
Solvent extraction of copper and ammonia from ammoniacal solutions using sterically hindered β-diketone
HU Hui-ping(胡慧萍), LIU Chun-xuan(刘春轩), HAN Xue-tao(韩雪涛),
LIANG Qi-wen(梁啟文), CHEN Qi-yuan(陈启元)
School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 13 November 2009; accepted 2 March 2010
Abstract: Extraction of copper and ammonia from Cu2+-NH3-Cl--H2O solution using laboratorialy synthesized sterically hindered β-diketone (4,4-dimethyl-1-(4-dodecylphenyl)-1,3-pentanedione) was studied. The effects of the copper concentration, the total ammonia concentration, the initial pH in the aqueous phase, the phase ratio, and the temperature on copper extraction ratio and ammonia extraction in loaded organic phase were investigated using this sterically hindered β-diketone. Under the conditions of temperature 25 °C, contact time of two phases 30 min, phase ratio 1?1, concentration of copper 3 g/L, concentration of total ammonia 3 mol/L, aqueous pH 8.43, and the concentration of β-diketone in organic phase 20% (volume fraction), ammonia in aqueous phase is much lower to be extracted by organic phase (just 14.5 mg/L), while the extraction rate of copper is 95.09%.
Key words: ammoniacal solution; 4, 4-dimethyl-1-(4-dodecylphenyl)-1, 3-pentanedione; copper; solvent extraction
1 Introduction
Tangdan copper mine, located near Dongchuan City in Yunnan Province, China, having 1.16×106 t copper reserve, is a refractory oxidized ore with high-alkaline gangues. Its average copper content is 0.46% (mass fraction, the same below if not mentioned). This oxidized copper mineral has high alkaline gangue content (w(CaO+MgO)>40%) with above 50% of malachite and chrysocolla, and the rest of it is raw vulcanized copper, such as digenite, chalocite and bornite. Ammonia leaching method is applied to deal with this oxidized copper mineral, which has some advantages, e.g., high copper leaching ratio and low production cost. If acid-leaching method is adopted, it has high production cost because of huge acid consumption for the existence of alkaline gangue. In 1990s, to deal with Tangdan oxidized copper mineral, Beijing General Research Institute of Mining and Metallurgy cooperated with Dongchuan Copper Mines Administration used the “ammonia leaching-solvent extraction-electrowinning” process. A pilot plant with 500 t cathode copper production per year was built in 1995[1-3]. In the “ammonia leaching-solvent extraction-electrowinning” process, the commonly used extractants are P204, LIX54 and LIX84. P204 (di-2-ethylhexyl phosphate) is an acidic organophosphorous extractant. It is reported that under the conditions of temperature 25 °C, the contact time of two phases 10 min, phase ratio 1:1, ammoniacal solution (pH 10) and the concentration of P204 20% (volume fraction), the extraction rate of copper was 93.9%[4]. However, P204 is so soluble in alkaline solution that it is impossible to be commercially used in ammoniacal solution (pH >9). LIX54, a β-diketone extractant, has the virtue of higher saturation capacity of copper and lower ammonia extraction during the solvent extraction in ammoniacal solution[5]. But in 1995, Escondida of Chile applied LIX54 to extract copper from ammoniacal solution in a pilot scale. As a result of LIX54 deterioration, which might be led by the reaction between a keto group of LIX54 and ammonia to generate ketimine, the copper in loaded organic phase was difficult to back extract, and finally this pilot plant was forced to be closed[6]. Therefore, it is inappropriate to choose LIX54 to extract copper in ammoniacal system. LIX84, a hydroxyoxime extractant, has been proved to be the main extractant for the extraction of copper from ammoniacal solution[7-11]. However, hydroxyoxime such as LIX84 has a problem of serious ammonia extraction in ammoniacal solution[12-13]. The ammonia present in the loaded organic phase should be removed before attempting copper stripping with the spent electrolyte. Otherwise, ammonia in the loaded organic phase is transferred to the pregnant electrolyte after copper stripping, which may result in the accumulation and the precipitation of (NH4)2SO4 in the pregnant electrolyte[14]. In our research, TBP was added to LIX84, which just partially decreased ammonia extraction in the loaded organic phase[15].
In this work, a laboratory synthesized sterically hindered β-diketone was applied to reduce the ammonia extraction in the loaded organic phase. The effects of the concentration of copper ion, the total ammonia concentration, the initial pH in the aqueous phase, and the phase ratio on copper extraction ratio and ammonia extraction in loaded organic phase were also studied.
2 Experimental
2.1 Reagents
Sterically hindered β-diketone (4, 4-dimethyl-1-(4- dodecylphenyl)-1, 3-pentanedione) used as extractant for the extraction of copper ions was laboratorialy synthesized according to Ref.[16]. The diluent (260# kerosene) was supplied by Shanghai Rare-earth Chemical Co, Ltd, China. Aqueous phase was prepared with proper molar ratio of CuCl2·2H2O, NH3·H2O and NH4Cl. All the other reagents used were in AR grade.
2.2 Apparatus
Extraction studies were carried out in conical flask with a thermostatic water bath shaker to equilibrate the samples for 30 min to study the copper extraction or the ammonia stripping. The pH of aqueous solutions was measured with a digital Rex pH meter Model pHS-3C provided with combined glass electrode. The Pgeneral Model TAS-990 atomic absorption spectrophotometer (AAS) was used for analyzing copper content after proper dilutions with 0.3 mol/L HNO3. The ammonia content was determined with Nessler's Reagent spectrophotometry method using Model 722 visible spectrophotometer.
2.3 General extraction experiments
Desired volumes of the aqueous phase and organic phase were equilibrated in a thermostatic water bath shaker for 30 min and after phase disengagement, the aqueous phase was separated followed by the determination of copper concentrations in the raffinate by atomic absorption spectrophotometer[17]. The concentration of copper ions in the organic phase was calculated from the difference between the concentration in the aqueous phase before and after extraction. The loaded organic phases were centrifuged to remove all possible content of aqueous entrainment, stripped with distilled water at a phase ratio (O/A) of 1?5. Then, the two phases were separated with separatory funnel. The ammonia content of the aqueous phase was determined by the Nessler's Reagent Spectrophotometry[18].
3 Results and discussion
The extraction equilibrium for a divalent metal Cu2+ and a chelating extractant, such as sterically hindered β-diketone, is generally given by[19]
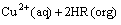
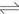
 (1)
(1)
If the aqueous solution contains copper ions and ammonium ions, at sufficiently high pH value where free ammonia is produced, copper ammine complexes in aqueous phase will be formed, which subjects to the following equilibrium[19]:
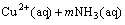
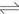
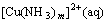 (2)
(2)
When the two phases contact, ammonia in aqueous phase may be extracted into the organic phase as [Cu(NH3)m]R2(org) which is extracted into the organic phase by β-diketone, which subjects to the following equilibrium[20]:

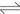
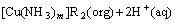 (3)
(3)
where HR(org) refers to sterically hindered β-diketone in organic phase; CuR2(org) refers to complexing compound of copper and sterically hindered β-diketone in organic phase; [Cu(NH3)m2+](aq) refers to the complexing species between ammine and copper ion in aqueous phase; and Cu(NH3)m2+]R2(org) refers to the extraction compound between Cu(NH3)m2+ and sterically hindered β-diketone in organic phase.
3.1 Rate of copper extraction
The extraction experiments were carried out for 3-20 min. The operating conditions of extraction were: [Cu2+]=3 g/L, [NH3]=1 mol/L, [NH4Cl]=2 mol/L, the concentration of β-diketone in organic phase 20% (volume fraction) and phase ratio (O/A)=1 at room temperature. The results are represented in Fig.1.
In Fig.1, the extraction rate of copper increases with the increase of the contact time. The extraction equilibrium is established after 10 min. In all the extraction experiments, a contact time of 30 min was chosen to ensure that the equilibrium could be reached.

Fig. 1 Effect of contact time on copper extraction rate
3.2 Effect of copper concentration on copper extraction rate and ammonia extraction
The effects of the initial copper concentration in the aqueous phase on the extraction of copper and ammonia in the loaded organic phase were studied (See Fig.2 and Fig.3). The operating conditions used were [NH3]=1 mol/L, [NH4Cl]=2 mol/L, the concentration of β- diketone in organic phase 20% (volume fraction), O/A phase ratio 1 and contact time 30 min at room temperature.

Fig.2 Effect of copper concentration of aqueous phase on copper extraction rate
In Fig.2, the extraction rate of copper decreases with the increase of the initial copper concentration in the aqueous phase. The variation of the initial copper concentration from 2.8 to 7.1 g/L leads to a decrease of copper extraction rate from 80.4% to 53.4%. The possible reason may be that the concentration of β-diketone in the initial organic phase is constant, while the concentration of β-diketone is declined with the increasing initial copper concentration in the aqueous phase, which results in the decrease of copper extraction rate.

Fig.3 Effect of copper concentration of aqueous phase on ammonia concentration in loaded organic phase
In Fig.3, the variation of the initial copper concentration from 2.8 to 7.1 g/L leads to an increase of extracted ammonia in loaded organic phase from 20.8 to 35.8 mg/L. Ammonia in aqueous phase is probably extracted to the organic phase by β-diketone as the species [Cu(NH3)m]R2(org). With the increase of the initial copper concentration in aqueous phase, the content of [Cu(NH3)m](aq) increases. And it is beneficial to the formation of [Cu(NH3)x]R2(org) when the two phases are contacted. Hence, there is a significant increase of the extracted ammonia content in the loaded organic phase.
3.3 Effect of total ammonia concentration on copper extraction rate and ammonia extraction
The effects of total ammonia concentration in the initial aqueous phase on the extraction of copper and ammonia are shown in Fig. 4 and Fig.5. The operating conditions used were [Cu2+]=3 g/L, the molar ratio of NH3 to NH4Cl =1:2, the concentration of β-diketone in organic phase 20% (volume fraction), phase ratio (O/A)=1 and contact time 30 min at room temperature.
In Fig.4, it can be observed that the extraction rate of copper decreases with the increase of the total ammonia concentration in the initial aqueous phase. The variation of the total ammonia concentration from 1.5 to 6 mol/L leads to an decrease of the extraction rate of copper from 98.7% to 17.2%. The decrease of extracted copper percentage can be explained that the ammine copper species formation of [Cu(NH3)m]2+ occurs in the aqueous solution and this formation affects the metal extraction by virtue of the resulting decrease in the concentration of free copper ions.
In Fig.5, the extracted ammonia concentration increases with the increase of the total ammonia concentration in the aqueous phase. The variation of the total ammonia concentration from 1.5 to 6 mol/L leads to
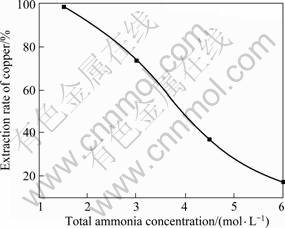
Fig.4 Effect of total ammonia concentration of aqueous phase on copper extraction rate

Fig.5 Effect of total ammonia concentration of aqueous phase on ammonia concentration in loaded organic phase
an increase of extracted ammonia concentration from 5.6 to 16.2 mg/L. These phenomena can be explained by the above equilibriums (2) and (3). The concentration of [Cu(NH3)m]2+(aq) increases with the increase of total ammonia concentration, which results in the increase of extracted ammonia in loaded organic phase.
3.4 Effect of initial pH on copper extraction rate and ammonia extraction
The extraction of copper and ammonia from ammoniacal medium was studied using β-diketone within the initial pH range from 7.77 to 10.03 (see Table 1). The operating conditions of extraction were: [Cu2+]=3 g/L, [NH3]+[NH4Cl]=3 mol/L, the concentration of β-diketone in organic phase 20% (volume fraction), phase ratio (O/A) =1 and contact time 30 min at room temperature.
In Table 1, when the pH value of the aqueous phase ranges from 7.77 to 10.03, pH has little effect on the extraction rate of copper. While the extracted ammonia concentration in loaded organic phase increases with
Table 1 Effect of pH on copper extraction rate and ammonia extraction

increasing pH value of the aqueous phase. As the initial pH value increases from 7.77 to 10.03, the highest extracted ammonia concentration in loaded organic phase is just 16.7 mg/L. These phenomena can be explained that the concentration of [Cu(NH3)m]2+(aq) increases with the increase of the pH value, which results in the increase of extracted ammonia content in loaded organic phase.
3.5 Effect of O/A phase ratio on copper extraction rate and ammonia extraction
The effect of O/A phase ratio on copper extraction ratio and ammonia extraction is shown in Fig.8 and Table 2. The operating conditions used were [Cu2+]=3 g/L, [NH3]=1 mol/L, [NH4Cl]=2 mol/L, the concentration of β-diketone in organic phase 20% (volume fraction) and contact time 30 min at room temperature. The O/A phase ratio is from 1?5 to 10?1.
In Fig.6, the extraction rate of copper increases with the increase of the phase ratio (O/A). The variation of the phase ratio from 1?10 to 2?1 leads to an increase in the rate of extracted copper from 28.0% to 94.2%. When the phase ratio is more than 2, the phase ratio has almost no effect on the extraction rate of copper. These phenomena can be explained by the equilibrium (1). The concentration of HR(org) in organic phase increases with the increase of the phase ratio (O/A), which results in the increase of copper extraction ratio.
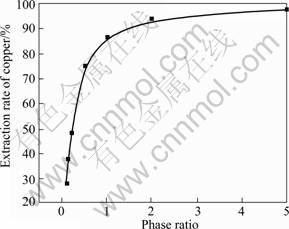
Fig.6 Effect of phase ratio on copper extraction rate
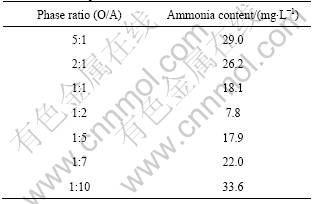
Table 2 shows that the extracted ammonia content in the loaded organic phase decreases from 29.0 to 7.8 mg/L as the phase ratio (O/A) descends from 5?1 to 1?2. However, the extracted ammonia concentration in loaded organic phase increases from 7.8 mg/L to 33.6 mg/L as the phase ratio (O/A) descends from 1?2 to 1?10. The lowest extracted ammonia concentration in loaded organic phase was achieved at O/A ratio of 1?2.
Table 2 Effect of phase ratio on ammonia extraction
3.6 Effect of temperature on copper extraction rate and ammonia extraction
The effects of temperature on copper extraction ratio and ammonia extraction are shown in Table 3. The operating conditions used were [Cu2+]=3 g/L, [NH3]=1 mol/L, [NH4Cl]=2 mol/L, the concentration of β-diketone in organic phase 20% (volume fraction), phase ratio (O/A) =1 and contact time 30 min. The temperature was 25-50 °C.
Table 3 Effect of temperature on copper extraction rate

Table 3 shows that the extraction rate of copper basically unchanged in the temperature range of 25-50 °C. The extracted ammonia concentrations in loaded organic phases are very low (less than 12 mg/L) at 25, 30 and 50 °C.
4 Conclusions
1) The laboratorialy synthesized sterically hindered β-diketone has been proved to be an effective extractant for the extraction of copper with much lower ammonia concentration extracted in loaded organic phase. With the increase of copper concentration and total ammonia concentration in the aqueous phase, the copper extraction rate decreases. With the increase of initial pH value and phase ratio, the copper extraction rate increases. Temperature has almost no effect on the copper extraction rate. The extracted ammonia concentration in loaded organic phase increases with the increase of copper concentration, total ammonia concentration and initial pH value in the aqueous phase.
2) Under the conditions of temperature 25 °C, contact time of two phases 30 min, phase ratio 1?1, concentration of copper 3 g/L, concentration of total ammonia 3 mol/L, aqueous pH 8.43, and concentration of β-diketone in organic phase 20% (volume fraction), ammonia in aqueous phase is much lower to be extracted by the organic phase (just 14.5 mg/L), while the extraction rate of copper is 95.09%. Though the extracted copper rate of β-diketone is a little lower than that of LIX84, the extracted ammonia concentration of β-diketone is much lower than that of LIX84.
References
[1] CHENG Qiong, ZHANG Xiao-lin, LIU Dian-wen, ZHANG Wen-bin. Ammonia leaching of oxidized copper ore at normal temperature and pressure [J]. Hydrometallurgy of China, 2006, 25(2): 74-77. (in Chinese)
[2] CHENG Qiong, ZHANG Wen-bi. Tedmieal progress in ammonia leaching of Tangdan oxidized copper ore containing alkaline gangues [J]. Yunnan Metallurgy, 2005, 34(6): 17-20. (in Chinese)
[3] LIU Da-xing, ZHAO Bing-zhi, JIANG Kai-xi. Study on treatment of Tangdan refractory copper oxide ore with high content of alkali gangues [J]. Mining and Metallurgy of China, 2003, 12(2): 49-52. (in Chinese)
[4] QIAN Dong, WANG Kai-yi, CAI Chun-lin, PAN Chun-yue, TANG You-gen, JIANG Jin-zhi. Separation of nickel, cobalt and copper by solvent extraction with P204 [J]. Transactions of Nonferrous Metals Society of China, 2001, 11(5): 803-805.
[5] ALGUACIL F J, ALONSO M. Recovery of copper from ammoniacal ammonium sulfate medium by LIX 54 [J]. Journal of Chemical Technology and Biotechnology, 1999, 74(12): 1171-1175.
[6] ZHU Tun. Extraction and ion exchange [M]. Beijing: Metallurgical Industry Press, 2005: 280-281. (in Chinese)
[7] REDDY R B, PRIYA N D. Process development for the separation of copper(II), nickel(II) and zinc(II) from sulphate solutions by solvent extraction using LIX84I [J]. Separation and Purification Technology, 2005, 45(2): 163-167.
[8] MACKENZIE M, VIRNIG M, FEATHER A. The recovery of nickel from high-pressure acid leach solutions using mixed hydroxide product-LIX?84-INS technology [J]. Minerals Engineering, 2006, 19(12): 1220-1233.
[9] SARANGI K, PARHI P K, PADHAN E, PALAI A K, NATHSARMA K C, PARK K H. Separation of iron(III), copper(II) and zinc(II) from a mixed sulphate/chloride solution using TBP, LIX84I and Cyanex 923 [J]. Separation and Purification Technology, 2007, 55(1): 44-49.
[10] PARHIA P K, SARANGI K. Separation of copper, zinc, cobalt and nickel ions by supported liquid membrane technique using LIX 84I, TOPS-99 and Cyanex 272 [J]. Separation and Purification Technology, 2008, 59(2): 169-174.
[11] SENGUPTA B, BHAKHAR M S, SENGUPTA R. Extraction of copper from ammoniacal solutions into emulsion liquid membranes using LIX 84 I? [J] Hydrometallurgy, 2007, 89(3/4): 311-318.
[12] PARIJA C, REDDY B R, BHASKARA SARMA P V R. Recovery of nickel from solutions containing ammonium sulphate using LIX 84-I [J]. Hydrometallurgy, 1998, 49(3): 255-261.
[13] PARIJA C, BHASKARA SARMA P V R. Separation of nickel and copper from ammoniacal solutions through co-extraction and selective stripping using LIX84 as the extractant [J]. Hydrometallurgy, 2000, 54(2/3): 195-204.
[14] RITCEY G M, ASHBROOK A W. Solvent extraction: Principles and applications to process metallurgy [M]. SUN Fang-jiu, transl. Beijing: Atomic Energy Press, 1985: 111-119. (in Chinese)
[15] LIU Chun-xuan, HU Hui-ping, HAN Xue-tao. Study on the copper and ammonia extraction from Cu2+-NH3-Cl--H2O solutions with the mixture of LIX84 and TBP [J]. The Chinese Journal of Process Engineering. 2009, 9(6): 86-92. (in Chinese)
[16] FU Weng. The synthesis and characterization of four kinds of beta-diketone [D]. Changsha: Central South University. 2008: 35-56. (in Chinese)
[17] GB/T 2466. 1-1996. Pyrites and concentrate-determination of copper content [S]. (in Chinese)
[18] GB/T18204. 25-2000. Methods for determination of ammonia in air of public places [S]. (in Chinese)
[19] ALGUACIL F J, ALONSO M. The effect of ammonium sulphate and ammonia on the liquid-liquid extraction of zinc using LIX 54 [J]. Hydrometallurgy, 1999, 53: 203-209.
[20] FLETT D S, MELLING J. Extraction of ammonia by commercial copper chelating extractants [J]. Hydrometallurgy, 1979, 4: 135-146.
(Edited by LI Xiang-qun)
Foundation item: Project(2007CB613601) supported by the National Basic Research Program of China; Project(NCET-08) supported by Program for New Century Excellent Talents in University, China
Corresponding author: HU Hui-ping; Tel: +86-731-88876034; E-mail: phhuiping@hotmail.com
DOI: 10.1016/S1003-6326(09)60412-X