Adsorption removal of endosulfan through Saccharum officinarum derived activated carbon from selected soils
来源期刊:中南大学学报(英文版)2019年第1期
论文作者:Khuram Shahzad AHMAD
文章页码:146 - 157
Key words:soil; endosulfan; adsorption; activated carbon; sugarcane husk
Abstract: Pesticide contamination causes precarious implications on human health and environment. Thus the investigation of its sorption phenomenon is highly imperative. Endosulfan insecticide was examined for its adsorption behavior on ten assorted soils through batch equilibrium method. Adsorption coefficient values (Kd) ranged from 1.4 μg/mL to 18 μg/mL. The highest Kd value was obtained for Peshawar soil owing to the presence of highest amount of organic matter (1.4%). Negative values of Gibbs free energy displayed a low interaction between soil and pesticide, exhibiting that the reaction was physiosorption and exothermic in nature. Statistical analysis showed a negative correlation of soil pH and Kd (R2=–0.77 and p=0.03) and a positive correlation with organic matter (R2=0.96).Activated carbon prepared from Saccharum officinarum bagasse removed significant amount pesticide. The maximum removal observed was 93% and 97% in 5×10–6 and 7.5×10–6, respectively. Activated carbon prepared from biomass for removal purposes was proved to be highly efficient and cost effective.
Cite this article as: Khuram Shahzad AHMAD. Adsorption removal of endosulfan through Saccharum officinarum derived activated carbon from selected soils [J]. Journal of Central South University, 2019, 26(1): 146–157. DOI: https://doi.org/10.1007/s11771-019-3989-7.

J. Cent. South Univ. (2019) 26: 146-157
DOI: https://doi.org/10.1007/s11771-019-3989-7
Khuram Shahzad AHMAD
Department of Environmental Sciences, Fatima Jinnah Women University,The Mall, 46000, Rawalpindi, Pakistan
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: Pesticide contamination causes precarious implications on human health and environment. Thus the investigation of its sorption phenomenon is highly imperative. Endosulfan insecticide was examined for its adsorption behavior on ten assorted soils through batch equilibrium method. Adsorption coefficient values (Kd) ranged from 1.4 μg/mL to 18 μg/mL. The highest Kd value was obtained for Peshawar soil owing to the presence of highest amount of organic matter (1.4%). Negative values of Gibbs free energy displayed a low interaction between soil and pesticide, exhibiting that the reaction was physiosorption and exothermic in nature. Statistical analysis showed a negative correlation of soil pH and Kd (R2=–0.77 and p=0.03) and a positive correlation with organic matter (R2=0.96). Activated carbon prepared from Saccharum officinarum bagasse removed significant amount pesticide. The maximum removal observed was 93% and 97% in 5×10–6 and 7.5×10–6, respectively. Activated carbon prepared from biomass for removal purposes was proved to be highly efficient and cost effective.
Key words: soil; endosulfan; adsorption; activated carbon; sugarcane husk
Cite this article as: Khuram Shahzad AHMAD. Adsorption removal of endosulfan through Saccharum officinarum derived activated carbon from selected soils [J]. Journal of Central South University, 2019, 26(1): 146–157. DOI: https://doi.org/10.1007/s11771-019-3989-7.
1 Introduction
Many plants and insect species are in great threat from the last 50 years due to increase in agricultural activities. Agricultural expansion causes extinction of natural herbs, plants, insects, animals and other vital land elements. When farm and agricultural field increase, the natural landscape diminishes. Pesticide use generates negative impacts on our ecosystem. Despite of the harmful effects, pesticides are used in agricultural activities for the protection of food crops [1]. Pesticide exposure leads to too many serious health effects, increases the invulnerability of pest community, decreases the population of many natural beneficial plants, parasites and herbs [2, 3]. Pesticides are also beneficial because they protect our environment from the harmful organisms and increase the food production but on the other hand they also cause dreadful impacts on the ecosystem by damaging the beneficial insects and herbs. Developing countries utilize pesticides in excessive amount and the exposure to pesticides causes huge number of deaths annually [4]. On the basis of chemical nature, pesticides are classified as organic and inorganic. Leaching of pesticides depends upon the type and concentration hence non-polar and less soluble pesticides have low adsorption phenomenon as compare to the polar and more soluble pesticides [5]. Pesticides remain in the soil as they take time to degrade and accumulate into the soil. Huge amount of pesticides are present in the soil [6]. Pesticides are used in Pakistan in many agricultural activities. Pesticides are found to contaminate the soil and groundwater of Punjab and Sindh areas. It is because farmers use large amount of pesticides that also causes damaging health effects due to greater exposure to them. The use of pesticides should be limited to reduce soil contamination [7]. Limiting the damaging effects of pesticides in environment and controlling or reducing these harmful effects requires profound understanding and knowledge about the sorption processes along with the interaction of pesticides with soil. The adsorption of pesticides into soil depends greatly upon the physiochemical properties of soil and the major parameters include, organic matter, clay, pH, electric conductivity and solubility of soil. Adsorption process handles the movement of pesticides into soil by leaching and runoff [8]. Excessive use of pesticides in turn greatly affects the ecosystem, contaminates the water and reduces the soil fertility. Highly toxic pesticides are called persistent organic pollutants. Due to anthropogenic activities, these pesticides are released into the environment from many industries [9].
Endosulfan is an organochlorine insecticide (Figure 1). These organochlorine pesticides are highly toxic [10]. Adsorption property of endosulfan is inversely linked to the pH of soil. Its adsorption is increased if the soil has low pH [11]. endosulfan (6, 7, 8, 9, 10, 10-hexachloro-1, 5, 5a, 6, 9, 9a-hexahydro-6, 9-methano-2, 3, 4-benzo- dioxathiepin-3-oxide) is used to kill insects that damage fruit, oil seed, vegetables, grains and cotton crops. Endosulfan has two isomers; α- and β- isomers, but the α-isomer is more toxic than the β-isomer. The solubility of α-isomer is 0.53 mg/mL and β-isomer is 0.28 mg/mL. It causes many defects in humans thus damaging the human health [12]. The molecular formula of endosulfan is C9H6Cl6O3S and its molecular weight is 406.9 g/mol with a melting point of 106 °C and density of 1.7 g/cm3 [13]. Previously extensive studies have been performed on the biodegradative pathways for the removal of endosulfan from soils and aqueous media. Numerous literature is present that articulates the remediation of endosulfan contaminated soils via microbial degradation. LI et al isolated Achromobacter xylosoxidans from activated sludge for the detoxification of endosulfan [14]. Similarly, Aspergillus niger, Pseudomonas aeruginosa, Ochrobacterum sp., Arthrobacter sp., and Burkholderia sp., have also been reported to detoxify Endosulfan contaminated soils and aqueous solutions [15–17]. However, removal through biodegradation is an arduous and intricate task, due to the involvement of sensitive microbes. Removal through non-biodegradative routes is rather preferable. GUPTA et al [18] had used carbon slurry emanated from industrial generators to remove endosulfan from water. MISHRA et al [19] utilized sal wood charcoal for the decontamination of endosulfan.
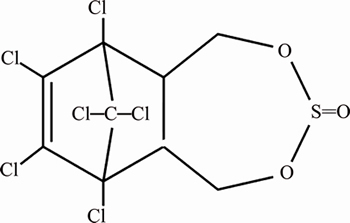
Figure 1 Chemical structure of endosulfan
The objective of this study was to analyze the interaction of endosulfan with the diverse range of soils of varying physicochemical properties and its removal from soils using a cost effective and environmental friendly method, i.e., by activated carbon [20]. Activated carbon was prepared from Saccharum officinarum bagasse. Usage of Saccharum officinarum bagasse for the removal of pesticide also assists in reducing the waste [21, 22]. Biomass based activated carbon has not been used previously for the removal of endosulfan from soils thus this study can prove to be a pioneer for pesticide remediation by activated carbon from soils.
2 Materials and methods
Endosulfan analytical standard (99% pure) was purchased from Dr. Ehrenstorfer, Germany. Anhydrous sodium chloride (NaCl) was utilized. Methanol used was 99% pure. Standard stock solution of endosulfan was prepared in distilled water. Weighing balance, hot plate, pH meter, EC meter, orbital and incubator shaker, octagonal sieve shaker, centrifuge, UV-visible spectrophotometer and atomic spectrophotometer were used in the current study.
The soil samples were collected from ten distinct areas from Pakistan including Deer, Gujranwala, Karachi, Attock, Mirpur, Sohawa, Chakwal, Taxila, Peshawar and Hafizabad. Temperature was around 20 °C over all locales and no precipitation was seen during the soil sampling. Soil samples were collected through random sampling. After the collection, the soil samples were stored in polythene bags. Soil samples were air dried in the green house for 2–3 d. The dried specimens were ground with pestle and mortar then sieved through 2 mm mesh. The sieved samples were stored in petri dishes for further examination and experimentation.
2.1 Adsorption experiment of endosulfan
Stock solution of endosulfan was set up according to the standard protocol [23–25]. Diluted solutions were prepared of different concentrations (0, 0.25×10–6, 0.5×10–6, 0.75×10–6, 1×10–6, 2.5×10–6, 5×10–6, 7.5×10–6). All samples were run in duplicates. In each dilution, 10 mL of 0.1 mmol sodium chloride solution was added [26]. 0.5 g of soil was also added in each centrifuge tube and labelled. Same procedure was followed for duplicate vials. 10 mL of pesticide solution was added into the labeled plastic vial. The vials were kept in the shaker for 24 h at 150 r/min. After shaking, the samples were centrifuged for 15 min at 3000 r/min. The solution was filtered by using nylon filters. This filtered solution was analyzed by UV spectrophotometer [24].
2.2 Preparation of activated carbon
Activated carbon (AC) was prepared by using Saccharum officinarum bagasse (Figure 2). Initially, Saccharum officinarum bagasse were washed with tap water and then with distilled water. It was dried in oven for 48 h at 110 °C. After drying, the Saccharum officinarum bagasse was ground first with pestle and mortar and later in a grinding machine to convert it into a fine powder. After drying and grinding, the mass left was approximately 300 g. Concentrated sulfuric acid was added in equal ratio 1:1. 300 mL sulfuric acid was slowly added in ground Saccharum officinarum bagasse in fume hood and stirred continuously until sulfuric acid was properly mixed with it. This mixture was kept overnight for the proper mixing of sulfuric acid with ground Saccharum officinarum. Followed by mixing, the pH of activated carbon was neutralized by washing it with cold distilled water and soaking in 15% of sodium bicarbonate solution. The mixture was left overnight. It was washed again by distilled water and the process of washing was continued until the pH of activated carbon reached 7. It was kept for drying in oven at 110 °C for 12 h and finally stored in an airtight media bottle [27].
2.3 Iodine test
This test was performed by dissolving 2 g of iodine salt into 50 mL distilled water. It was stirred for 10–15 min. When the salt was completely dissolved in water, 5 g of activated carbon was added into it and stirred again for 1 h. The iodine solution was filtered through a filter paper. Before filtration its color was brown and after the filtration it turned yellow. This test showed that iodine was absorbed by the activated carbon. This test was used for confirmation of accurate preparation of activated carbon [28].
2.4 FTIR characterization
FTIR spectrometry was used to examine the change in functional groups before and after preparation of activated carbon. These active functional groups provide sites for the attachment of pesticides. FTIR spectra were acquired before the preparation of activated carbon (Saccharum officinarum bagasse) and after its preparation (activated carbon). Then both were compared. The calculations of activated carbon were carried out using FTIR-8400 which ranged from 400–4000 cm–1. For this purpose, pellets of activated carbon were prepared using pre-heated KBr in powder form. Activated carbon prepared was well mixed with KBr with the help of mortar and pestle. The mixture was then subsequently given high pressure through the hydraulic pump [29].

Figure 2 Activated carbon preparation from Saccharum officinarum bagasse
2.5 Removal of endosulfan by activated carbon
Two dilutions of 5×106 and 7.5×106 concentrations were prepared from the 10×10–6 stock solution. 0.5 g of each soil sample was added into plastic vials followed by 10 mL of each dilution and their absorbance was observed through UV-spectrometer. The vials were left for 3 h. After 3 h their absorbance was checked by UV- spectrophotometer. Then same experiment was performed to check absorbance after 6 h [30].
2.6 Data analysis
The concentration of endosulfan adsorbed in soil samples was determined by difference in final and equilibrium concentration by using the relation (Eq. (1)):
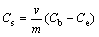 (1)
(1)
where Cs is the concentration of pesticide adsorbed, m is the amount of soil in g and v is volume of the solution. Ce is the equilibrium concentration of the supernatant and Cb is the equilibrium concentration of blank. Linear isotherms equation can be derived as Eq. (2):
 (2)
(2)
where Kd is linear sorption coefficient and Ce is endosulfan concentration at equilibrium in μg/mL.
The isotherms of adsorption of pesticides in selected soil samples were fitted to Freundlich equation. The Freundlich equation can be written as (Eq. (3)):
 (3)
(3)
where Cs is endosulfan concentration adsorbed in soil, Ce is the equilibrium concentration in μg/mL, and Kf and 1/n is the Freundlich constant. Kfoc is calculated by Eq. (4) given below:
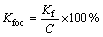 (4)
(4)
By using formula Koc and Kom values can be calculated by:
 (5)
(5)
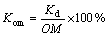 (6)
(6)
Gibbs free energy was calculated by Eq. (7):
 (7)
(7)
In Eq. (7), R is the universal gas constant, T is the temperature. The nature of adsorption can be determined with the help of its value. The value ≤–40 kJ/mol represents the physical binding of pesticide with soil. Gibbs free energy is a thermodynamic parameter [31].
3 Results and discussion
3.1 Soil properties
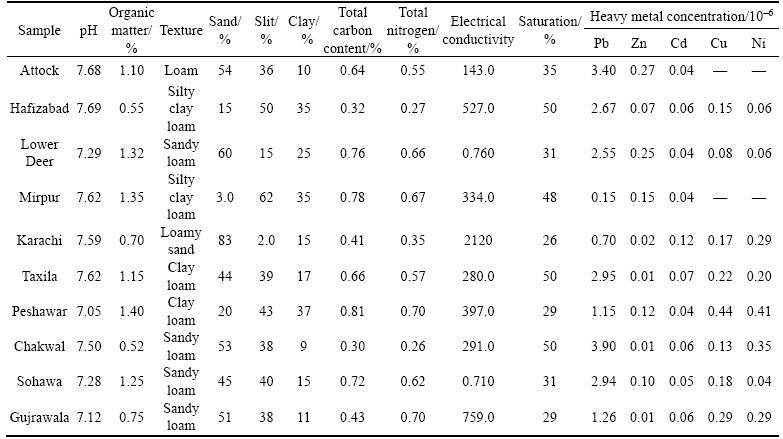
The soil samples were analyzed for their physiochemical properties (Table 1). Physiochemical properties of soil play vital role to define the relationship of pesticide with soil. These properties also help in determining the incorporation of pesticides into the soil. Physiochemical properties of ten soil samples included; pH, electrical conductivity, soil texture, clay, sand and silt content, organic carbon content and amount of organic matter in the soil. The pH of all soil samples ranged from 7.05 to 7.69. Lowest soil pH was observed from Peshawar soil sample while the highest pH was obtained from Hafizabad. Previous research by IMTIAZ et al [32] also reported high pH of Hafizabad among all collected samples [32]. Soil samples were also assessed for their organic matter content (OM). OM of soil samples ranged from 0.52% to 1.4%. The highest OM was observed in soil from Peshawar (1.4%). The highest OM in Peshawar soil might be due to the fertile nature of soils in that region. Two rivers pass through the region; Kabul and Bara rivers provide the rich nature of land [33]. Generally, these soils were found to possess an average amount of organic matter. It has been observed from previous studies that soils with pH values in alkaline range possess a lower range of OM [34]. High pH values in soils obstruct the uptake of water by plants which subsequently increases soil salinity, resulting in reduced OM [35, 36]. The textural analysis of soils grouped them into different classes including loamy, silty clay loam, sandy loam, loamy sand and clay loam. The highest sand content was found in Karachi soil (83%) owing to its specific geographical location of being a coastal city [37]. Soil samples were also investigated for their electrical conductivities (EC). The high EC value indicates the salinity hazard for soil [38]. According to the analysis, sample from Karachi displayed the highest EC value (2120 μS/cm). Soils were further subjected to heavy metal analysis including lead, zinc, cadmium, nickel and copper. The highest lead content was found in soil from Chakwal region (3.9×10–6). RAZA et al [39] attributed the origin of lead content in Chakwal soil mainly to the ground rock sources while to some extent it was also a consequence of fallout from the atmosphere due to emissions from vehicles and industrial effluents. Zinc content was found highest in soil from Attock region (0.277). Although it is the highest concentration among all samples yet this value is considered to be quite low according to the generalized guidelines [40]. ALVI et al [41] concluded in their study related to Attock soil that soils with elevated pH and low OM were usually deficient in soil micronutrients including zinc.
Table: 1 Physiochemical properties of soil
3.2 Adsorption isotherms
Endosulfan adsorption was analyzed by UV-vis spectrophotometer (Table 2). The values from UV-spectrophotometer were then utilized to plot the comparative linear and Freundlich adsorption isotherms (Figure 3). The linear adsorption coefficient values (Kd) ranged from 1.4 μg/mL to 18 μg/mL with the following trend:
S5>S10>S2>S3>S8>S9>S4=S6>S1=S7
The sample S5 displayed the highest Kd value among all (18 μg/mL). KUMAR et al [11] utilized a highly concentrated Endosulfan solution for adsorption experiments with their Kd values ranging between 0.6 and 2.3 mg/g. They concluded from their results that initially endosulfan was adsorbed quickly onto the soils displaying a surface phenomenon. Since endosulfan is hydrophobic, it occupies the spaces between the soil particles rapidly exhibiting a more linear trend [42]. It was analyzed from previous researches that endosulfan adsorbed much rapidly at the initial stage of phenomenon while its adsorption rate decelerated, with very insignificant amount being utilized in later phases. S5 exhibited the lowest value of pH and the highest amount of organic matter content which can be the reason for its highest adsorption among all samples [21]. DORES et al [43] displayed that endosulfan was adsorbed in a high amount in Brazilian soils which can be attributed to the presence of high OM in soils of that region. The R2 values ranged from 0.74–0.96 displaying a good fit with the linear model. Pesticide mobility was evaluated by the values of Koc. Koc values have been grouped into different classes for pesticide mobility [44]. Koc values lower than 50 indicate a highest mobility group, while its value between 50 and 100 depict a high mobility group and from 150 to 500 indicate medium mobility of pesticide in soil [30]. Koc values in the current experiment ranged from 299.4 to 2699 μg/mL. Thus the soils in the current study displayed medium to low mobility of endosulfan. A significant variation in the Koc values has been observed in Indian soil as well depending upon the physicochemical properties of each soil [45]. It has been observed from the experiments of PARKIPAN et al [46] that Koc displayed an inverse relation with pesticide mobility [46]. Thus soils with high values of Koc displayed more capacity for adsorption. Adsorption distribution coefficient normalized for organic matter (Kom) was also assessed for the soils. The highest Kom was found in sample S5 also displaying the highest rate of adsorption (Kd=18 μg/mL) and highest organic matter (1.4%). The soil-pesticide interaction was further assessed by the thermodynamic parameter Gibbs free energy (ΔG). Its value ≤–40 kJ/mol represents physiosorption, also implying the reaction to be exothermic and spontaneous [31]. The ΔG in the current study ranged from –12 to –18 kJ/mol which represented the phenomena of physisorption indicating that the interaction between soil and pesticides occurred through weak Van der Waal’s forces.
Table 2 Comparative parameter of linear and Freundlich models are given below
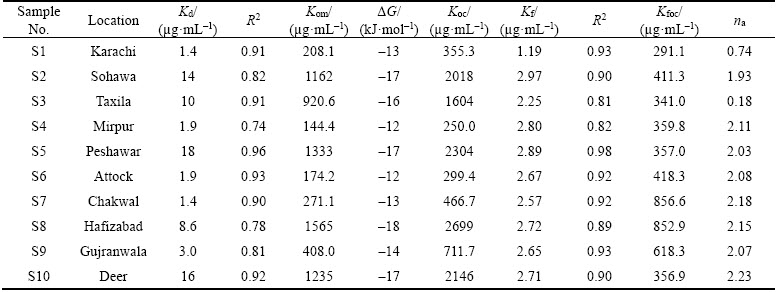
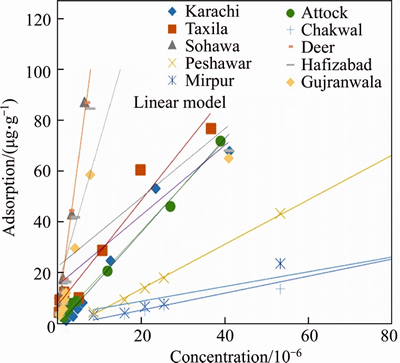
Figure 3 Graphical representation of linear model comparative adsorption of endosulfan on selected soils
Freundlich adsorption isotherm was also used to assess the adsorption of endosulfan on soils. The Freundlich distribution coefficient (Kf) values were lower than the Kd values and lied between 1.19 and 2.89 μg/mL. ATASOY et al [47] exhibited Freundlich adsorption of endosulfan on Turkish soils showing a good rate of adsorption. The R2 values for Freundlich constant ranged from 0.81 to 0.96, indicating a better fit than linear model. The adsorption on Indian soils displayed an ‘L’ type curve [48]. This depicted a non-linearity between the adsorption mechanism and the equilibrium concentration of the solution. The curve of the current isotherm showed linearity thus depicting a ‘C’ type curve. The better fit to Freundlich in the current experiment can be compared to the similar fit by PARKPIAN et al [46] inferring that a logarithmic decline occurred of the energy of adsorption when the ratio of covered surface increased which can be attributed to surface heterogeneity. Furthermore, Parkipan also indicated that the small quantity of soil samples used in the experiment might be the reason for achieving linearity in the isotherms. This can be justified in the current experiments as the amount of soil taken was 0.5 g. The na values were also evaluated by the regression equation to study the extent of adsorption irreversibility.
3.3 Effect of soil physicochemical properties on adsorption
Physiochemical properties play an important role to find the adsorption behavior of pesticides and also determine the soil fertility and the relation of pesticide with soil. In this study, the soil of Peshawar contained the highest amount of organic matter (1.40) and organic carbon (0.81) while its pH was the lowest 7.05 hence the highest Kd value (18 μg/mL) was observed. Karachi soil’s pH was 7.59 and it possessed low organic matter (0.70) and organic carbon (0.41). Kd value of Karachi sample was found to be less (1.4 μg/mL). Attock soil displayed low Kd (1.9 μg/mL) as well which can be attributed to its high pH 7.68 and 54% sand in its texture thus displaying low adsorption. A high percentage of sand content in soils deters the binding of pesticide molecules to soil particles thus decreasing the rate of adsorption. SINGH et al [48] in their study displayed the dependence of clay content and organic matter on the rate of adsorption [48]. This study showed that the soil with high level of Kd had high organic matter and organic carbon and low pH.
Statistical analysis (regression and correlation) was performed on all soil samples for the Kd values and their corresponding physiochemical properties including pH, OM and TOC. pH was indirectly proportional to adsorption rate (R2=–0.77) while OM (R2=0.96) and TOC (R2=0.96) are directly proportional to it (Table 3). Hence, increasing OM and TOC will increase the adsorption of soil while increasing pH will have inverse effect. According to the results, the soil sample with highest value of Kd also had the highest amount of organic matter in it. This justified the direct relation between organic matter and adsorption capacity of soil.
The relationship of physiochemical properties of soils, i.e., pH, OM and TOC with Kd was further assessed in Minitab 17. The residual graphs plotted determined the goodness of fit in ANOVA. From these residual plots it is determined that the ordinary least square assumptions are being encountered. Sustaining these assumptions specifies that the ordinary least squares regression will produce unbiased coefficient estimates with the minimum variance. Normal probability plots of residuals, residuals versus fits and residuals versus order of data were plotted in Minitab (Figure 4). Normal probability plot of residuals displays that the data are distributed normally. The residuals versus fits plot determines that the data have a constant variance.
3.4 Characterization of activated carbon and removal of endosulfan
Fourier transform-infrared spectroscopy was used to analyze functional groups. These functional group can act as an active site for the attachment of pesticide. It identified the change in bonds before and after the transformation of the activated carbon. The C—Br bending peak was observed at 563.23 cm–1 and at 597.95 cm–1 while C—H stretching vibrations appeared at 2931 cm–1. The peak at 1732.13 cm–1 was assigned as C=O stretching vibration. An absorption peak at 1323 cm–1 was due to C—N (aromatic amines) stretch. The N—H stretching peaks were observed at 908.5 cm–1. Absorption at 868 cm–1 was attributed to the C—H stretching vibrations (Figure 5 and Table 4).
Iodine test showed that iodine was absorbed by the activated carbon. This test was used for confirmation of accurate preparation of activated carbon (Figure 6). The brown colored iodine solution was transformed to yellow color by the action of activated carbon.
Table 3 Linear regression and correlation for endosulfan adsorption

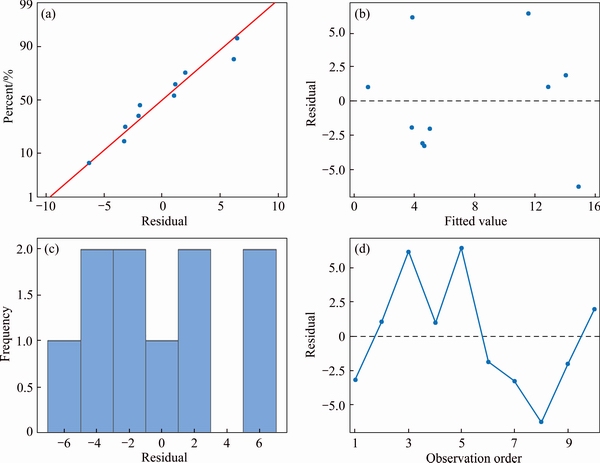
Figure 4 ANOVA histogram, versus fit, versus order and normal probability residual plots of soil samples with physiochemical properties pH, TOC and OM with the response of Kd
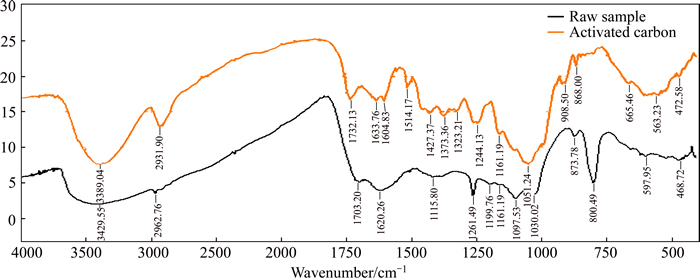
Figure 5 FTIR-spectrum of activated carbon sample
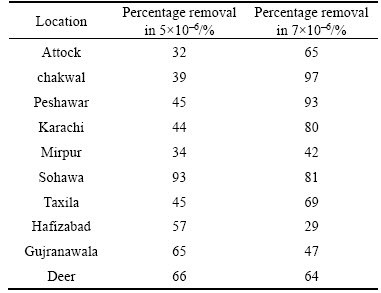
The removal of pesticide (endosulfan) done from the selected soil samples with the help of activated carbon taking two initial concentrations; 5×10–6 and 7.5×10–6 (Table 5). Absorbance was checked before the addition of activated carbon by UV visible spectrophotometer. Later, absorbance was also checked after the addition of activated carbon after 3 and 6 h. The graphs were plotted of absorbance versus time using Microsoft Excel. In 5×10–6 concentration, it was noted that most of the removal percentage was 50% to 60%, while the maximum removal was from the soil of Sohawa (93%) (Figure 7). From Gujranawala, Hafizabad and Deer soils, pesticide removal percentage was 60%–65% while minimum removal was from Attock soil (32%). In 7.5×10–6 concentration, maximum removal was observed in Chakwal (97%) and minimum removal was observed in Hafizabad soil (29%). From this study, it is concluded that the factors, time and concentration affect the rate of removal and absorbance of activated carbon. Previously, MISHRA [19] have utilized sal wood based activated carbon for removal of endosulfan from aqueous solution after leaving the solution for 24 h. The removal efficiency of Saccharum officinarum bagasse based activated carbon used in the current experimentation was based on time period of 3 and 6 h only. It showed significant potential of the AC prepared in this experiment for endosulfan remediation from soils after such a short time duration.
Table 4 Functional group in activated carbon identified with help of FT-IR

Figure 6 Iodine test for activated carbon displaying efficiency of AC by converting the iodine solution from brown to yellow
Table 5 Removal of endosulfan by activated carbon from selected soils in 5×10–6 and 7.5×10–6 concentrations
4 Conclusions
Soils from various different areas were analyzed for the adsorption of endosulfan and its remediation by indigenously prepared Saccharum officinarum bagasse based activated carbon. Adsorption of endosulfan is highly influenced by the physiochemical properties of soil that include pH, organic matter and organic carbon content. The soil with the highest amount of organic carbon and organic matter also possessed high Kd and Kf. High Kd and Kf values of soil showed greater ability of soils for adsorption. Furthermore, soil texture was also found to affect the adsorption and removal of endosulfan from soils. The removal of endosulfan with the help of activated carbon emanated biosorbent is economically beneficial and an environmental friendly method. Maximum 97% of pesticide was removed with the help of this method. endosulfan removal results deduced that time and concentration of pesticide greatly affect the remediation rate. Removal with the help of activated carbon prepared by Saccharum officinarum bagasse is a promising method because it is not only less costly but environmental friendly as well aiding in waste management. Its potential can be further assessed for decontamination of soils and water bodies from various other pollutants.
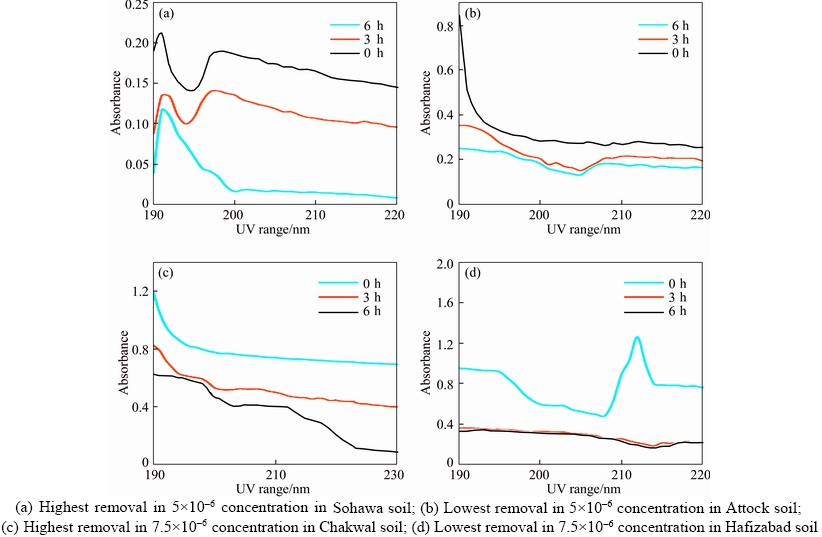
Figure 7 UV spectrums of endosulfan removal by activated carbon:
References
[1] GEIGER F, BENGTSSON J, BERENDSE F, WEISSER W W, EMMERSON M, MORALES M B, EGGERS S. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland [J]. Basic and Applied Ecology, 2010, 11(2): 97–105.
[2] SHEIKH S A. Pesticides and associated impact on human health: A case of small farmers in southern Sindh [J]. Pakistan Journal of Pharmacy and Nutrition Sciences, 2011, 1(1): 82–86.
[3] PINGALI P L, ROGER P A. Impact of pesticides on farmer health and the rice environment (Vol. 7) [M]. New York: Springer Science & Business Media, 2012.
[4] SARWAR M. The dangers of pesticides associated with public health and preventing of the risks [J]. International Journal of Bioinformatics and Biomedical Engineering, 2015, 1(2): 130–136.
[5] ELDRIDGE B F. Pesticide application and safety training for applicators of public health pesticides [R].Vector-Borne Disease Section, California Department of ublic Health Vector Control Technician Certification Training Manual, Category A, 2008.
[6] SYED J H, MALIK R N. Occurrence and source identification of organochlorine pesticides in the surrounding surface soils of Ittehad Chemical Industries Kalashah Kaku, Pakistan [J]. Environmental Earth Sciences, 2011, 62(6): 1311–1321.
[7] TARIQ M I, AFZAL S, HUSSAIN I, SULTANA N. Pesticides exposure in Pakistan: A review [J]. Environment International, 2007, 33(8): 1107–1122.
[8] SHAHMAT M, SEAMAN A, WOODBINE M. Influence of sodium chloride, pH and temperature on the inhibitory activity of sodium nitrite on L. monocytogenes [M]// GOULD G W, CORRY E L, ed., Survival in Extremes of Environment, 1980: 227–237.
[9] GAVRILESCU M. Fate of pesticides in the environment and its bioremediation [J]. Engineering in Life Sciences, 2005, 5(6): 497–526.
[10] MUNNECKE D M, JOHNSON H W, TAL B. Microbial metabolism and enzymology of selected pesticides in Biodegradation and the detoxification of environmental pollutants [M]. Boca Raton: CRC Press, 1982.
[11] KUMAR M, PHILIP L. Adsorption and desorption characteristics of hydrophobic pesticide endosulfan in four Indian soils [J]. Chemosphere, 2006, 62: 1064–1077.
[12] SAIYED H, DEWAN A, BHATNAGAR V, SHENOY U, SHENOY R, RAJMOHAN R. Effect of endosulfan on male reproductive development [J]. Environmental Health Perspective, 2003, 111: 1958–1962.
[13] GOEBEL H, GORBACH S, KNAUF W, RIMPAU R H, HUTTENBACH H. Properties, effects, residues, and analytics of the insecticide Endosulfan [M]. New York: Springer, 1982.
[14] LI W, DAI Y, XUE B, LI Y, PENG X, ZHANG J, YAN Y. Biodegradation and detoxification of endosulfan in aqueous medium and soil by Achromobacter xylosoxidans strain CS5 [J]. Journal of Hazardous Materials, 2009, 167(1–3): 209–216.
[15] BHALERAO T S, PURANIK P R. Biodegradation of organochlorine pesticide, endosulfan, by a fungal soil isolate, Aspergillus niger [J]. International Biodeterioration & Biodegradation,2007, 59(4): 315–321.
[16] KUMAR M, LAKSHMI C V, KHANNA S. Biodegradation and bioremediation of endosulfan contaminated soil [J].Bioresource Technology,2008, 99(8): 3116–3122.
[17] ARSHAD M, HUSSAIN S, SALEEM M. Optimization of environmental parameters for biodegradation of alpha and beta endosulfan in soil slurry by Pseudomonas aeruginosa [J]. Journal of Applied Microbiology, 2008, 104(2): 364–370.
[18] GUPTA V K, ALI I. Removal of endosulfan and methoxychlor from water on carbon slurry [J]. Environmental Science & Technology, 2008, 42(3): 766–770.
[19] MISHRA P C, PATEL R K. Removal of endosulfan by sal wood charcoal [J]. Journal of Hazardous Materials, 2008, 152(2): 730–736.
[20] AHMADPOUR A, DO D D. The preparation of activated carbon from macadamia nutshell by chemical activation [J]. Carbon, 1997, 35: 1723–1732.
[21] PUSINO A, FIORI MG, BRASCHI I, GESSA C. Adsorption and desorption of triasulfuron by soil [J]. Journal of Agriculture and Food Chemistry, 2003, 51: 5350–5354.
[22] GARBA A, BASRI H, NASRI N S, ISMAIL A R. Synthesis and characterization of porous carbon from biomass using KOH and K2CO3 Chemical activation [J]. ARPN Journal of Engineering and Applied Science, 2016, 11(3): 1613–1617.
[23] OECD. Guideline for the testing of chemicals. adsorption- desorption using a batch equilibrium method [S]. 2005.
[24] AHMAD K S. Green electrokinetic remediation of Thiabendazole adsorbed soils via mineralization [J]. Agrochimica, 2017, 61(3): 190–205.
[25] AHMAD K S. Pedospheric sorption investigation of sulfonyl urea herbicide Triasulfuron via regression correlation analysis in selected soils [J]. South African Journal of Chemistry, 2017, 70(1): 163–170.
[26] SHARIFF R M. Kinetic and thermodynamic study for Adsorption-Desorption of Diazinon with copper in the presence of Surfactant [J]. Global Journal of Science and Frontier Research, 2012, 12(4): 17–31.
[27] XU T, LIU X. Peanut shell activated carbon: characterization, surface modification and adsorption of Pb2+ from aqueous solution [J]. Chinese Journal of Chemical Engineering, 2008, 16: 401–406.
[28] HASSLER J W. Activated carbon [M]. New York, USA: Chemical Publishing Co, 1963.
[29] NABIYOUNI G, GHANBARI D. Thermal, magnetic, and optical characteristics of ABS-Fe2O3 nanocomposites [J]. Journal of Applied Polymer Science, 2012, 125: 3268–3274.
[30] AHMAD K S. Evaluating the adsorption potential of Alachlor and its subsequent removal from soils via activated carbon [J]. Soil and Sediment Contamination: An International, 2018, 27: 249–266. DOI: 10.1080/15320383. 2018.1470604.
[31] MICHALKOVA A, GORB L, HILL F, LESZCZYNSKI J. Can the Gibbs free energy of adsorption be predicted efficiently and accurately: An MO5-2X DFT Study [J]. Journal of Physical Chemistry, 2011, 115: 2423–2430.
[32] IMTIAZ M, ALLOWAY B J, ASLAM M, MEMON M Y, KHAN P, SIDDIQUI S U H, SHAH S K H. Zinc sorption in selected soils [J].Communications in Soil Science and Plant Analysis, 2006, 37: 1675–1688.
[33] KHAN A N. Analysis of 2010-flood causes, nature and magnitude in the Khyber Pakhtunkhwa, Pakistan [J]. Natural Hazards, 2013, 66: 887–904.
[34] UZOMA K C, INOUE M, ANDRY H, FUJIMAKI H, ZAHOOR A, NISHIHARA E. Effect of cow manure biochar on maize productivity under sandy soil condition [J]. Soil Use and Management, 2011, 27: 205–212.
[35] MAVI M S, MARSCHNER P, CHITTLEBOROUGH D J, COX J W, SANDERMAN J. Salinity and sodicity affect soil respiration and dissolved organic matter dynamics differentially in soils varying in texture [J]. Soil Biology and Biochemistry, 2012, 45: 8–13.
[36] PERALTA R M, AHN C, GILLEVET P M. Characterization of soil bacterial community structure and physicochemical properties in created and natural wetlands [J]. Science of the Total Environment, 2013, 443: 725–732.
[37] SIDDIQUI M N, MAAJID S. Monitoring of geomorphological changes for planning reclamation work in coastal area of Karachi, Pakistan [J]. Advance Space Research, 2004, 33: 1200–1205.
[38] BAUDER T A, WASKOM R M, SUTHERLAND P L, DAVIS J G, FOLLETT R H, SOLTANPOUR P N. Irrigation water quality criteria. Service in action; no. 0.506, 2011 [R]. Colorado State University Extension, 2011.
[39] RAZA N, NIAZI S B, SAJID M, IQBAL F, ALI M. Studies on relationship between season and inorganic elements of Kallar Kahar Lake (Chakwal), Pakistan [J]. Journal of Research Bahauddin Zakariya University, 2007, 18: 61–68.
[40] ZIA H M, AHMED R, KHALIQ I, JAVED H A. Micronutrients status and management in orchards soils: applied aspects [C]// Proceeding of Plant Nutrition Management for Horticultural Crops under Water Stressed Conditions. Quetta, Pakistan: Agriculture Research Institute, Sariab, 2004: 62–73.
[41] ALVI S, KHALID R, RASHID M, WAHEED A. Soil micronutrient status in Hazro area of district attock, Pakistan [J]. Pakistan Journal of Industrial Research Series A Physical Sciences, 2011, 54(1): 45–47.
[42] LAN J, CHENG Y, ZHAO Z, Effective organochlorine pesticides removal from aqueous systems by magnetic nanospheres coated with polystyrene [J]. Journal of Wuhan University of Technology: Material Sciences Edition, 2014, 29: 168–173.
[43] DORES E F, SPADOTTO C A, WEBER O L, DALLA VILLA R, VECCHIATO A B, PINTO A A. Environmental behavior of chlorpyrifos and endosulfan in a tropical soil in central Brazil [J]. Journal of Agriculture and Food Chemistry, 2015, 64: 3942–3948.
[44] LIU P S, KAO L S, LIN M K. Organophosphates inhibit catecholamine secretion and calcium influx in bovine adrenal chromaffin cells [J].Toxicology, 1994, 90: 81–91.
[45] SHIVARAMAIAH H M. Adsorption, desorption and movement of endosulfan in agricultural soil [J]. International Journal of Food, Agriculture and Veterinary Science, 2014, 4: 53–61.
[46] PARKPIAN P, ANURAKPONGSATORN P, PAKKONG P, PATRICK W H Jr. Adsorption, desorption and degradation of α-endosulfan in tropical soils of Thailand [J]. Journal of Environmental Science and Health: Part B, 1998, 33: 211–233.
[47] ATASOY A D, MERMUT A R, KUMBUR H,  NCE F, ARSLAN H, AVCI E D. Sorption of alpha and beta hydrophobic endosulfan in a Vertisol from southeast region of Turkey [J]. Chemosphere, 2009, 74: 1450–1456.
NCE F, ARSLAN H, AVCI E D. Sorption of alpha and beta hydrophobic endosulfan in a Vertisol from southeast region of Turkey [J]. Chemosphere, 2009, 74: 1450–1456.
[48] SINGH R P, SINGH S. Adsorption and movement of endosulfan in soils: A verification of the co-solvent theory and a comparison of batch equilibrium and soil thin layer chromatography results [J]. Adsorption Science and Technology, 2008, 26: 185–199.
(Edited by HE Yun-bin)
中文导读
甘蔗制作活性炭去除指定土壤中的硫丹吸附
摘要:农药污染对人体健康和环境造成极大的危害,因此,对其吸附现象的研究十分必要。采用间歇平衡法考察了硫丹杀虫剂在10种不同土壤中的吸附性能。吸附系数(Kd)范围为1.4 ~18 μg/mL。白沙瓦土壤由于有机质含量最高(1.4%),其Kd值最高。吉布斯自由能为负值表明土壤与农药的相互作用较低,说明该反应本质上是物理吸附和放热反应。经统计学分析,土壤pH与Kd呈负相关(R2=–0.77, P=0.03),与有机质呈正相关(R2=0.96)。从甘蔗渣中提取的活性炭,可去除大量农药。在5×10–6和7.5×10–6浓度的最大去除率分别为93%和97%。以生物质为原料制备的活性炭具有高效、经济的特点。
关键词:土壤;硫丹;吸附;活性炭;甘蔗皮
Received date: 2017-10-26; Accepted date: 2018-06-15
Corresponding author: Khuram Shahzad AHMAD, PhD; Tel: +92-51-9292900-169; E-mail: chemist.phd33@yahoo.com, dr.k.s.ahmad@fjwu.edu.pk; ORCID: 0000-0001-9171-8904

