采用负载Ni0.5Zn0.5Fe2O4磁性纳米粒子芦荟壳灰从水溶液中快速高效去除Ag(I)
来源期刊:中国有色金属学报(英文版)2016年第8期
论文作者:Parisa BEIGZADEH Farid MOEINPOUR
文章页码:2238 - 2246
关键词:吸附;Ag+离子;Ni0.5Zn0.5Fe2O4; 芦荟
Key words:adsorption; Ag+ ions; Ni0.5Zn0.5Fe2O4; aloe vera
摘 要:采用负载Ni0.5Zn0.5Fe2O4磁性纳米粒子芦荟壳灰从水溶液中去除Ag(I)。采用XRD、SEM、BET等温线、振荡试样磁力计(VSM)和傅里叶变换红外光谱(FT-IR)表征该吸附剂。采用该吸附剂在不同pH值(2~7)、吸附剂量(0.01~0.5 g)、Ag+浓度(50, 100, 200, 300, 500, 700 和1000 mg/L)下测定Ag(I)的吸光度。在最佳条件(30 min, pH=5)下,得到最高的Ag+去除率。在50 mL 100 mg/L Ag+溶液中,最佳吸附剂量是0.20 g,去除率为98.3%。基于 Langmuir等温线,得到最大单层饱和吸附量为243.90 mg/g。表征结果表明,吸附剂的比表面积和孔体积分别为814.23 m2/g 和 0.726 cm3/g。实验数据与Langmuir和Freundlich等温线模型吻合。合成的吸附剂对水溶液中Ag(I)吸附具有理想的表面积和吸附容量。
Abstract: Silver (I) was removed from aqueous environment by aloe vera shell ash supported Ni0.5Zn0.5Fe2O4 magnetic nanoparticles. The adsorbent was characterized by several methods including X-ray diffraction (XRD), scanning electron microscopy (SEM), BET isotherm, vibrating sample magnetometer (VSM) and Fourier transform infrared spectroscopy (FT-IR). To determine the absorption of silver (I) by this adsorbent, different pH values (2-7), adsorbent dose (0.01-0.5 g), concentrations of Ag+ (50, 100, 200, 300, 500, 700 and 1000 mg/L) and exposure time (5-100 min) were experimented. The highest removal efficiency of Ag+ was achieved under optimum condition (30 min and pH=5). The optimum adsorbent dose was 0.20 g (in 50 mL of 100 mg/L Ag+ solution), which achieved a removal efficiency of 98.3%. The maximum monolayer adsorption capacity based on the Langmuir isotherm is 243.90 mg/g. Characterization results revealed that specific surface area and porous volume were 814.23 m2/g and 0.726 cm3/g, respectively. The experimental data were fitted well with the Langmuir and Freundlich isotherm models. Synthesized adsorbent has desired surface area and adsorptive capacity for silver (I) adsorption in aquatic environment.
Trans. Nonferrous Met. Soc. China 26(2016) 2238-2246
Parisa BEIGZADEH, Farid MOEINPOUR
Department of Chemistry, Bandar Abbas Branch, Islamic Azad University, Bandar Abbas 7915893144, Iran
Received 16 August 2015; accepted 4 March 2016
Abstract: Silver (I) was removed from aqueous environment by aloe vera shell ash supported Ni0.5Zn0.5Fe2O4 magnetic nanoparticles. The adsorbent was characterized by several methods including X-ray diffraction (XRD), scanning electron microscopy (SEM), BET isotherm, vibrating sample magnetometer (VSM) and Fourier transform infrared spectroscopy (FT-IR). To determine the absorption of silver (I) by this adsorbent, different pH values (2-7), adsorbent dose (0.01-0.5 g), concentrations of Ag+ (50, 100, 200, 300, 500, 700 and 1000 mg/L) and exposure time (5-100 min) were experimented. The highest removal efficiency of Ag+ was achieved under optimum condition (30 min and pH=5). The optimum adsorbent dose was 0.20 g (in 50 mL of 100 mg/L Ag+ solution), which achieved a removal efficiency of 98.3%. The maximum monolayer adsorption capacity based on the Langmuir isotherm is 243.90 mg/g. Characterization results revealed that specific surface area and porous volume were 814.23 m2/g and 0.726 cm3/g, respectively. The experimental data were fitted well with the Langmuir and Freundlich isotherm models. Synthesized adsorbent has desired surface area and adsorptive capacity for silver (I) adsorption in aquatic environment.
Key words: adsorption; Ag+ ions; Ni0.5Zn0.5Fe2O4; aloe vera
1 Introduction
Heavy metals as raw materials or catalysts are used extensively in many industrial processes, such as mining, metallurgy, electrolysis, electroplating and leather [1]. They can be afterward discharged into the environment via wastewater, which has become a hazard to human and local environment [2]. Because the heavy metals in water are difficult to be bio-degenerated and tend to cumulate in living structures through the food chain [3], the wastewater including heavy metals should be cleaned up before evacuation. Wastewater comprising heavy metals is usually cleaned up via precipitation, membrane separation, ion exchange, and adsorption [4-7]. Among them, adsorption was established as an important and economically practical treatment technology for removing the Ag+ ions from water and wastewater. Activated carbon is usually used adsorbent for the removal of Ag+ ions from aqueous solution. Despite the abundance applications of activated carbon, its use is sometimes limited due to its high cost and also for loss during its re-formation [8-10]. Therefore, the researchers are on the search for new low-cost substitute adsorbents for the water pollution control, especially, where cost acts an important role. Much effort has been done towards the development of another adsorbents that are effective and low-cost. They can be produced from a wide diversity of raw materials, which are abundant and have high carbon and low inorganic content. Owing to the low cost and high accessibility of these materials, it is not essential to have complex regeneration processes. Such low cost adsorption methods have attracted many researchers. Often, the adsorption capabilities of such adsorbents are not large, therefore, the study and investigation of more and more new adsorbents are still under development. Several common adsorbents of different origin, primarily including activated carbons, clays, zeolites, biomass, and polymeric materials, have been used for the removal of heavy metal from the industrial wastewater [11-15]. In recent years, due to economic problems, creating a cheap and efficient alternative method of wastewater treatment instead of expensive and inefficient methods is of great importance. One of the most efficient, technical and economic methods in this context, is the use of magnetic adsorbent. These adsorbents have magnetic properties and by using an external magnetic field they can be easily separated from the solution. In the magnetic separation, high costs of separation, such as centrifugation and filtration are not included [16]. Extensive researches in the field of magnetization of materials such as chitosan [17], silica [18], polymer [19] and activated carbon [20] have been conducted for water contaminants removal. The use of this property in the nanoparticles, due to their high specific surface area and adsorption capacity is very good [21-23]. Nickel-zinc ferrites have drawn noticeable consideration of researchers as a result of their remarkable magnetic properties, large permeability, and very high electrical resistivity [24]. They have extensive potential applications such as high–density information storage devices, microwave devices, transformer cores, and magnetic fluids [25]. The use of activated carbon to remove chlorine, separating gases and air pollution treatment, recycling of heavy metals from aqueous solutions has many applications. But because of the high cost, other options have been suggested as an alternative. Ash due to the low cost of production is a good alternative to activated carbon [26-30]. Ashes can be produced from a wide range of carbon materials, such as wood, coal, shell, walnut shell, fruit stones, and agricultural waste [31].
In this work, we became interested to investigate the capability of the surface modified Ni0.5Zn0.5Fe2O4 magnetic nanoparticles with ash prepared from aloe vera shell (Ni0.5Zn0.5Fe2O4/ASA) as a low-cost adsorbent for removal of Ag+ ions from aqueous solution and also to study the adsorption mechanism of Ag+ ions onto this adsorbent. For this purpose, a set of batch adsorption experiments (pH, contact time, adsorbent dosage and initial Ag+ ions concentration) on the Ag+ ions removal using this adsorbent were carried out at optimum conditions. The characterization of the adsorbent was described by FT-IR, XRD and SEM analyses.
2 Experimental
2.1 Materials and methods
Analytical-grade salt AgNO3 was obtained from Merck. A 1000 mg/L stock solution of the salt was prepared in deionized water. All working solutions were prepared by diluting the stock solution with deionized water. Deionized water was prepared using a Millipore Milli-Q (Bedford, MA) water purification system. All reagents (Fe(NO3)3·9H2O, Zn(NO3)2·6H2O and Ni(NO3)2·6H2O, NaOH and HNO3) used in the study were of analytical grade and purchased from Aldrich. Before each experiment, all glassware were cleaned with dilute nitric acid and repeatedly washed with deionized water. X-ray diffraction analysis (XRD) was carried out using a PAN analytical X’Pert Pro X-ray diffractometer. Surface morphology and particle size were studied using a Hitachi S-4800 SEM instrument. FT-IR spectra were determined as KBr pellets on a Bruker model 470 spectrophotometer. The specific surface (SBET) of Ni0.5Zn0.5Fe2O4/ASA was determined by a micrometrics apparatus (Gemini 2375) by adsorption of nitrogen at 77 K according to the traditional method of Brunauer Emmet and Teller or BET.
All the metal ion concentrations were measured with a Varian AA240FS atomic absorption spectrophotometer.
2.2 Synthesis of aloe vera shell ash (ASA)
Aloe vera is grown in southern Iran. Aloe vera shells were collected from, Qeshm Island, Hormozgan, Iran, and were applied as a raw material for the preparation of surface modified adsorbent. The collected aloe vera shells were washed and dried in an air oven at 80 °C for 24 h and then ground and sieved to the desired particle size (2–3 mm). The resultant sieved powder was carbonized in a furnace at 700 °C at heating rate of 10 °C/min for 2 h. The methods for producing carbon materials are similar to other studies also have been used [26].
2.3 Synthesis of Ni0.5Zn0.5Fe2O4/ASA
At first, Ni-Zn ferrite was prepared using stoichiometric ratios of metal nitrates to freshly extracted egg-white [32]. The metal nitrates (Fe(NO3)3·9H2O, Zn(NO3)2·6H2O and Ni(NO3)2·6H2O) and 2 g ASA were dissolved together in a minimum amount of double distilled water to get a clear solution. 60 mL of extracted egg-white dissolved in 40 mL of double distilled water while vigorous stirring, was added to nitrate mixture at ambient temperature. After constant stirring for 30 min, the resultant sol-gel was evaporated at 80 °C until dry precursor was obtained. The dried precursors were ground and calcined in a muffle furnace at 550 °C for 2 h.
2.4 Adsorption experiments
Batch adsorption of silver ions onto the adsorbent (Ni0.5Zn0.5Fe2O4/ASA) was investigated in aqueous solutions under various operating conditions viz pH 2-7, at a temperature of 298 K, for an initial Ag+ ion concentration of 100 mg/L. About 0.20 g adsorbent was added to 50 mL of silver nitrate solution (100 mg/L). Then, the mixture was agitated on a shaker at 250 r/min. The initial pH values of the silver solutions were adjusted from 2 to 7 with 0.1 mol/L HNO3 or 0.1 mol/L NaOH solutions using a pH meter. After equilibrium, the samples were centrifuged and the adsorbent (Ni0.5Zn0.5Fe2O4/ASA) was removed magnetically from the solution. The Ag+ concentration and final pH in the supernatant were measured by flame atomic absorption spectrometer and a pH meter, respectively. The effects of several parameters, such as contact time, initial concentration, pH and adsorbent dose on extent of adsorption of Ag+ were investigated. Each datum point was taken as the average of three measurements.
The Ag+ removal rate (R) was calculated as
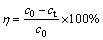 (1)
(1)
where c0 and ct (mg/L) are the concentrations of Ag+ in the solution at initial and equilibrium time, respectively.
The amount of Ag+ adsorbed (Qe) was calculated using
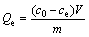 (2)
(2)
where ce is the equilibrium concentration of Ag+ (mg/L), m is the mass of adsorbent (g), and V is the volume of solution (L).
To specify the regeneration of the adsorbent (Ni0.5Zn0.5Fe2O4/ASA) sample, adsorption/desorption cycles were repeated 5 times using the same adsorbent sample. Na4-EDTA (0.1 mol/L) was used as a desorption agent. Adsorbent (Ni0.5Zn0.5Fe2O4/ASA) samples carrying 99.95 mg/g Ag+ were placed in this desorption medium (25 mL) and stirred magnetically at 25 °C for 1 h. After 1 h, the aqueous phase was separated from the adsorbent and the concentration of Ag+ in that phase was measured.
2.5 Adsorption isotherms
Adsorption isotherms were obtained by using 0.20 g of adsorbent and 50 mL of silver nitrate solution with different concentrations (50-1000 mg/L) at 298 K. These solutions were buffered at an optimum pH (pH=5) for adsorption and agitated on a shaker at 250 r/min until they reached adsorption equilibrium (30 min). The quantity of Ag+ adsorbed was derived from the concentration change.

Fig. 1 FTIR spectra of Ni0.5Zn0.5Fe2O4/ASA (a), ASA (b), and Ni0.5Zn0.5Fe2O4 (c)

Fig. 2 XRD patterns of Ni0.5Zn0.5Fe2O4

Fig. 3 SEM image of Ni0.5Zn0.5Fe2O4/ASA nanocomposite
3 Results and discussion
3.1 Characterization of Ni0.5Zn0.5Fe2O4/ASA magnetic nanoparticles
Ni0.5Zn0.5Fe2O4 nano-crystallites were prepared according to the reported procedure by GABAL et al [32]. Ni0.5Zn0.5Fe2O4/ASA nanocrystallites were characterized by FT-IR (Fig. 1), XRD (Fig. 2) and SEM (Fig. 3). FT-IR spectra of Ni0.5Zn0.5Fe2O4, Ni0.5Zn0.5- Fe2O4/ASA and ASA are compared in Fig. 1. In the FT-IR spectrum of Ni0.5Zn0.5Fe2O4/ASA (Fig. 1(a)), most of the bands of Ni0.5Zn0.5Fe2O4 (Fig. 1(c)) and ASA (Fig. 1(b)) with a slight shift for some of them, are observable, which shows that ASA has been supported well on the Ni0.5Zn0.5Fe2O4. The bands are in the low-frequency region (1000-500 cm-1) due to iron oxide skeleton, which is in agreement with the magnetite spectrum. The peak at 1444.85 cm-1 showed the existence of Fe—O [33]. In Fig. 1(a), the presence of —OH stretching mode is evident by the peak close to 3446.75 cm-1. To confirm the Ni ferrite formation in the synthesized magnetic nanoparticles, the XRD spectrum of the sample was studied. The XRD patterns (Fig. 2) show that Ni0.5Zn0.5Fe2O4 nanoparticles have the spinel structure, with all the major peaks matching the standard pattern of bulk Ni0.5Zn0.5Fe2O4 (JCPDS 08-0234). The particle size of adsorbent was investigated by SEM. The SEM photograph of sample (Fig. 3) shows that average size of Ni0.5Zn0.5Fe2O4/ASA is approximately less than 100 nm.
The magnetic properties of the Ni0.5Zn0.5Fe2O4/ASA were evaluated by a vibrating sample magnetometer (VSM). As shown in Fig. 4, the saturation magnetic moments of the Ni0.5Zn0.5Fe2O4/ASA reached about 17 kA/m. It showed superparamagnetic behavior that would enable easy recovery of the adsorbent from solution under an applied magnetic field.
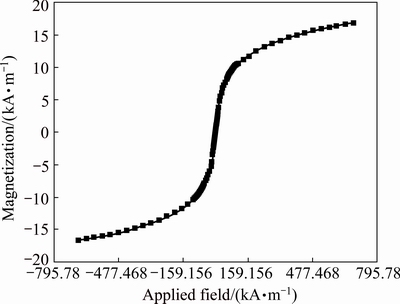
Fig. 4 VSM curve of Ni0.5Zn0.5Fe2O4/ANSA at room temperature
Some properties of the Ni0.5Zn0.5Fe2O4/ASA are presented in Table 1. The Ni0.5Zn0.5Fe2O4/ASA has a surface area about 814.23 m2/g and a porous volume equal to 0.726 cm3/g.
Table 1 Properties of Ni0.5Zn0.5Fe2O4/ASA

3.1 Adsorption and removal of Ag+ from aqueous solution
3.1.1 Effect of contact time
The effect of contact time on the amount of silver adsorbed was studied at 100 mg/L initial concentration of silver. It could be observed from Fig. 5 that with the increase of contact time, the adsorption also increased. Minimum adsorption was 90.0% for 5 min and maximum adsorption value was 97.0% for 30 min. The adsorption characteristic indicated a rapid uptake of the silver. The adsorption rate, however, reduced to a constant value with an increase in contact time because all available sites were covered, and no active site was present for adsorbing.
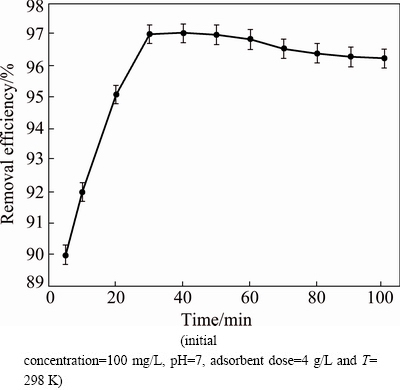
Fig. 5 Effect of contact time on removal efficiency
3.1.2 Effect of pH
The acidity of the aqueous solution applies a considerable effect on the adsorption process because it can affect the solution chemistry of contaminants and the state of functional groups on the surface of adsorbents [34-36]. The effect of solution pH on Ag+ adsorption was studied at pH 2-7 at 298 K. As shown in Fig. 6, the adsorption rate of Ag+ is enhanced with increasing pH from 2 to 7. However, at low pH values, hydrogen ions (H+) are likely to compete with Ag+ and thus lower the amount of Ag+ removed. Therefore, the great Ag+ adsorption occurring at higher pH could be described to a decrease in competition between H+ and Ag+ at the same adsorption site of the adsorbent beads. At pH>6, the Ag+ ions begin to hydrolyze and then form insoluble silver hydroxide. At this time, both adsorption and precipitation are effective mechanisms in the removal of Ag+ ions from aqueous solution [37]. Therefore, the maximum adsorption occurs at around pH 5.0 and it is therefore selected for all adsorption experiments in this study. In order to check the stability of Ni0.5Zn0.5Fe2O4/ ASA in acidic medium, the reaction mixture in pH 5 (optimized pH) was subjected to the elemental analysis by ICP-AEM technology and no Ni, Zn and Fe were detected, which indicated that the spinel structure of Ni0.5Zn0.5Fe2O4 was stable.
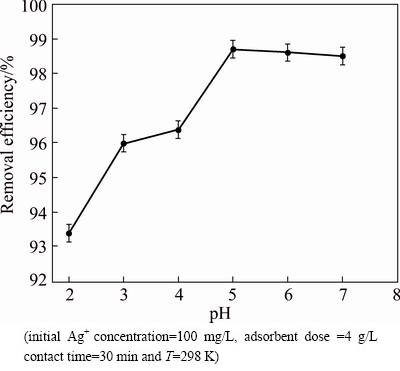
Fig. 6 Effect of pH on Ag+ removal at different pH values
3.1.3 Effect of adsorbent dosage
The effect of change in the adsorbent amount on the process adsorption of Ag+ was investigated, with different adsorbent doses in the range of 0.01-0.50 g. The results obtained are shown in Fig. 7. From Fig. 7, it is considered that as the adsorbent dose is enhanced, the removal rate also increases, until it approaches a saturation point, where the enhancement in adsorbent dose does not alter the removal rate. An increase in adsorption rate with adsorbent quantity can be ascribed to the increased surface area and the availability of more adsorption sites. The best removal rate of Ag+ is about 98.3%, using an adsorbent dosage of 0.20 g in 50 mL of 100 mg/L Ag+ solution (4 g/L).
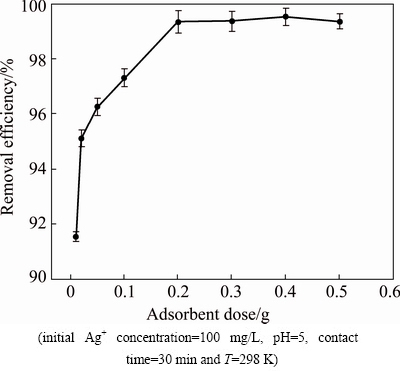
Fig. 7 Effect of adsorbent dosage on removal efficiency of Ag+ ions
3.1.4 Effect of initial Ag+ concentration
Batch adsorption experiments were performed at different initial Ag+ concentrations (50, 100, 200, 300, 500, 700 and 1000 mg/L), while other experimental parameters were constant. Figure 8 shows that adsorption capacity of Ag+ increases, but the removal efficiency of Ag+ does not increase too much, indicating that the adsorption of Ag+ onto Ni0.5Zn0.5Fe2O4/ASA is related to initial Ag+ concentration. This observation can be described considering the fact that by increasing the initial Ag+ concentration, more Ag+ ions are available, while the amount of active sites on adsorbent is constant, which causes to decrease or remain constant R.
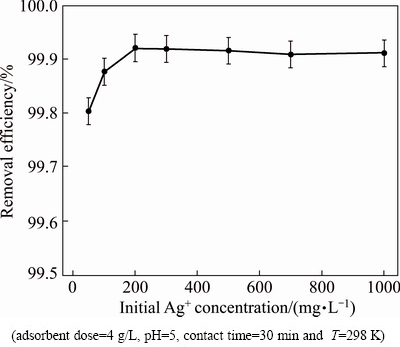
Fig. 8 Effect of initial Ag+ concentration on removal efficiency of Ag+
3.2 Adsorption isotherms
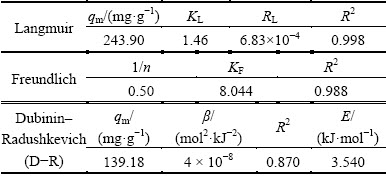
Isotherms study can explain how an adsorbate interacts with adsorbent. The experimental data were corresponded by Langmuir, Freundlich and Dubinin- Radushkevich models as shown in Table 1.
Langmuir isotherm model, which defines a monolayer adsorption, is given in Eq. (3):
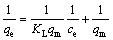 (3)
(3)
where qe is the amount of Ag+ adsorbed per unit mass at equilibrium (mg/g); qm is the maximum amount of adsorbent that can be adsorbed per unit mass adsorbent (mg/g); ce is the concentration of adsorbent (in the solution at equilibrium, mg/L); KL is the adsorption equilibrium constant.
A plot of 1/qe versus 1/ce gives a straight line, with a slope of 1/(KLqm) and intercept of 1/qm.
The main characteristics of the Langmuir isotherm can be expressed in terms of a dimensionless constant separation factor RL that is given by Eq. (4) [38]:
 (4)
(4)
where c0 is the highest initial concentration of adsorbate (mg/L), and KL (L/mg) is the Langmuir constant. The value of RL shows the shape of the isotherm to be either unfavorable (RL>1), linear (RL=1), favorable (0
Freundlich isotherm is expressed by Eq. (5). This isotherm model defines a heterogeneous adsorption with different surface energy sites and supposes the change of uptake with exponential distribution of adsorption sites and energies [39-41].
lg qe=lg KF+1/n lg ce (5)
where ce (mg/L) and qe (mg/g) are the equilibrium concentration of adsorbent in the solution and the amount of adsorbent adsorbed at equilibrium respectively; KF (mg1-1/n·L1/n·g-1) and n are the Freundlich constant which indicate the adsorption capacity for the adsorbent and adsorption intensity, respectively.
A plot of lg qe versus lg ce gives a straight line of slope 1/n and intercept lg KF. The value of 1/n mentions the adsorption intensity and the type of isotherm to be favorable (0.1<1/n<0.5) or unfavorable (1/n>2).
In order to discern between physical and chemical adsorption, the sorption data were analyzed using Dubinin–Radushkevich (D-R) equation, which is given by Eq. (6):
lg qe=ln qm-βε2 (6)
where β is a constant related to the mean energy of adsorption (mol2/kJ2), qm is the maximum adsorption capacity of metal ions (mg/g), ε is the Polanyi potential given by Eq. (7):
ε=RTln(1+1/ce) (7)
where R is the mole gas constant (8.314 J/(mol·K) and T is the temperature (K). By plotting lnqe versus ε2 with experimental data, a straight line is obtained. From the intercept and slope, the values of qm and β are determined. With the value of β, the mean energy E, which is the free energy transfer of 1 mol of solute from infinity to the surface of adsorbent, can be obtained by Eq. (8):
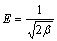 (8)
(8)
For E<8 kJ/mol, the adsorption process might be performed physically, while chemical adsorption when E>8 kJ/mol [42]. All the parameters are listed in Table 2.
Table 2 Langmuir, Freundlich, D-R isotherm constants for adsorption of Ag+ ions onto Ni0.5Zn0.5Fe2O4/ASA
3.3 Mechanism of adsorption
From Table 2, in which the Langmuir, Freundlich, D–R isotherm constants for the adsorption of Ag+ are summarized, it can be derived from R2 that the Langmuir and Freundlich models matched the experimental data better than D-R model. The values can conclude that the maximum adsorption corresponds to a saturated monolayer of adsorbate in plane of the adsorbent surface. To confirm the favorability of the adsorption process, the separation factor (RL) was determined and given in Table 2. In this study, RL value of 6.83×10-4 shows the favorable adsorption between Ni0.5Zn0.5Fe2O4/ASA and Ag+. However, the multilayer adsorption of Ag+ through the adsorption process may be possible. The Freundlich parameter, 1/n, is related to the adsorption intensity of the adsorbent. When 0.1<1/n ≤0.5, the adsorption of the adsorbate is easy; when 0.5<1/n ≤1, there is a difficulty with the adsorption; when 1/n>1, it is quite difficult to adsorb [43,44]. In our study, the value of 1/n (0.50) shows the favorable adsorption of Ag+ on Ni0.5Zn0.5Fe2O4/ASA. Moreover, from the D-R isotherm constants, it is clear that the adsorption of Ag+ by Ni0.5Zn0.5Fe2O4/ASA may be explained as physical adsorption process for the value of E is 3.54 kJ.
3.4 Desorption studies
In metal ion adsorption process, it is significant to easily desorb the adsorbed metal ions under appropriate conditions. In the desorption studies, 0.1 mol/L Na4-EDTA was used as desorption agent. The Ni0.5Zn0.5Fe2O4/ASA samples loaded with the maximum amount of Ag+ ions were placed in desorption medium and the amount of ions desorbed within 1 h measured. Figure 9 shows the data of repeated adsorption/ desorption cycles for Ag+ ions after 5 cycles. The data show that there is a slight decrease in the adsorption capacity of the Ni0.5Zn0.5Fe2O4/ASA with progressing cycles. Therefore, we can conclude that adsorbent, Ni0.5Zn0.5Fe2O4/ASA, can be used repeatedly without significant loss of its adsorption capacity.
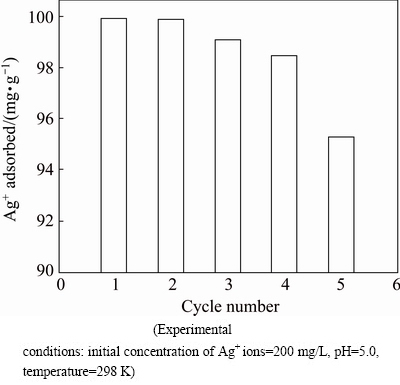
Fig. 9 Adsorption capacity of Ni0.5Zn0.5Fe2O4/ASA to Ag+ ions during repeated adsorption/desorption cycles
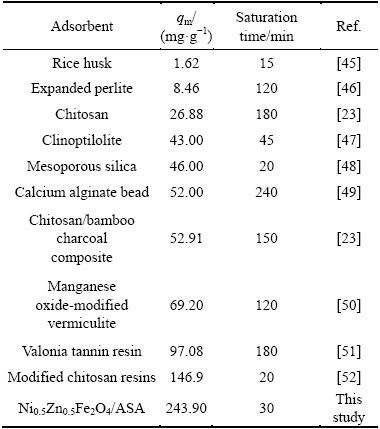
The adsorption capacity is a significant parameter which determines the performance of an adsorbent. Table 3 compares the maximum adsorption capacity of Ni0.5Zn0.5Fe2O4/ASA for Ag+ adsorption with that of other adsorbents in the literature.
Table 3 Maximum adsorption capacity of different adsorbents for Ag+ removal
4 Conclusions
Ni0.5Zn0.5Fe2O4/ASA magnetic nanoparticles were used in adsorption of Ag+ ions from aqueous systems and the maximum Ag+ adsorption occurred in the pH 5 with maximum adsorption capacity of 243.90 mg/g at 25 °C. The adsorption isotherm fitted the Langmuir and Freundlich models well. The prepared magnetic adsorbent can be well dispersed in the aqueous solution and easily separated from the solution with the aid of an external magnet after adsorption. The process of water treatment described here is clean and safe using the magnetic nanoparticles. Thus, this adsorbent was found to be useful and valuable for controlling water pollution due to Ag+ ions.
Acknowledgments
The authors acknowledge the Islamic Azad University-Bandar Abbas Branch for financial support of this study.
References
[1]  Adsorption of heavy metal ions by beech sawdust–kinetics, mechanism and equilibrium of the process [J]. Ecological Engineering, 2013, 58: 202-206.
Adsorption of heavy metal ions by beech sawdust–kinetics, mechanism and equilibrium of the process [J]. Ecological Engineering, 2013, 58: 202-206.
[2] XU M Y, YIN P, LIU X G, TANG Q H, QU R J, XU Q. Utilization of rice husks modified by organomultiphosphonic acids as low-cost biosorbents for enhanced adsorption of heavy metal ions [J]. Bioresource Technology, 2013, 149: 420-424.
[3] SAYGIDEGER S, GULNAZ O, ISTIFLI E S, YUCEL N. Adsorption of Cd (II), Cu (II) and Ni (II) ions by Lemna minor L: Effect of physicochemical environment [J]. Journal of Hazardous Materials, 2005, 126: 96-104.
[4] KALIN M, FYSON A, WHEELER W N. The chemistry of conventional and alternative treatment systems for the neutralization of acid mine drainage [J]. Science of the Total Environment, 2006, 366: 395-408.
[5] YANAGISAWA H, MATSUMOTO Y, MACHIDA M. Adsorption of Zn (II) and Cd (II) ions onto magnesium and activated carbon composite in aqueous solution [J]. Applied Surface Science, 2010, 256: 1619-1623.
[6] ZHANG Ming-liang. Adsorption study of Pb (II), Cu (II) and Zn (II) from simulated acid mine drainage using dairy manure compos [J]. Chemical Engineering Journal, 2011, 172: 361-368.
[7] LUPTAKOVA A, UBALDINI S, MACINGOVA E, FORNARI P, GIULIANO V. Application of physical-chemical and biological–chemical methods for heavy metals removal from acid mine drainage [J]. Process Biochemistry, 2012, 47: 1633-1639.
[8] EL-ASHTOUKHY E S, AMIN N, ABDELWAHAB O. Removal of lead (II) and copper (II) from aqueous solution using pomegranate peel as a new adsorbent [J]. Desalination, 2008, 223: 162-173.
[9] BAYSAL Z. Equilibrium and thermodynamic studies on biosorption of Pb (II) onto Candida albicans biomass [J]. Journal of Hazardous Materials, 2009, 161: 62-67.
[10] SENTHIL KUMAR P. Adsorption isotherms, kinetics and mechanism of Pb (II) ions removal from aqueous solution using chemically modified agricultural waste [J]. The Canadian Journal of Chemical Engineering, 2013, 91: 1950-1956.
[11] ABDEL-HALIM E, AL-DEYAB S S. Removal of heavy metals from their aqueous solutions through adsorption onto natural polymers [J]. Carbohydrate Polymers, 2011, 84: 454-458.
[12] BULGARIU D, BULGARIU L. Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass [J]. Bioresource Technology, 2012, 103: 489-493.
[13] CELIS R, HERMOSIN M C, CORNEJO J. Heavy metal adsorption by functionalized clays [J]. Environmental Science & Technology, 2000, 34: 4593-4599.
[14] JI F. Preparation of cellulose acetate/zeolite composite fiber and its adsorption behavior for heavy metal ions in aqueous solution [J]. Chemical Engineering Journal, 2012, 209: 325-333.
[15] KOBYA M. Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone [J]. Bioresource Technology, 2005, 96: 1518-1521.
[16] SHEN Y, TANG J, NIE Z, WANG Y, REN Y, ZUO L. Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification [J]. Separation and Purification Technology, 2009, 68: 312-319.
[17] CHO D W, JEON B H, CHON C M, KIM Y J, SCHWARTZ F W, LEE E S, SONG H. A novel chitosan/clay/magnetite composite for adsorption of Cu (II) and As (V) [J]. Chemical Engineering Journal, 2012, 200: 654-662.
[18] SHI Guo-long, SUN Bai, JIN Zhen, LIU Jin-hua, LI Min-qiang. Synthesis of SiO2/Fe3O4 nanomaterial and its application as cataluminescence gas sensor material for ether [J]. Sensors and Actuators B: Chemical, 2012, 171: 699-704.
[19] DALLAS P, GEORGAKILAS V, NIARCHOS D, KOMNINOU P, KEHAGIAS T, PETRIDIS D. Synthesis, characterization and thermal properties of polymer/magnetite nanocomposites [J]. Nanotechnology, 2006, 17: 2046.
[20] MOHAN D, SARSWAT A, SINGH VINOD K, ALEXANDRE- FRANCO M, PITTMAN C U. Development of magnetic activated carbon from almond shells for trinitrophenol removal from water [J]. Chemical Engineering Journal, 2011, 172: 1111-1125.
[21] MAK S Y, CHEN D H. Binding and sulfonation of poly (acrylic acid) on iron oxide nanoparticles: A novel, magnetic, strong acid cation nano-dsorbent [J]. Macromolecular Rapid Communications, 2005, 26: 1567-1571.
[22] NITAYAPHAT W, JINTAKOSOL T. Removal of silver (I) from aqueous solutions by chitosan/carbon nanotube nanocomposite beads [J]. Advanced Materials Research, 2014, 893: 166-169.
[23] NITAYAPHAT W, JINTAKOSOL T. Removal of silver (I) from aqueous solutions by chitosan/bamboo charcoal composite beads [J]. Journal of Cleaner Production, 2015, 87: 850-855.
[24] SHARMA S, VERMA K, CHAUBEY U, SINGH V, MEHTA B. Influence of Zn substitution on structural, microstructural and dielectric properties of nanocrystalline nickel ferrites [J]. Materials Science and Engineering: B, 2010, 167: 187-192.
[25] VIRDEN A, O’GRADY K. Structure and magnetic properties of NiZn ferrite nanoparticles [J]. Journal of Magnetism and Magnetic Materials, 2005, 290: 868-870.
[26] MANE S, VANJARA A, SAWANT M. Removal of phenol from wastewater using date seed carbon [J]. Journal of the Chinese Chemical Society, 2005, 52: 1117-1122.
[27] PANDAY K K, PRASAD G, SINGH V. Copper (II) removal from aqueous solutions by fly ash [J]. Water Research, 1985, 19: 869-873.
[28] VAZQUEZ-RIVERA NATALIA I, SOTO-PEREZ L, St JOHN JULIANA N, MOLINA-BAS OMAR I, HWANG S S. Optimization of pervious concrete containing fly ash and iron oxide nanoparticles and its application for phosphorus removal [J]. Construction and Building Materials, 2015, 93: 22-28.
[29] HADDABI M A, AHMED M, AL JEBRI Z A, VUTHALURU H, ZNAD H, KINDI M I. Boron removal from seawater using date palm (Phoenix dactylifera) seed ash [J]. Desalination and Water Treatment, 2016, 57: 5130-5137.
[30] AGRAFIOTI E, KALDERIS D, DIAMADOPOULOS E. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge [J]. Journal of Environmental Management, 2014, 133: 309-314.
[31] BANAT F, AL-ASHEH S, AL-MAKHADMEH L. Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters [J]. Process Biochemistry, 2003, 39: 193-202.
[32] GABAL M A, EL-SHISHTAWY R M, AL ANGARI Y. Structural and magnetic properties of nano-crystalline Ni-Zn ferrites synthesized using egg-white precursor [J]. Journal of Magnetism and Magnetic Materials, 2012, 324: 2258-2264.
[33] POL VILAS G, DAEMEN LUKE L, VOGEL SVEN, CHERTKOV G. Solvent-free fabrication of ferromagnetic Fe3O4 octahedra [J]. Industrial & Engineering Chemistry Research, 2009, 49: 920-924.
[34] REN Yue-ming, WEI Xi-zhu, ZHANG M. Adsorption character for removal Cu (II) by magnetic Cu (II) ion imprinted composite adsorbent [J]. Journal of Hazardous Materials, 2008, 158: 14-22.
[35] ZHOU Yu-ting, NIE Hua-li, BRANFORD-WHITE C, HE Zhi-yan, ZHU L M. Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid [J]. Journal of Colloid and Interface Science, 2009, 330: 29-37.
[36] SHENG Guo-dong, LI Jia-xing, SHAO Da-dong, HU Jun, CHEN Chang-lun, CHEN Yi-xue, WANG X. Adsorption of copper (II) on multiwalled carbon nanotubes in the absence and presence of humic or fulvic acids [J]. Journal of Hazardous Materials, 2010, 178: 333-340.
[37] SALAM M A, MAKKI M M S I, ABDELAAL M Y A. Preparation and characterization of multi-walled carbon nanotubes/chitosan nanocomposite and its application for the removal of heavy metals from aqueous solution [J]. Journal of Alloys and Compounds, 2011, 509: 2582-2587.
[38] HALL KENNETH R, EAGLETON LEE C, ACRIVOS A, VERMEULEN T. Pore-and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions [J]. Industrial & Engineering Chemistry Fundamentals, 1966, 5: 212-223.
[39] CHEN Hao, ZHAO Jie, WU Jun-yong, DAI G. Isotherm, thermodynamic, kinetics and adsorption mechanism studies of methyl orange by surfactant modified silkworm exuviae [J]. Journal of Hazardous Materials, 2011, 192: 246-254.
[40] HOU Mei-fang, MA Cai-xia, ZHANG Wei-de, TANG Xiao-yan, FAN Yan-ning, WAN H F. Removal of rhodamine B using iron-pillared bentonite [J]. Journal of Hazardous Materials, 2011, 186: 1118-1123.
[41] KERKEZ  , BAYAZIT S S. Magnetite decorated multi-walled carbon nanotubes for removal of toxic dyes from aqueous solutions [J]. Journal of Nanoparticle Research, 2014, 16: 1-11.
, BAYAZIT S S. Magnetite decorated multi-walled carbon nanotubes for removal of toxic dyes from aqueous solutions [J]. Journal of Nanoparticle Research, 2014, 16: 1-11.
[42] TAN Yan-qing, CHEN Man, HAO Y. High efficient removal of Pb (II) by amino-functionalized Fe3O4 magnetic nano-particles [J]. Chemical Engineering Journal, 2012, 191: 104-111.
[43] LUO Xiao-gang, ZHANG L. High effective adsorption of organic dyes on magnetic cellulose beads entrapping activated carbon [J]. Journal of Hazardous Materials, 2009, 171: 340-347.
[44] SAMIEE S, GOHARSHADI E K. Graphene nanosheets as efficient adsorbent for an azo dye removal: Kinetic and thermodynamic studies [J]. Journal of Nanoparticle Research, 2014, 16: 1-16.
[45] ZAFAR S, KHALID N, MIRZA M L. Potential of rice husk for the decontamination of silver ions from aqueous media [J]. Separation Science and Technology, 2012, 47: 1793-1801.
[46] GHASSABZADEH H, MOHADESPOUR A. Adsorption of Ag, Cu and Hg from aqueous solutions using expanded perlite [J]. Journal of Hazardous Materials, 2010, 177: 950-955.
[47] AKGUL M, KARABAKAN A, ACAR O, YURUM Y. Removal of silver (I) from aqueous solutions with clinoptilolite [J]. Microporous and Mesoporous Materials, 2006, 94: 99-104.
[48] YANG Hong, XU Ran, XUE Xiao-ming, LI Feng-ting, LI G T. Hybrid surfactant-templated mesoporous silica formed in ethanol and its application for heavy metal removal [J]. Journal of Hazardous Materials, 2008, 152: 690-698.
[49] TORRES E, MATA Y N, BLAZQUEZY M L, MUNOZ J A, GONZALEZ F, BALLESTER A. Gold and silver uptake and nanoprecipitation on calcium alginate beads [J]. Langmuir, 2005, 21: 7951-7958.
[50] SARI A, TUZEN M. Adsorption of silver from aqueous solution onto raw vermiculite and manganese oxide-modified vermiculite [J]. Microporous and Mesoporous Materials, 2013, 170: 155-163.
[51] YURTSEVER M, SENGL A. Adsorption and desorption behavior of silver ions onto valonia tannin resin [J]. Transactions of Nonferrous Metals Society of China, 2012, 22: 2846-2854.
[52] ELWAKEEL KHALID Z, EL-SAYED GAMAL O, DARWEESH R S. Fast and selective removal of silver (I) from aqueous media by modified chitosan resins [J]. International Journal of Mineral Processing, 2013, 120: 26-34.
Parisa BEIGZADEH, Farid MOEINPOUR
Department of Chemistry, Bandar Abbas Branch, Islamic Azad University, Bandar Abbas 7915893144, Iran
摘 要:采用负载Ni0.5Zn0.5Fe2O4磁性纳米粒子芦荟壳灰从水溶液中去除Ag(I)。采用XRD、SEM、BET等温线、振荡试样磁力计(VSM)和傅里叶变换红外光谱(FT-IR)表征该吸附剂。采用该吸附剂在不同pH值(2~7)、吸附剂量(0.01~0.5 g)、Ag+浓度(50, 100, 200, 300, 500, 700 和1000 mg/L)下测定Ag(I)的吸光度。在最佳条件(30 min, pH=5)下,得到最高的Ag+去除率。在50 mL 100 mg/L Ag+溶液中,最佳吸附剂量是0.20 g,去除率为98.3%。基于Langmuir等温线,得到最大单层饱和吸附量为243.90 mg/g。表征结果表明,吸附剂的比表面积和孔体积分别为814.23 m2/g 和 0.726 cm3/g。实验数据与Langmuir和Freundlich等温线模型吻合。合成的吸附剂对水溶液中Ag(I)吸附具有理想的表面积和吸附容量。
关键词:吸附;Ag+离子;Ni0.5Zn0.5Fe2O4; 芦荟
(Edited by Xiang-qun LI)
Corresponding author: Farid Moeinpour; Tel/Fax: +98-761-6670242; E-mail: f.moeinpour@gmail.com; fmoeinpour52@iauba.ac.ir
DOI: 10.1016/S1003-6326(16)64341-8

