): 437-438.
[144] SONG D, JING X, WANG J, YANG P, WANG Y, ZHANG M. The assembly of ZSM-5 layer on Mg-Li alloy by hot-pressing using silane coupling agent as bond and its corrosion resistance [J]. Journal of Alloys and Compounds, 2012, 510(1): 66-70.
[145] SONG D, JING X, WANG J, WANG Y, YANG P, ZHAO M, ZHANG M. Corrosion-resistant ZSM-5 zeolite coatings formed on Mg-Li alloy by hot-pressing [J]. Corrosion Science, 2011, 53(5): 1732-1737.
[146] LIU K, ZHANG M, ZHAI J, WANG J, JIANG L. Bioinspired construction of Mg-Li alloys surfaces with stable superhydrophobicity and improved corrosion resistance [J]. Applied Physics Letters, 2008, 92(18): 183103-183103-3.
[147] CHEN D, JIN N, CHEN W, WANG L, ZHAO S, LUO D. Corrosion resistance of Ni/Cu/Ni-P triple-layered coating on Mg-Li alloy [J]. Surface & Coatings Technology, 2014, 254(18): 440-446.
[148] LI Z, JING X, YUAN Y, ZHANG M. Composite coatings on a Mg-Li alloy prepared by combined plasma electrolytic oxidation and sol-gel techniques [J]. Corrosion Science, 2012, 63: 358-366.
[149] SUN P, LU Y, YUAN Y, JING X, ZHANG M. Preparation and characterization of duplex PEO/MoC coatings on Mg-Li alloy [J]. Surface & Coatings Technology, 2011, 205(19): 4500-4506.
[150] XU D K, ZU T T, YIN M, XU Y B, HAN E H. Mechanical properties of the icosahedral phase reinforced duplex Mg-Li alloy both at room and elevated temperatures [J]. Journal of Alloys and Compounds, 2014, 582(1): 161-166.
孙月花,王日初,彭超群,冯 艳,杨 明
中南大学 材料科学与工程学院,长沙 410083
摘 要:Mg-Li合金作为超轻金属结构材料,由于其具有较高的比强度、较好的成形性和良好的电磁屏蔽性能,在航空航天以及军事领域具有良好的发展前景。综述Mg-Li合金的研究进展并指出其面临的三大问题。针对Mg-Li合金较差的耐蚀性,重点总结合金的腐蚀行为,并详细地介绍包括电镀、化学镀、等离子喷涂、熔盐置换、化学转化膜、阳极氧化、微弧氧化、有机涂层以及有机-无机杂化涂层等表面处理技术。最后,探讨Mg-Li合金腐蚀与防护的发展方向。
关键词:Mg-Li合金;比强度;成形性;电磁屏蔽;腐蚀行为;表面处理;杂化涂层
(Edited by Sai-qian YUAN)
Foundation item: Project (2017zzts005) supported by the Fundamental Research Funds for the Central Universities of Central South University
Corresponding author: Ming YANG; Tel/Fax: +86-731-88836638; E-mail: 13507469742@163.com
DOI: 10.1016/S1003-6326(17)60167-5

