生物浸铀中影响嗜酸氧化亚铁硫杆菌活性的氟毒物活性形态
来源期刊:中国有色金属学报(英文版)2013年第3期
论文作者:彭志俊 余润兰 邱冠周 覃文庆 顾帼华 王清良 李 乾 刘学端
文章页码:812 - 817
关键词:氟毒性;生物浸出;铀矿;嗜酸氧化亚铁硫杆菌
Key words:fluorine toxicity; bioleaching; uranium ore; Acidithiobacillus ferrooxidans
摘 要:为了确定浸矿菌耐氟的机制,在氟化物存在的条件下,驯化铀矿浸出菌嗜酸氧化亚铁硫杆菌 ATCC 23270,研究溶液中含不同氟浓度、不同pH值时铀矿浸出菌的活性变化,以及有无蛋白酶K处理时铀矿浸出菌细胞内氟浓度的变化情况。采用铂电极和Ag/AgCl参比电极测量氧化还原电位,以作为细菌不同活性的参照指标,采用氟离子选择性电极测定细胞内的氟浓度。结果表明,真正影响铀矿浸出菌活性的是HF,溶液pH值增加以及溶液中与氟有较强络合能力的离子浓度的变化,也会引起耐氟菌假象的出现。浸矿菌的耐氟能力可能与细胞壁和细胞膜上的一些蛋白密切相关。
Abstract: In order to determine the mechanism of bacterial tolerance to fluorine, Acidithiobacillus ferrooxidans ATCC 23270 was domesticated and studied under the conditions of different fluorine concentrations and pH values with or without treatment by Proteinase K. The bacterial activities were observed through measuring the changes of solution potentials by platinum electrode with Ag/AgCl reference electrode and the intracellular fluorine was determined by ?uorine ion-selective electrode. The results indicated that the tolerance of Acidithiobacillus ferrooxidans ATCC 23270 to fluorine could be obviously improved by domestication, HF was the effective form of fluorine to affect the bacterial activity, and pH increase or concentration change of ions of strong complex ability with fluorine ions in solution could result in false appearance of high fluorine-resistant strain. Some proteins located in cell wall or cell membrane were intimately relative with the bacterial fluorine tolerance.
Trans. Nonferrous Met. Soc. China 23(2013) 812-817
Zhi-jun PENG1,2, Run-lan YU1,2, Guan-zhou QIU1,2, Wen-qing QIN1,2, Guo-hua GU1,2, Qing-liang WANG1,2, Qian LI1,2, Xue-duan LIU1,2
1. School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
2. Key Laboratory of Biometallurgy of Ministry of Education, Central South University, Changsha 410083, China
Received 16 November 2011; accepted 28 April 2012
Abstract: In order to determine the mechanism of bacterial tolerance to fluorine, Acidithiobacillus ferrooxidans ATCC 23270 was domesticated and studied under the conditions of different fluorine concentrations and pH values with or without treatment by Proteinase K. The bacterial activities were observed through measuring the changes of solution potentials by platinum electrode with Ag/AgCl reference electrode and the intracellular fluorine was determined by fluorine ion-selective electrode. The results indicated that the tolerance of Acidithiobacillus ferrooxidans ATCC 23270 to fluorine could be obviously improved by domestication, HF was the effective form of fluorine to affect the bacterial activity, and pH increase or concentration change of ions of strong complex ability with fluorine ions in solution could result in false appearance of high fluorine-resistant strain. Some proteins located in cell wall or cell membrane were intimately relative with the bacterial fluorine tolerance.
Key words: fluorine toxicity; bioleaching; uranium ore; Acidithiobacillus ferrooxidans
1 Introduction
Utilizing low-grade uranium ores is a great challenge with the depletion of high-grade uranium ores and the increasing demand of nuclear energy for uranium material. Conventional acid heap leaching, which has been widely used in uranium mine, requires large amount of H2SO4 acid and also often brings environmental problems [1]. The microbial leaching in uranium industry has many advantages, such as adaptation to low-grade ores, short leaching cycle, relatively low cost and low contamination [2].
Fluorine element, the 13th most abundant element on the earth’s crust [3], is widely present in many ores, such as phosphorite [4], silicate mines [5,6], copper ores [7-9] and uranium ores [10]. Fluorine is one of the great deleterious substances in bioleaching uranium ore. As a strong hydrogen bonding species, fluorine is capable of interacting with most of the cellular components and thus has a plethora of effects on cell metabolism [11]. Many literatures [12-15] reported the potential inhibitory effects of fluorine on these microorganisms, including aquatic organisms [16], wastewater treatment micro- organisms [17] and animal rats microorganisms [18,19]. van LOVEREN et al [15] found that enolase and ATPase activity were influenced at different degrees, and the enolase genes of the fluoride-resistant and fluoride- sensitive strains were identical. It was also reported that the toxicity of fluorine was related to pH value of culture medium [13,14]. HE et al [20] suggested that fluorine absorption from the intestine was less sensitive to pH and maybe occur via a carrier-mediated process (i.e. facilitated diffusion). WILK-BLASZCZAK et al [21] reported the fluorine permeability via anion channels in airway epithelial cells.
However, information about the effect of fluorine toxicity on bioleaching microorganisms was very limited in bioleaching uranium ore. Very low concentration of fluoride could inhibit ferrous- and sulfur-oxidizing bacterium, Acidithiobacillus ferrooxidans [22-24]. SUZUKI et al [25] demonstrated that the growth of Acidithiobacillus thiooxidans on elemental sulphur was totally inhibited by 4.2 mg/L fluoride at pH 2.3, but 840 mg/L NaF did not significantly inhibit the sulphur oxidation rate at pH=7. HF membrane permeability ranged from 10-4 to 10-3 cm/s, 5-7 orders of magnitude higher than F- and H+ [26]. SUNDKVIST et al [27] also speculated that the transport of fluorine through biological cell membranes mainly occurred by passive non-ionic diffusion of the protonated form of fluorine, HF. GUNNERIUSSON et al [28] found that the formation of jarosite reduced the fluorine concentration in solution due to fluorine ions exchange with hydroxide ions in jarosite. LI et al [29] showed that a fluorine-resistant strain was obtained by domestication at different fluorine levels or through ultraviolet radiation.
According to the previous work mentioned above, the reported fluorine concentrations of Acidithiobacillus ferrooxidans tolerance are very different. At the same time, there are many forms of fluorine such as free fluorine ion, HF and complex fluorine. Ions strength also maybe affects bacterial tolerance to fluorine. The mechanism of bacterial fluorine-resistance has still not been explained clearly so far. The objective of this work is trying to further verify and understand which active form of fluorine really affects the activity of Acidithiobacillus ferrooxidans and whether the bacterial tolerance to fluorine is related to cell wall or cell membrane.
2 Experimental
2.1 Bacteria and media
Acidithiobacillus ferrooxidans ATCC 23270 was obtained from Key Laboratory of Biometallurgy of Education Ministry, China. The composition of 9K medium was as follows: 3 g/L (NH4)2SO4, 0.1 g/L KCl, 0.5 g/L K2HPO4, 0.5 g/L MgSO4·7H2O, 0.01 g/L Ca(NO3)2, 25 g/L FeSO4·7H2O.
2.2 Tolerance experiment
Acidithiobacillus ferrooxidans ATCC 23270 was inoculated in 9K medium containing different concentrations of fluorine, 0, 5, 10, 20 and 50 mg/L, respectively. These cultures were incubated in 100 mL 9K medium at pH 2.0 with a shaking speed of 170 r/min at 30 °C. NaF was utilized in these experiments to provide fluorine. The activity of bacteria was determined by measuring solution redox potential.
In order to exclude the effect of Na+ ions on the cells due to the addition of NaF, the cells were also cultured in solution containing 0, 6.05, 12.1, 24.2 and 60.5 mg/L Na+ ions respectively through adding Na2SO4 as control, because sulphate ions have the smallest detrimental effect on bioleaching bacteria [8].
2.3 Domestication experiment
The experiments were carried out to domesticate a higher tolerant strain through gradual addition of fluorine in the media. First, Acidithiobacillus ferrooxidans ATCC 23270 was inoculated and incubated in a series of fresh media containing 0, 10, 20, 30, 40, 50 and 60 mg/L fluorine, respectively. The redox potentials of these solutions were measured by platinum electrode and Ag/AgCl reference electrode every day. When the solution potentials reached about 600 mV (vs Ag/AgCl), the ferrous ions in the medium were thought to be almost completely oxidized. Then, the culture was inoculated and incubated again in the solution containing higher concentration of fluorine. Finally, a new higher fluorine-resistant strain was obtained, and named as Frs.
2.4 pH effect experiment
In order to exclude the effect of pH, Frs was first inoculated in 9K media with different pH values containing no fluorine. And then, to decide the active form of fluorine on bacterial tolerance, Frs was inoculated in 9K medium containing 40 mg/L fluorine at pH values of 1.5 and 2.0, respectively. Last, Frs was inoculated in 9K medium containing 50 mg/L fluorine at pH values of 2.0 and 2.5, respectively. Their redox potentials were measured to observe the effect of different pH values. Free fluorine ions in solutions were analysed using a fluoride ion-selective electrode (PF-1, Shanghai Constant Magnetic Electronic Technology Company, China) [30].
2.5 Intracellular fluorine experiment
For the purpose of measurement of intracellular fluorine, Frs was cultured in pH=2.0 9K medium containing 40 mg/L fluorine up to later logarithmic phase. Partial cells were harvested from the culture medium by filtering and centrifugation at 10000 r/min for 20 min, and the pellets were suspended again in distilled water. Then cells were concentrated again by centrifugation at 12000 r/min for 20 min, and the suspension and centrifugation procedures were repeated three times. At last, the pellets were diluted again to about 1010 cell/mL with disruption buffer (50 μL of 7.5 mol/L urea, 2.5 mol/L thiourea, 1.25 mmol/L EDTA, 1.75 g/L Pepstatin A, protease inhibitor cocktail) [31] and then were smashed by ultrasonic wave. Last, intracellular fluorine was determined by fluoride ion-selective electrode.
Other partial cells were incubated continuously to stationary phase, and then intracellular fluorine was determined similarly.
With the aim to measure the intracellular fluorine concentration of treated Frs by Proteinase K, the same procedure was proceeded as above mentioned. Just those cells, before incubated continuously to stationary phase, were treated with Proteinase K at later logarithmic phase for 4 h at 37 °C [32].
In order to test the activity of treated Frs, it was inoculated respectively into two kinds of 9K media, one without fluorine and another containing 40 mg/L fluorine. A control test was also carried out by inoculating non-treated cells into 40 mg/L fluorine medium. Finally, the activities of cells were observed.
3 Results and discussion
3.1 Tolerance of Acidithiobacillus ferrooxidans ATCC 23270 to fluorine
The influence of fluorine on the growth of Acidithiobacillus ferrooxidans ATCC 23270 is presented in Fig. 1. It shows that fluorine has an apparently negative effect on the strain. There was no obvious effect on the strain at 5 mg/L fluorine. The apparent inhibition was found when the concentration of fluorine increased to 10 mg/L, and the growth of the cells was very slow when the total fluorine was over 20 mg/L. However, this result was not consistent with the result of LIU et al [33], who got a very high fluorine-resistant strain (1.48 g/L). The reason may be that the strain had adapted for a long time in acid mine drainage containing a great deal of fluorine. However, the strain Acidithiobacillus ferrooxidans ATCC 23270 here was conserved in our laboratory with very low fluorine-resistance capability.
The result of Na+ control test is shown in Fig. 2. It shows that the strain grew well even though Na+ concentration was 60.5 mg/L. The result indicates that the toxic action mainly results from fluorine but not Na+ ions.
3.2 Bacterial domestication and its tolerance in solution containing fluorine
As seen from Table 1, for Acidithiobacillus ferrooxidans ATCC 23270, it needed about 4 d when the solution potential was up to about 600 mV in 10 mg/L fluorine medium, while it needed only 1 d after the first domestication. It is indicated that the tolerance of Acidithiobacillus ferrooxidans ATCC 23270 to fluorine could be obviously improved by adaptation for a long time. At last, a strain (i.e. Frs) was obtained, which could resist fluorine up to 40 mg/L.
3.3 Effect of fluorine on Frs activity at different pH values
The effect of pH or Frs activity is shown in Fig. 3. It is indicated that pH value just has a little influence on the Frs growth.
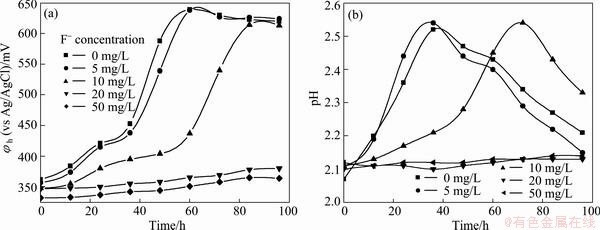
Fig. 1 Effect of fluorine concentration on growth of Acidithiobacillus ferrooxidans ATCC 23270
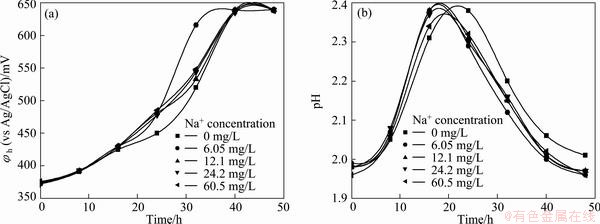
Fig. 2 Influence of Na+ concentration on growth of Acidithiobacillus ferrooxidans ATCC 23270
Table 1 Domestication of Acidithiobacillus ferrooxidans ATCC 23270
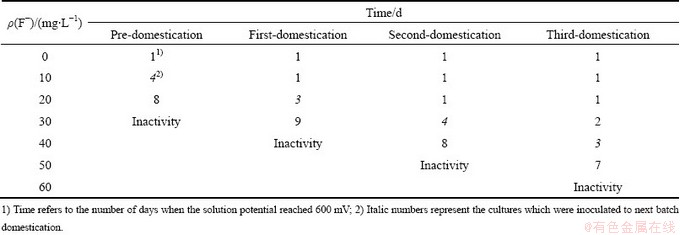
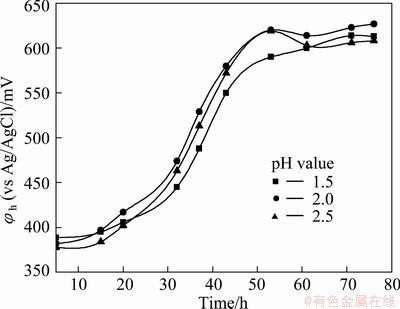
Fig. 3 Effect of pH on Frs activity
The growth of Frs incubated in the media with different concentrations of fluorine and pH values is shown in Fig. 4. The result was quite different from Fig. 3. It is indicated that the great difference results from the fluorine effect rather than pH change. In 9K media containing 50 mg/L fluorine, Frs could almost not grow at pH 2.0 but grow well at pH 2.5. Similarly, Frs actually could not survive at pH 1.5 in 40 mg/L fluorine media just because of the change of pH. So it could be inferred that HF should be active form of fluoride which affects the bacterial activity and pH value plays an important role in the active form of fluorine
There are many forms of fluorine in leaching solution, such as free fluorine ions, complex fluorine and HF. HF concentration can be calculated from the Henderson-Hasselbalch equation [11]:
HF H++F-, (1)
H++F-, (1)
pH=pKa+lg[c(F-)/c(HF)] (2)
6F-+Fe3+ (FeF6)3-, (3)
(FeF6)3-, (3)
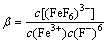 (4)
(4)
c(F)=c(HF)+c(F-)+6c(C) (5)
where c(F) is the total concentration of fluorine, c(HF) is the equilibrium concentration of HF, c(F-) is the equilibrium concentration of F- ions, c(C) is the equilibrium concentration of complex ions, and β is the stable constant of complex ions.
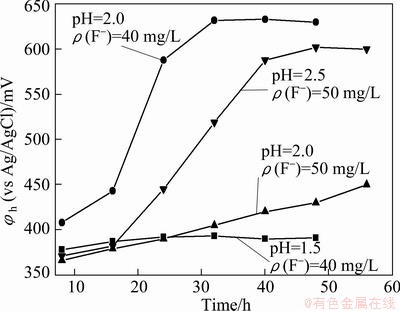
Fig. 4 Effect of fluorine on Frs activity at different pH values
In the case of same total fluorine concentration, according to Eqs. (1)-(5), the equilibrium concentration of HF at pH 2.0 must be greater than that at pH 2.5. The concentration of HF is lower as a result of higher pH, leading to the reduction of fluorine influence on the strain. Meanwhile, when pH value of the solution becomes higher, the capability of ferric ions complexing with F- also is enhanced [27]. According to the result of Fig. 4, it is suggested that the really active form or inhibitory substance is HF. Hence, pH value increase or concentration change of ions of strong complex ability with fluorine ions in solution could result in false appearance of high fluorine-resist strain.
3.4 Intracellular fluorine
The change of intracellular fluorine concentration is presented in Fig. 5. Without Proteinase K treatment, the concentration of fluorine was just 1.41 mg/L in stationary phase and it was 5.3 mg/L in logarithmic phase. In contrast, after Proteinase K treatment, it was 6.2 mg/L and 9.9 mg/L, respectively.
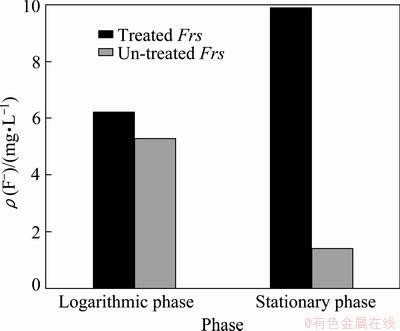
Fig. 5 Intracellular fluorine concentration at different phases
The reason may be that fluorine traverses the membrane likely by assistant diffusion [34], which needed some proteins to help to accomplish the transport course. The treatment of Proteinase K would destroy part of outer membrane proteins involved in free fluorine ion’s exclusion at neutral conditions. Hence, when some kinds of proteins were damaged, intracellular fluorine increased.
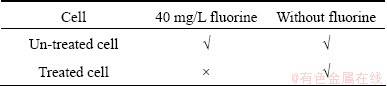
Frs treated by Proteinase K was inoculated again into 9K medium without fluorine and with 40 mg/L fluorine, respectively. The results are shown in Table 2, in which “√” means that the cells can survive, in contrast, “×” means not. Survivorship here was defined as the ability to raise solution redox potential to about 600 mV in 3 d. It shows that Frs could not survive normally in solution containing fluorine. However, the ability of growth in 9K medium without fluorine demonstrates that the treated Frs was still active. These results indicate that the tolerance of Frs to fluorine maybe was related to cell wall or cell membrane proteins, and it is necessary to clarify what proteins of cell wall or membrane are related to the tolerance of the cells to fluorine in the next work.
Table 2 Treated and un-treated cells inoculated in different media
4 Conclusions
1) The tolerance of Acidithiobacillus ferrooxidans ATCC 23270 to fluorine could be improved by domestication.
2) HF was the effective form of fluorine to affect the bacterial activity, pH increase or concentration change of ions of strong complex ability with fluorine ions in solution could result in false appearance of high fluorine-resistant strain.
3) Some proteins of cell wall or cell membrane were intimately relative with the bacterial fluorine tolerance.
References
[1] WANG Xue-gang, LI Jiang, SUN Zhan-xue, LIU Ya-jie. An industrial heap bioleaching of fluorine-bearing uranium ore [C]// QIU Guan-zhou, LIU Jian-she, LIU Xue-duan, YANG Yu. The 19th International Biohydrometallurgy Symposium. Changsha, China: Central South University Press, 2011: 601-603.
[2] WU Wei-rong, ZHENG Zhi-hong, LIU Jin-hui, HE Xiao-yu, GU Shi-fei. Study on thiobacillus thiooxidans domestication of fluorine enduring [J]. Nonferrous Metals: Extractive Metallurgy, 2007(3): 38-40. (in Chinese)
[3] MASON B, MOORE C B. Principles of geochemistry [M]. New York: John Willey & Sons, 1982.
[4] CHEN Mao-chun, LIANG Bin, ZHANG Yong-kui. The effect of fluoride ions to thiobacillus thiooxidans [J]. Sichuan Chemical Industry and Corrosion Control, 2001, 4(2): 14-17. (in Chinese)
[5] DOPSON M, HALINEN A K, RAHRMEN N,  D, SUNDKVIST J E, RIEKKOLA-VANHANEN M, KAKSONEN A H, PUHAKKA J A. Silicate mineral dissolution during heap bioleaching [J]. Biotechnology and Bioengineering, 2008, 99(4): 811-820.
D, SUNDKVIST J E, RIEKKOLA-VANHANEN M, KAKSONEN A H, PUHAKKA J A. Silicate mineral dissolution during heap bioleaching [J]. Biotechnology and Bioengineering, 2008, 99(4): 811-820.
[6] DOPSON M, VGREN L L,  D. Silicate mineral dissolution in the presence of acidophilic microorganisms: Implications for heap bioleaching [J]. Hydrometallurgy, 2009, 96(4): 288-293.
D. Silicate mineral dissolution in the presence of acidophilic microorganisms: Implications for heap bioleaching [J]. Hydrometallurgy, 2009, 96(4): 288-293.
[7] BRIERLEY J A, KUHN M C. From laboratory to application heap bioleach or not [C]//DONATI E R, VIERA M R, TAVANI E L, GIAVENO M A, LAVALLE T L, CHIACCHIARINI P A. The 18th International Biohydrometallurgy Symposium. Bariloche, Argentia: Lulea Tekniska Universitet Press, 2009: 311-317.
[8] BRIERLEY J A, KUHN M C. Fluoride toxicity in a chalcocite bioleach heap process [J]. Hydrometallurgy, 2010, 104(3-4): 410-413.
[9] SICUPIRA L, VELOSO T, REIS F,  V. Assessing metal recovery from low-grade copper ores containing fluoride [J]. Hydrometallurgy, 2011, 109(3-4): 202-210.
V. Assessing metal recovery from low-grade copper ores containing fluoride [J]. Hydrometallurgy, 2011, 109(3-4): 202-210.
[10] LIU Jin-hui, WU Wei-rong, LIU Ya-jie, SUN Zhan-xue. Study on the fluorine resistance of thiobacillus thiooxidans in uranium leaching [J]. Metal Mine, 2009, 395: 50-53. (in Chinese)
[11] BHATNAGAR M, BHATNAGAR A. Algal and cyanobacterial responses to fluoride [J]. Fluoride, 2000, 33(2): 55-65.
[12] BUNICK F J, KASHKET S. Enolases from fluoride-sensitive and fluoride-resistant streptococci [J]. Infection and Immunity, 1981, 34(3): 856-863.
[13] KASHKET S, KASHKET E R. Dissipation of the proton motive force in oral streptococci by fluoride [J]. Infection and Immunity, 1985, 48(1): 19-22.
[14] LESHER R J, BENDER G R, MARQUIS R E. Bacteriolytic action of fluoride ions [J]. Antimicrobial Agents and Chemotherapy, 1997, 12: 339-345.
[15] van LOVEREN C, HOOGENKAMP M A, DENG D M, TEN CATE J M. Effects of different kinds of fluorides on enolase and atpase activity of a fluoride-sensitive and fluoride-resistant streptococcus mutans strain [J]. Caries Research, 2008, 42(6): 429-434.
[16] CAMARGO J A. Fluoride toxicity to aquatic organisms: A review [J]. Chemosphere, 2003, 50: 251-264.
[17] OCHOA-HERRERA V, BANIHANI Q,  G, KHATRI C, FIELD J A, SIERRA-ALVAREZ R. Toxicity of fluoride to microorganisms in biological wastewater treatment systems [J]. Water Research, 2009, 43(13): 3177-3186.
G, KHATRI C, FIELD J A, SIERRA-ALVAREZ R. Toxicity of fluoride to microorganisms in biological wastewater treatment systems [J]. Water Research, 2009, 43(13): 3177-3186.
[18] REYNOLDS K E, WHITFORD G M, PASHLEY D H. Acute fluoride toxicity: The influence of acid-base status [J]. Toxicology and Applied Pharmacology, 1978, 45: 415-427.
[19] BRUSSOCK S M, KRAL T A. Effects of pH on expression of sodium fluoride resistance in streptococcus mutans [J]. Journal of Dental Research, 1987, 66(10): 1594-1596.
[20] HE H, GANAPATHY V, ISALES C M, WHITFORD G M. pH-dependent fluoride transport in intestinal brush border membrane vesicles [J]. Biochim Biophys Acta, 1998, 1372: 244-254.
[21] WILK-BLASZCZAK M A, FRENCH A S, MAN S F. Halide permeation through 10 pS and 20 pS anion channels in human airway epithelial cells [J]. Biochimica Et Biophysica Acta, 1992, 1104(1): 160-166.
[22] RAZZELL W E, TRUSSELL P C. Isolation and properties of an iron-oxidizing thiobacillus [J]. Bacteriol J, 1963, 85: 595-603.
[23] SCHIPPERS A, von REGE H, SAND W. Impact of microbial diversity and sulfur chemistry on safeguarding sulfidic mine waste [J]. Minerals Engineering, 1996, 9(10): 1069-1079.
[24] HE Xiao-yu, LIU Ya-jie, WU Wei-rong, XU Ling-ling. Effect of Na+, F- and shear force on a moderately thermoacidophilic iron-oxidizing bacterial strain [J]. Nonferrous Metal: Extractive Metallurgy, 2006(5): 4. (in Chinese)
[25] SUZUKI I, LEE D, MACKAY B, HARAHUC L, OH J K. Effect of various ions, pH, and osmotic pressure on oxidation of elemental sulfur by thiobacillus thiooxidans [J]. Applied and Environmental Microbiology, 1999, 65(11): 5163-5168.
[26] GUTKNECHT J, WALTER A. Hydrofluoric and nitric acid transport through lipid bilayer membranes [J]. Biochim Biophys Acta, 1981, 644(1): 153-156.
[27] SUNDKVIST J E,  GUNNERIUSSON L, RJE
GUNNERIUSSON L, RJE  E B. Fluorine toxicity in bioleaching systems [C]//HARRISON S T L, RAWLINGS D E, PETERSEN J. The 16th International Biohydrometallurgy Symposium. Cape Town, South Africa, 2005: 19-28.
E B. Fluorine toxicity in bioleaching systems [C]//HARRISON S T L, RAWLINGS D E, PETERSEN J. The 16th International Biohydrometallurgy Symposium. Cape Town, South Africa, 2005: 19-28.
[28] GUNNERIUSSON L,  , HOLMGREN A, KUZMANN E, KOVACS K, RTES A V. Jarosite inclusion of fluoride and its potential significance to bioleaching of sulphide minerals [J]. Hydrometallurgy, 2009, 96(1-2): 108-116.
, HOLMGREN A, KUZMANN E, KOVACS K, RTES A V. Jarosite inclusion of fluoride and its potential significance to bioleaching of sulphide minerals [J]. Hydrometallurgy, 2009, 96(1-2): 108-116.
[29] LI Guang-yue, LIU Yu-long, DING De-xin, WANG You-tuan, WANG Yong-dong, NIE Xiao-qin, HU Nan. Studies on ultraviolet mutation of thiobacillus ferrooxidans [J]. Uranium Mining and Metallurgy, 2009, 28(2): 78-81. (in Chinese)
[30] XU Chun-yan, HUANG Ji-zu. A research of measurement of ions concentration with pH meter [J]. Journal of North China Institute of Technology, 1997, 18(3): 271-273. (in Chinese)
[31] HE Zhi-guo, ZHONG Hui, LI Qing-hua, GU Guo-hua, HU Yue-hua, LI Gui-yuan. Research on the proteome response of Acidithiobacillus ferrooxidans to phosphate starvation by SELDI-Protein chip technologies [J]. Progess in Biochemistry and Biophysies, 2008, 35(1): 77-84.
[32] XIA Le-xian. Function of sulfur metabolism of thiobacillus in the bioleaching of sulfide minerals and properties of key sulfur-oxidizing enzymes [D]. Changsha: Central South University, 2008. (in Chinese)
[33] LIU Ya-jie, LI Jiang, NIU Jian-guo, LI Xue-li, SHI Huai-jun, LIU Yan, HE Xiao-yu. Influence of fluorine ion to iron-sulfur oxidizing bacteria in the uranium bioleaching [J]. Non-Ferrous Mining and Metallurgy, 2006, 22(2): 18-21. (in Chinese)
[34] BARBIER O, ARREOLA-MENDOZA L,  DEL RAZO L. Molecular mechanisms of fluoride toxicity [J]. Chemico-Biological Interactions, 2010, 188(2): 319-333.
DEL RAZO L. Molecular mechanisms of fluoride toxicity [J]. Chemico-Biological Interactions, 2010, 188(2): 319-333.
彭志俊1,2,余润兰1,2,邱冠周1,2,覃文庆1,2, 顾帼华1,2, 王清良1,2, 李 乾1,2, 刘学端1,2
1. 中南大学 资源加工与生物工程学院,长沙 410083;
2. 中南大学 教育部生物冶金重点实验室,长沙 410083
摘 要:为了确定浸矿菌耐氟的机制,在氟化物存在的条件下,驯化铀矿浸出菌嗜酸氧化亚铁硫杆菌 ATCC 23270,研究溶液中含不同氟浓度、不同pH值时铀矿浸出菌的活性变化,以及有无蛋白酶K处理时铀矿浸出菌细胞内氟浓度的变化情况。采用铂电极和Ag/AgCl参比电极测量氧化还原电位,以作为细菌不同活性的参照指标,采用氟离子选择性电极测定细胞内的氟浓度。结果表明,真正影响铀矿浸出菌活性的是HF,溶液pH值增加以及溶液中与氟有较强络合能力的离子浓度的变化,也会引起耐氟菌假象的出现。浸矿菌的耐氟能力可能与细胞壁和细胞膜上的一些蛋白密切相关。
关键词:氟毒性;生物浸出;铀矿;嗜酸氧化亚铁硫杆菌
(Edited by Sai-qian YUAN)
Foundation item: Project (2010CB630903) supported by the National Basic Research Program of China
Corresponding author: Run-lan YU; Tel: +86-731-88836943; E-mail: yrl715@sina.com.cn
DOI: 10.1016/S1003-6326(13)62533-9

