J. Cent. South Univ. Technol. (2009) 16: 0434-0439
DOI: 10.1007/s11771-009-0073-8
Hybrid supercapacitor based on polyaniline doped with lithium salt and activated carbon electrodes
FANG Jing(方 静), CUI Mu(崔 沐), LU Hai(卢 海), ZHANG Zhi-an(张治安),
LAI Yan-qing(赖延清), LI Jie(李 劼)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract: Polyaniline(PANI) nanofiber was synthesized by interfacial polymerization utilizing the interface between HCl and CCl4. The hybrid type supercapacitors (PLi/C) based on Li-doping polyaniline and activated carbon electrode were fabricated and compared with the redox type capacitors (PLi/PLi) based on two uniformly Li-doping polyaniline electrodes. The electrochemical performances of the two types of supercapacitors were characterized in non-aqueous electrolyte. PLi/C supercapacitors have a wider effective energy storage potential range and a higher upper potential. At the same time, the PLi/C supercapacitor exhibits a specific capacity of 120.93 F/g at initial discharge and retains 80% after 500 cycles. The ohmic internal resistance (RES) of PLi/C supercapacitor is 5.0 Ω, which is smaller than that of PLi/PLi capacitor (5.5 Ω). Moreover, it can be seen that Et4NBF4 organic solution is more suitable for using as organic electrolyte of PLi/C capacitor compared with organic solution containing LiPF6.
Key words: polyaniline; Li salt; hybrid supercapacitor; conducting polymer; doping
1 Introduction
Supercapacitor is a kind of new storage energy devices that can provide higher specific power and longer cycle life than batteries, and higher specific energy than conventional dielectric capacitors because of the high specific capacitance of its electrode materials [1-3]. Recent interests in supercapacitors have been stimulated by their potential applications such as power storage devices operating in parallel with batteries in hybrid electric and electric vehicles [4-6].
Two types of superapacitors with different energy storage mechanisms are under study currently: the electric double layer capacitor (EDLC) and the redox supercapacitor. The capacitance of the EDLC arises from the separation of electronic and ionic charges at the interface between high specific area carbon electrodes and an aqueous or organic electrolyte [7-8]. The capacitance of redox supercapacitor arises from the Faradic charge transfer taking place at the electrode materials like lithium rechargeable battery. Transition metal oxides such as ruthenium oxide and conducting polymers, such as polyaniline, polypyrrole, and polythiophene, can be used as electrode materials for redox supercapacitor [9-11].
Among the conducting polymers, polyaniline has attracted much attention, because of its desirable chemical stability, good conductivity and high Faradic pseudo capacitance, as well as low cost [12-13].
Long-term stability during cycling and high power density are major demands for supercapacitors used in hybrid electric vehicles and electric vehicles. However, it is well known that polyaniline requires the insertion/deinsertion of counter ions, which is related with the cycle life of polyaniline capacitors [14]. And the capacitors based on two polyaniline electrodes show poor electrochemical properties at a higher discharge current, because of their large discharge voltage drop [15]. Therefore, it is necessary to utilize the composite structures of polyaniline and carbon. The capacitors possessing advantages of both electric double layers capacitance and Faradic pseudo capacitance can exhibit long cycle life and large power density but less specific capacitance loss [16-18].
In this work, the hybrid type supercapacitors (PLi/C) based on Li-doping polyaniline and activated carbon electrode were fabricated and compared with the redox type supercapacitor (PLi/PLi). Electrochemical measurements were also carried out in order to evaluate and compare the electrochemical properties of these two types of supercapacitors.
2 Experimental
2.1 Preparation of material
Aniline of 0.02 mol was dissolved in 100 mL CCl4, and this solution is named as A. Ammonium persulfate
of 1.14 g was dissolved in a concentration of 1.0 mol/L HCl solution of 100 mL and this solution is named as B. Then, the two solutions were carefully transferred to a beaker in turn, generating an interface between the two layers. After 1-3 min, green polyaniline was formed at the interface and then diffused gradually toward the aqueous phase. The whole reaction system was kept still at 25 ℃ for 5 h. Then the aqueous phase was filtered, and the obtained product was washed by water and acetone several times until the filtered liquid became colorless. The PANI-HCl powder was dried in vacuum oven at 40 ℃, then dropped into 1 mol/L ammonia. The solution was stirred for 24 h. Then the PANI-EB powder was washed by water and acetone several times and dried at 60 ℃ in vacuum oven. The PANI-EB powder was immersed in 1 mol/L LiPF6 solution at volume ratio of EC to EMC to DMC of 1?1?1 for 72 h in a glove box under Ar atmosphere. After that, the doped powder was washed by acetone and dried at 60 ℃ under vacuum. Finally PANI-LiPF6(PLi) powder was obtained.
Activated carbon powder was prepared in our laboratory. With coal-tar pitch as raw material, KOH and CO2 as activated agents, activated carbon was prepared by chem-physical activation. The properties of activated carbon were shown as follows: specific surface area is 1 905 m2/g; total pore volume is 1.059 cm3/g; mesopore volume is 0.211 4 cm3/g; ratio of mesopore is 19.96%; and average pore diameter is 2.224 nm.
2.2 Preparation of electrodes
For the preparation of PLi electrode sheets, the PLi powder was mixed with 10% (mass fraction) of carbon black as a conductor, and 20% of polyvinylidene fluoride (PVDF) as a binder, which were dissolved in N-methyl- 2-pyrrolidone (NMP) to obtain slurry. For the preparation of activated carbon electrode sheets, the activated carbon powder was mixed with carbon black and PVDF by the same proportion, which was dissolved in NMP to obtain slurry. These two kinds of viscous slurry were laminated on 20 μm-thickness aluminum foil using a doctor blade apparatus respectively, and dried in a vacuum oven at 60 ℃ for 12 h. These electrode sheets were cut into circle with an area of 0.785 cm2.
2.3 Fabrication of supercapacitor
The redox-type supercapacitor (PLi/PLi) consisted of two PLi electrodes, a separator of porous polyethylene film (Celgard 2300), and an electrolyte of 1 mol/L LiPF6 solution in EC/EMC/DMC(volume ratio of 1?1?1). For the hybrid type (PLi/C), an asymmetric-type supercapacitor was prepared with PLi and activated carbon electrodes as positive and negative electrodes, respectively, a separator of porous polyethylene film (Celgard 2300) and an electrolyte of 1 mol/L LiPF6 solution in EC/EMC/DMC(volume ratio of 1?1?1) or 1 mol/L Et4NBF4 in PC solution. Electrode, separator, and electrolyte solution were assembled in sequence as a sandwich and enveloped in a glove box.
All electrochemical characterization was performed in the two-electrode system. Constant-current charge- discharge and cycle life measurements were performed on a CT2001A-5V rapid sampling battery testing system (LAND, China) at room temperature. Cyclic voltammograms and alternating current (AC) impedance measurement were performed in the Potentionsat M273A and 5210 model (EG&G Princeton Applied Research, USA). The electrochemical impedance spectroscopy was recorded in the range from 20 kHz to 2 mHz.
3 Results and discussion
3.1 Cyclic voltammetery
To identify the oxidation and reduction potentials and electrochemical reactions of the cells, cyclic voltammetery was performed for the PLi/C and PLi/PLi supercapacitors. Cyclic voltammograms of the PLi/C and PLi/PLi supercapacitors in different potential ranges are shown in Fig.1. In the range of 0-1.0 V, the current—
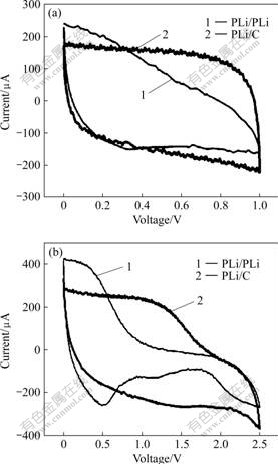
Fig.1 Cyclic voltammograms of supercapacitors composed of PLi/PLi and PLi/C electrodes at scan rate of 5 mV/s in different ranges of effective energy storage potential: (a) 0-1.0 V; (b) 0-2.5 V
voltage(CV) curve of PLi/C shows more rectangular-like shape than that of PLi/PLi, as shown in Fig.1(a), which indicates that charge and discharge processes of PLi/C occur more reversibly.
Fig.1(b) shows the CV curves of PLi/C and PLi/PLi supercapacitors in the range of 0-2.5 V. It can be seen that when the cut-off voltage approaches 2.0 V, PLi/PLi supercapacitor suffers from the oxidation of electrolyte, electrode, and other compositions. However, this phenomenon cannot be found in PLi/C supercapacitor until the cut-off voltage approaches 2.5 V. This shows that PLi/C supercapacitor can work in larger potential range than PLi/PLi supercapacitor. In addition, for PLi/PLi supercapacitor, the scanning area is relatively narrow in the range of 1.0-2.5 V, indicating that the capacitance is small in this potential range and contributes a little to the total capacitance. For PLi/C supercapacitor, however, the capacitance contribution to the total capacitance is relatively large in the range of 1.0-2.5 V. So the electrochemical performance of PLi/C supercapacitor is better than that of PLi/PLi supercapacitor in a high potential range.
3.2 Electrochemical impedance spectroscopy
The technique of AC impedance spectroscopy can be used to measure the resistance associated with the specific power of PLi/PLi and PLi/C supercapacitors. Impedance spectra of these cells are shown in Fig.2. Fig.2 shows that the spectra are almost similar, which consist of a distorted semicircle in the high-frequency region due to the porosity of the electrode and a linear part at the low frequency end due to the diffusion- controlled doping and dedoping of anions that are resulted from Warburg behavior. The high frequency intercept of the semi-circle on the real axis gives the ohmic internal resistance (RES) of the cell for the sum of resistance of the electrolyte solution, the intrinsic resistance of the active material, and the contact resistance at the interface of active material/current collector. And the semicircle in the high-frequency region is related to the charge-transfer resistance of the electrode and electrolyte interface in the newly assembled supercapacitors, while the diameter provides the charge transfer resistance (Rct) of the cell. The RES values of PLi/C and PLi/PLi supercapacitors are 5.0 and 5.5 Ω, respectively. And Rct is 9.0 Ω for PLi/C supercapacitor and 11.0 Ω for PLi/PLi supercapacitor. The RES and the RCT of PLi/C supercapacitor are smaller than those of PLi/PLi supercapacitor.

Fig.2 Impedance spectra of supercapacitors composed of PLi/C and PLi/PLi electrodes
3.3 Charge-discharge characteristics
The charge-discharge behaviors of PLi/C and PLi/PLi supercapacitors were examined by chronopotentiometry. Figs.3(a) and (b) show the charge- discharge curves of PLi/PLi and PLi/C supercapacitors in the range of 0-1.0 V and 0-2.5 V, respectively. In Fig.3(a), the charge-discharge curves of both of supercapacitors types are nearly triangle, indicating that both types of supercapacitors exhibit good charge-discharge characteristics under constant current in the range of 0-1.0 V. In Fig.3(b), the discharge curve of PLi/PLi supercapacitor between 2.5 and 1.0 V decreases
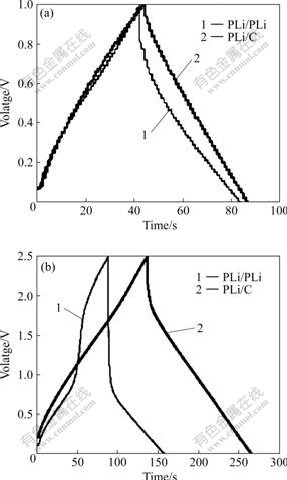
Fig.3 Charge-discharge curves of supercapacitors composed of PLi/PLi and PLi/C electrodes in different effective energy storage potential ranges: (a) 0-1.0 V; (b) 0-2.5 V
abruptly. That is, the capacitance between 2.5 and 1.0 V is small and negligible, and contributes little to the total capacitance, which is in a good agreement with the result of cyclic voltammetry. The specific capacitance of PLi/C supercapacitor is 91.88 F/g in the range of 0-1.0 V and 120.93 F/g in the range of 0-2.5 V. However, the specific capacitance of PLi/PLi supercapacitor is 128 F/g in the range of 0-1.0 V and 118 F/g in the range of 0-2.5 V. On the other hand, for PLi/C supercapacitor, the discharge curve decreases abruptly in the range of 2.0-2.5 V, but changes smoothly in the range of 0-2.0 V. It can be concluded that PLi/C supercapacitor is preferred to have a wider effective energy storage potential range and a higher upper potential, compared with PLi/PLi supercapacitor.
The energy density (E) of the supercapacitors is
calculated according to E=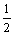 CU2, where C is the specific
CU2, where C is the specific
discharge capacitance, and U is the work voltage. Because the upper potential of PLi/C supercapacitor increases up to 2.5 V, which is larger than that of PLi/PLi supercapacitor, its energy density is larger than that of PLi/PLi supercapacitor.
3.4 Cycle life
Since long cycle life is very important for the electrode materials to be used as the supercapacitors, the cycle charge-discharge test was conducted to examine the service life of PLi/PLi and PLi/C supercapacitors. Fig.4 shows the specific discharge capacitances of those cells as a function of cycle numbers. The specific capacitances of both types supercapacitors are almost equal at the initial circle. After 500 cycles, the capacitance of PLi/C supercapacitor remains 80%, much higher than that of PLi/PLi supercapacitor that remains 70%. These results reveal that the activated carbon electrode can improve the cycle life of PLi/C supercapacitor, because the capacitor behavior of the
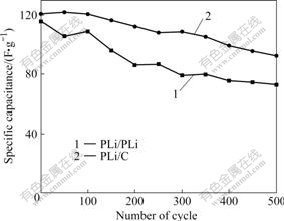
Fig.4 Cycle life characteristics of supercapacitors composed of PLi/PLi and PLi/C electrodes
activated carbon electrode is likely attributed more by double layer capacitance arising from physical separation of electronic and ionic changes at interface between electrode and electrolyte.
3.5 Choice of non-aqueous electrolyte
The properties of supercapacitor, such as capacitance and resistance, are all related to the type of non-aqueous electrolyte. The charge-discharge curves of PLi/C supercapacitors using 1 mol/L LiPF6 in EC/EMC/ DMC solution and 1 mol/L Et4NBF4 in PC solution as electrolytes were evaluated (see Fig.5). As shown in Fig.5(a), although using different types of electrolytes, the charge-discharge curve of PLi/C supercapacitor using 1 mol/L LiPF6 as electrolyte is in a good agreement with that of using Et4NBF4 as electrolyte. And the specific capacitance of the PLi electrode using LiPF6 as electrolyte is 120.93 F/g, which is almost equal to that of using Et4NBF4/PC as electrolyte(119.39 F/g).
Discharge curve of PLi/C supercapacitor using Et4NBF4 in PC solution as electrolyte changes more
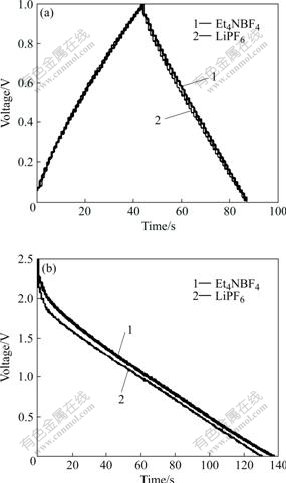
Fig.5 Charge-discharge curves of PLi/C supercapacitor using LiPF6 in EC/EMC/DMC solution and Et4NBF4 in PC solution as electrolytes in different effective energy storage potential ranges: (a) 0-1.0 V; (b) 0-2.5 V
smoothly than that of PLi/C supercapacitor using LiPF6 in EC/EMC/DMC solution as electrolyte, as shown in Fig.5(b). Thus the RES of the former is smaller than that of the latter. This can also be observed from impedance spectra in Fig.6. The internal resistances of PLi/C supercapacitor are 5.0 and 2.5 Ω respectively, when LiPF6 in EC/EMC/DMC solution and Et4NBF4 in PC solution are used as electrolytes.
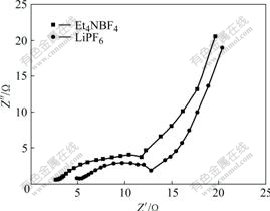
Fig.6 Impedance spectra of PLi/C supercapacitors using LiPF6 in EC/EMC/DMC solution and Et4NBF4 in PC solution as electrolytes
The properties, such as specific discharge capacitance, discharging time, and potential drop of the PLi/C supercapacitors using different kinds of electrolyte, are shown in Table 1. It can be found that the specific discharge capacitances of PLi/C capacitors increase from 90.62 to 119.39 F/g for Et4NBF4 and from 91.88 to 120.93 F/g for LiPF6 with the upper potential increasing from 1.0 to 2.5 V. Moreover, the specific discharge capacitance of PLi/C capacitors using different kinds of electrolyte solution is nearly equal in the same potential range. But considering the internal resistance of supercapacitor, it can be concluded that the electrochemical performance of supercapacitor using Et4NBF4 in PC solution as electrolyte is better than that using LiPF6 in EC/EMC/DMC solution as electrolyte.
Table 1 Properties of PLi/C supercapacitors using LiPF6 in EC/EMC/DMC solution and Et4NBF4 in PC solution as electrolytes

4 Conclusions
(1) Two types of supercapacitors are built: redox type supercapacitors (PLi/PLi) composed of two PANI-LiPF6 electrodes, and hybrid type supercapacitors (PLi/C) composed of a PANI-LiPF6 electrode and an activated carbon electrode. Using cyclic voltammetry, AC impedance, charge and discharge characteristics, and cycle life, the electrochemical properties of the two supercapacitors are studied.
(2) Compared with PLi/PLi supercapacitors, PLi/C supercapacitors provide a wider effective energy storage potential range (0-2.0 V) and a higher upper potential (2.5 V). The RES and Rct of PLi/C supercapacitors are 5.0 and 9.0 Ω, respectively, which are smaller than those of PLi/PLi supercapacitors.
(3) From cycle life curves, PLi/C supercapacitors offer better cyclic characteristics than PLi/PLi supercapacitors over 500 cycles. After 500 cycles, the capacitance of PLi/C supercapacitor remains 80%, much higher than that of PLi/PLi supercapacitor that remains 70%.
(4) Two kinds of electrolyte solution, that is, LiPF6 in EC/EMC/DMC solution and Et4NBF4 in PC solution are tested. The specific capacitance of PLi/C using LiPF6 in EC/EMC/DMC solution as electrolyte is 120.93 F/g, which is almost equal to that using Et4NBF4 in PC solution as electrolyte. The RES of the former is 5.0 Ω, which is larger than that of the latter. So PLi/C supercapacitor using Et4NBF4 in PC solution as electrolyte shows better electrochemical performance.
References
[1] K?TZ R, CARLEN M. Principles and applications of electrochemical capacitors [J]. Electrochimica Acta, 2000, 45(15/16): 2483-2498.
[2] LEWANDOWSKI A, GALINSKI M. Practical and theoretical limits for electrochemical double-layer capacitors [J]. Journal of Power Source, 2007, 173(2): 822-828.
[3] VIX-GUTERL C, SAADALLAH S, JUREWICZ K, FRACKOWIAK E, REDA M, PARMENTIER J, PATARIN J, BEGUIN F. Supercapacitor electrodes from new ordered porous carbon materials obtained by a templating procedure [J]. Materials Science and Engineering B, 2004, B108(1/2): 148-155.
[4] THOMAS E R, DEMISA H J, ZHU Z H, LU G Q. Nanoporous carbon electrode from waste coffee beans for high performance supercapacitors [J]. Electrochemistry Communications, 2008, 10(10): 1594-1597.
[5] PAYMAN A, PIERFEDERICI S, MEIBODY-TABAR F. Energy control of supercapacitor/fuel cell hybrid power source [J]. Energy Conversion and Management, 2008, 49(2): 1637-1644.
[6] TIAN Yong-ming, SONG Yan, TANG Zhi-hong, GUO Quan-gui, LIU Liang. Influence of high temperature treatment of porous carbon on the electrochemical performance in supercapacitor [J]. Journal of Power Sources, 2008, 184(2): 675-681.
[7] LAI Yan-qing, LI Jing, SONG Hai-shen, ZHANG Zhi-an, LI Jie, LIU Ye-xiang. Preparation of activated carbons from mesophase pitch and their electrochemical properties [J]. Journal of Central South University of Technology, 2007, 14(5): 601-606.
[8] ZHOU Shao-yun, WANG Zhi-xing, GUO Hua-jun, PENG Wen-jie. Effect of activated carbon and electrolyte on properties of supercapacitor [J]. Transactions of Nonferrous Metals Society of China, 2007, 17(6): 1328-1333.
[9] PARK J H, KO J M, PARK O O, KIM D W. Capacitance properties of graphite/polypyrrole composite electrode prepared by chemical polymerization of polpyrrole on graphite fiber [J]. Journal of Power Source, 2002, 105(1): 20-25.
[10] LAFORGUE A, SIMON P, SARRAZIN C, FAUVARQUE J. Polythiophene-based supercapacitors [J]. Journal of Power Sources, 1999, 80(1/2): 142-148.
[11] RYU K S, HONG Y S, PARK Y J, WU X, KIM KM, LEE Y, CHANG S H, LEE S J. Polyaniline doped with dimethylsulfate as a polymer electrode for all solid-state power source system [J]. Solid State Ionics, 2004, 175(1/4): 759-763.
[12] KO J M, SONG R Y, YU H J, YOON J W, MIN B G, KIM D W. Capacitive performance of the composite electrode consisted of polyaniline and activated carbons powder in a solid-like acid gel electrolyte [J]. Electrochimica Acta, 2004, 50(2/3): 873-876.
[13] PRASAD K R, MUNICHANDRAIAH N. Fabrication and evaluation of 450 F electrochemical redox supercapacitors using inexpensive and high-performance, polyaniline coated, stainless-steel electrodes [J]. Journal of Power Source, 2002, 112(2): 443-451.
[14] RYU K S, KIM K M, PARK N G, PARK Y J, CHANG S H. Symmetric redox supercapacitor with conducting polyaniline electrodes [J]. Journal of Power Source, 2002, 103(2): 305-309.
[15] RYU K S, KIM K M, PARK Y J, PARK N G, KANG M G, CHANG S H. Redox supercapacitor using polyaniline doped with Li salt as electrode [J]. Solid State Ionics, 2002, 152/153: 861-866.
[16] PARK J H, PARK O O. Hybrid electrochemical capacitors based on polyaniline and activated carbon electrodes [J]. Journal of Power Source, 2002, 111(1): 185-190.
[17] CHEN W C, WEN T C. Electrochemical and capacitance properties of polyaniline-implanted porous carbon electrode for supercapacitors [J]. Journal of Power Source, 2003, 117(1/2): 273-282.
[18] KHOMENKO V, FRAKOWIAK E, BEGUIN F. Determination of the specific capacitance of conducting polymer/nanotubes composite electrodes using different cell configurations [J]. Electrochimica Acta, 2005, 50(12): 2499-2506.
(Edited by CHEN Wei-ping)
Foundation item: Project(2008AA03Z207) supported by the National High-Tech Research and Development Program of China
Received date: 2008-08-26; Accepted date: 2008-10-14
Corresponding author: ZHANG Zhi-an, PhD; Tel: +86-731-8876454; E-mail: zhianzhang@sina.com