


Trans. Nonferrous Met. Soc. China 22(2012) 366-372
Preparation and tribological properties of surface modified Cu nanoparticles
YANG Guang-bin, CHAI Shan-tao, XIONG Xiu-juan, ZHANG Sheng-mao, YU Lai-gui, ZHANG Ping-yu
Key Laboratory of Ministry of Education for Special Functional Materials, Henan University,Kaifeng 475004, China
Received 28 March 2011; accepted 17 June 2011
Abstract: Cu nanoparticles surface-modified by dioctylamine dithiocarbamate (DTC8) were synthesized using a two-phase extraction route. The size, morphology and structure of resultant surface-capped Cu nanoparticles (coded as DTC8-Cu) were analyzed by means of X-ray diffraction, transmission electron microscopy and infrared spectrometry. The tribological behavior of DTC8-Cu as an additive in liquid paraffin was evaluated with a four–ball machine, and the surface topography of the wear scar was also examined by means of scanning electron microscopy. Results show that Cu nanoparticles modified by DTC8 have a small particle size and a narrow size distribution. Besides, DTC8-Cu as an additive in liquid paraffin has excellent antiwear ability, due to the deposition of nano-Cu with low melting point on worn steel surface leading to the formation of a self-repairing protective layer thereon.
Key words: copper nanoparticles; surface modification; tribological properties; additive; preparation
1 Introduction
Metal nanoparticles have been widely used in catalysis, photonics, magnetics, semiconductor and other fields by virtue of their unique physical and chemical properties. In recent years, inorganic nanoparticles have been extensively focused on the field of tribology [1-4]. Thanks to specific nature of nanoparticles such as small size effect, macro quantum tunnel effect, high surface area and activity, as well as isotropy, low melting point and shear strength, and good thermal stability, Cu nanoparticles as lubricant additives have been found to possess excellent tribological performance, self-repairing properties and environmental-friendliness [5-8]. However, inorganic nanoparticles (in particular, naked nanoclusters) have high surface activity and poor compatibility with lubricating oils and are easy to aggregate, which greatly hinders their application as additives in lubricating oils and greases. Fortunately, it is feasible to encapsulate inorganic nanoparticles by making use of in-situ surface-modification technique and hence effectively prevent the nanoparticles from aggregation and improve their compatibility with lubricating oils and greases [9].
It has been recognized that capping agents or surface modifying agents play an important role in stabilizing and controlling shape of nanoparticles [10, 11]. For example, citrate-protected copper colloidal nanoparticles turned olive-green and precipitated immediately upon exposure to air, indicating oxidation in an aerobic condition [12]. Moreover, the tribological properties of copper nanoparticles modified by organic compounds have been reported [5, 6, 13-16], but most of the surface modifying agents contain phosphorus element which leads to poisoning and failure of metallic catalysts in vehicular catalytic transformer, resulting in difficulty in control of harmful gas emissions. To meet the demands for sustainable development, environment protection and stringent emission regulations, many countries have limited the content of phosphorus in oils [17]. This urges us to develop novel Cu nanoparticle additives surface-capped with P-free organic compounds. Therefore, in the present research, CuSO4·5H2O was used as the starting material to synthesize Cu nanoparticles surface-capped by dioctylamine dithiocarbamate (DTC8) with bidentate nature and strong affinity to metal surface in the presence of hydrazine hydrate as the reducing agent and toluene plus water as the solvents. Two-phase extraction route was adopted to prepare stable dispersion of oil-soluble Cu nanoparticles, because among various methods for preparing inorganic nanoparticles, including irradiation, microemulsion, vacuum vapor deposition, metal vapor synthesis, chemical reduction, sonochemical reduction, thermal reduction, laser ablation, etc, chemical reduction in aqueous or organic solvents is simple and cost-effective, showing the greatest feasibility to be put into practical use [18]. The tribological properties of as-synthesized surface-capped Cu nanoparticles as lubricant additive were evaluated using a four-ball machine, and their crystal structure was analyzed by means of X-ray diffraction (XRD). Besides, the morphology and composition of worn steel surfaces were analyzed by means of scanning electron microscopy (SEM) and energy dispersive spectrometry (EDS).
2 Experimental
2.1 Preparation of surface-modified Cu nanoparticles
Dioctylamine dithiocarbamate was synthesized by following literature protocol [19]. Briefly, dioctylamine was dissolved in ethanol at a concentration of 10%, followed by dropwise addition of a stoichiometric amount of carbon disulfide (CS2). Resultant mixed solution was vigorously stirred at room temperature for 3 h, generating dioctylamine dithiocarbamate as the surface-capping agent for synthesizing Cu nanoparticles. The structure of DTC8 is shown in Fig. 1.
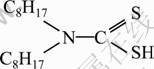
Fig. 1 Structure of DTC8
The details about the preparation of metallic Cu nanoparticles are described as follows. First, 20 mmol CuSO4·5H2O was completely dissolved in deionized water and the solution was adjusted to pH of 10 using ammonia solution. Then 120 mmol (10 mL) N2H4·H2O dissolved in 50 mL deionized water was added into the CuSO4 solution under magnetic stirring to change the solution color from blue to brown. Finally, a calculated amount of as-synthesized DTC8 ligand dissolved in toluene was added dropwise into the mixed solution of CuSO4 and N2H4·H2O to react for 3 h, accompanied by change of solution color to dark black. At the end of the reaction, the solution was separated into two phases. The organic phase was collected and rinsed with deionized water several times. After toluene solvent was removed by rotary evaporating, a sticky dark brown liquid of expected DDC8-capped Cu nanoparticles was obtained. Figure 2 schematically shows the structure of surface capped Cu nanoparticles.

Fig. 2 Suggested structure of surface-modified Cu nanoparticles
2.2 Characterization of surface-modified Cu nanoparticles
X-ray diffraction (XRD) analysis of surface- modified Cu nanoparticles was conducted with an X'Pert Philips X-ray diffractometer (Cu Kα radiation, operating at 40 kV and 40 mA, over a diffraction angle from 30° to 92° at a velocity of 0.04° per step).
A JEOL JEM-2010 microscope (TEM) was performed to analyze the microstructure of surface- modified Cu nanoparticles. The sample for TEM analysis was prepared by placing a drop of the toluene solution containing as-prepared target product onto a copper grid coated with a carbon film.
An Avatar 360 Fourier transform infrared (FT-IR) system was employed to confirm the chemical structure of as-prepared Cu nanoparticles.
2.3 Evaluation of tribological properties of surface- modified Cu nanoparticles
The tribological properties of as-prepared Cu nanoparticles as an additive in liquid paraffin were evaluated using a four-ball machine under conditions of a rotating speed of 1450 r/min, test duration of 30 min, and normal load of 300 N. AISI-52100 steel balls with a diameter of 12.7 mm and hardness of HRc 64-66 were used to comprise the frictional pair. Chemically pure paraffin oil with a boiling point above 300 °C was used as the base fluid. Continuous lubrication of the frictional pair was realized by immersing it in the oil cup containing a proper amount of to-be-tested lubricant. The wear scar diameters of the three lower balls were measured using an optical microscope to an accuracy of ±0.01 mm, and the friction coefficients were recorded automatically with a strain gauge attached to the test rig.
The morphology of the wear scars on worn steel balls was observed using a JSM 5600LV microscope (SEM, accelerating voltage 20 kV) equipped with an energy dispersive spectrometer (EDS) attachment.
朗读显示对应的拉丁字符的拼音字典 - 查看字典详细内容
3 Results and discussion
3.1 Structural characterization of surface-modified Cu nanoparticles
As-obtained sticky dark brown liquid can be well dissolved in apolar solvents including hexane, benzene, toluene, dichloromethane, and chloroform, but they cannot be dissolved in polar solvents such as alcohol, acetone, and water. Besides, they are free of color change or precipitation phenomenon during storage in atmosphere, indicting that as-synthesized surface-capped Cu nanoparticles are stable in ambient condition.
Figure 3 shows the XRD pattern of as-prepared surface-modified Cu nanoparticles. A large protrusion emerges at 2θ angles of 38-53°, which indicates that as-synthesized Cu nanoparticles should have a small particle size and contain a relatively high content of organic modifying layer [20].
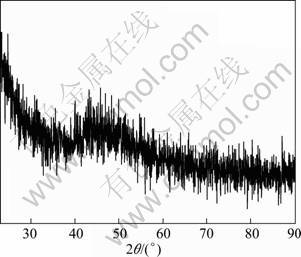
Fig. 3 XRD pattern of surface-modified Cu nanoparticles
Figure 4 shows a typical TEM image of as-prepared Cu nanoparticles. It is seen that surface-capped Cu nanoparticles appear as spheres and have a small average particle diameter (within a range of 2-6 nm) and narrow size distribution, and they show no obvious aggregating phenomenon. It is likely that the molecules of the organic modifying agent have been adsorbed on the surfaces of Cu nanoparticles to reduce their surface energy and prevent them from aggregation, resulting in well controlled particle size and improved dispersion capability of the nanoparticles in organic solvent [21]. In the meantime, additives with smaller particle size show better sedimentation stability, which provides more uniform distribution of the particles in oil [22, 23].
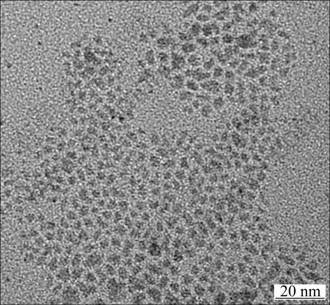
Fig. 4 TEM image of surface-modified Cu nanoparticles
The reduction reaction of aqueous CuSO4 by hydrazine hydrate under alkaline condition can be expressed as:
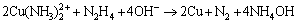
Hydrazine hydrate is much cheaper than other reducing agents like sodium borohydride and ascorbic acid. Furthermore, nitrogen gas produced during the reduction process may create an inert atmosphere automatically, which makes it feasible to synthesize metallic Cu nanoparticles in the absence of additional inert gases. As-prepared Cu nanoparticles have a small particle size and narrow size distribution, possibly because ammonia produced during the reduction reaction is able to not only adjust the pH of the CuSO4 solution but also function as a complexing agent generating chelated Cu(NH3)22+ ions and preventing severe reduction of Cu2+ and excessive growth of reduced Cu particles. Namely, there is a dynamical equilibrium between Cu2+ and Cu(NH3)22+, Cu2+ is gradually released due to dynamical equilibrium, and released Cu2+ is reduced by hydrazine hydrate in a mild manner to form Cu nanoparticles. Hopefully, such a facile synthetic approach might find promising application in large-scale production of copper nanoparticles.
One can envision that the choice of appropriate surface modifying agent is critical in the control of the eventual particle structures. Dithiocarbamate (DTC) ligand with bidentate nature was adopted in the present research, because it was anticipated to exhibit stronger affinity to the metal surface favoring the formation of smaller particle cores, also because it had been shown for the first time to stabilize the formation of gold nanoparticles, presumably via strong sulfur–gold interactions and a combination of steric and electrostatic repulsions between nanoparticle ligand shells preventing agglomeration [19].
Figure 5 shows the infrared (IR) spectra of surface-modified Cu nanoparticles and DTC8. Compared with the IR spectrum of DTC8, the IR spectrum of DTC8-Cu also has an absorption band at 2850-2950 cm–1, which is attributed to the C—H stretching vibration of —CH3 and —CH2. The strong absorption peaks at 1436 cm–1 and 1374 cm–1 correspond to the C—H bending vibration of —CH3 and —CH2—, respectively. The absorption peak at 723 cm–1 is assigned to the C—H bending vibration of (—CH2—)n (n≥4). The strong absorption peak at 1492 cm–1, located between the absorption peak of single bond C—N (1250-1350 cm–1) and double bond C=N (1640-1690 cm–1), is assigned to the characteristic absorption of C—N in dithiocarbamate, which indicates that C—N in the surface-capped Cu nanoparticles could have the nature of double bond C=N in some sense. Besides, a single absorption peak of vC—S emerges around 977 cm–1, which is lower than that of νC=S (1070-1200 cm–1) but higher than that of νC—S(860-930 cm–1). This implies that the electron cloud of the two carbon-sulfur bonds of dithiocarbamate in the surface-modified Cu nanoparticles tends to be averaged, forming two equivalent carbon- sulfur bonds [24].
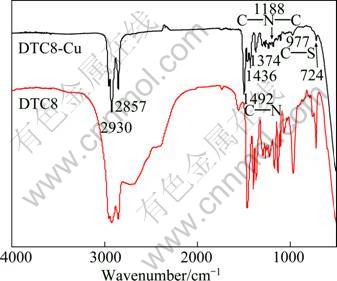
Fig. 5 FTIR spectra of surface-modified Cu nanoparticles
Based on the above-mentioned structural characterization results, we can primarily suppose that the molecules of the organic modifier are anchored to the surfaces of Cu nanoparticles via strong S—Cu interactions, forming an organic modifying layer containing long alkyl chain on the metal surface (see Fig. 2). This is why as-prepared Cu nanoparticles can be stably dispersed in organic solvents such as toluene, benzene, chloroform, petroleum ether and liquid paraffin.
3.2 Tribological properties of surface-modified Cu nanoparticles
Figure 6 shows the variation of friction coefficients with the content of Cu nanoparticles in liquid paraffin under a load of 300 N. The introduction of Cu nanoparticles in liquid paraffin led to a slight increase of the friction coefficient of the steel frictional pair, which might be closely related to the deposition of surface- capped Cu nanoparticles on the contact zone of the frictional pair. On one hand, when surface-capped Cu nanoparticles deposited on the steel sliding surface function as rolling balls benefit the formation of a good lubricating film thereon, the friction coefficient will be effectively reduced. On the other hand, when surface- capped Cu nanoparticles deposited on the steel sliding surface result in damage to the lubricating film, the friction coefficient will be increased [25]. We thought that Cu nanoparticles may destroy the oil film due to competing adsorption with liquid paraffin and cannot make up of continuous lubricating film, affect the compactness of the film formed by the base stock alone, resulting in high shear strength or surface roughness of the film containing Cu nanopartilces, resulting in increased friction coefficient [26, 27].
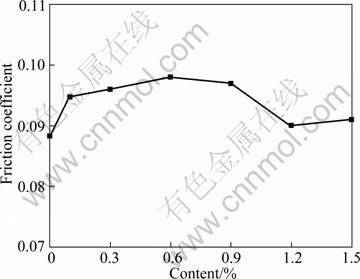
Fig. 6 Variation of friction coefficient with content of Cu nanoparticles in liquid paraffin (four-ball, 300 N, 1450 r/min, 30 min)
Figure 7 shows the wear scar diameter as a function of Cu nanoparticles content in liquid paraffin (four-ball tester, load 300 N, speed 1450 r/min, duration 30 min). The wear scar diameter is reduced from 0.665 mm under the lubrication of liquid paraffin alone to 0.460 mm under the lubrication of liquid paraffin containing 1.5% surface-capped Cu nanoparticles. This indicates that Cu nanoparticles as a lubricant additive have excellent antiwear ability, which might be closely related to the small size, low melting point and high reactivity of copper nanoparticles. Particularly in the form of a stable dispersion in oils, surface-capped copper nanoparticles are easy to enter into the rubbing surface and are deposited thereon to form a surface protective film under local high temperature and high contact pressure, resulting in significantly increased antiwear ability [25]. Furthermore, surface-capped Cu nanoparticles in liquid paraffin can fill up micro-pits and grooves of the friction surface to play a role in self-repairing, also resulting in increased antiwear ability. Subsequently, Cu nanoparticles with a small size are more liable to interact with the surfaces of the friction pairs to form a surface protective film, which contributes primarily to increased antiwear ability [25].

Fig. 7 Wear scar diameter as function of Cu nanoparticles content in liquid paraffin (four-ball, 300 N, 1450 r/min, 30 min)
Figure 8 shows the SEM micrographs of the wear scars lubricated by liquid paraffin alone and liquid paraffin containing 0.9% Cu nanoparticles (load 300 N, speed 1450 r/min, duration 30 min). It is seen that the wear scar under the lubrication of liquid paraffin containing surface-capped Cu nanoparticles is much smaller than that under the lubrication of liquid paraffin alone, well corresponding to relevant wear scar diameter shown in Fig. 7. Furthermore, the worn surface lubricated with liquid paraffin containing Cu nanoparticles is much smoother and flatter than that lubricated with liquid paraffin alone, and shows almost no sign of severe scuffing, well corresponding to good antiwear ability and self-repairing function of the surface-capped copper nanoparticles.
In order to further prove the presence of deposited Cu film on the worn surface of steel ball, we conducted EDS analysis. As shown in Fig. 9, Cu element was detected on worn steel surface, confirming the existence of Cu in the boundary lubricating film. Thus it can be concluded that the tribochemical reaction film in connection with deposited nano-Cu particulates of good antiwear ability and self-repairing function contributes to greatly reducing wear of the steel-steel pair.

Fig. 8 SEM images of worn steel surfaces lubricated with liquid paraffin (a, b) and liquid paraffin containing 0.9% surface-capped Cu nanoparticles (c, d)
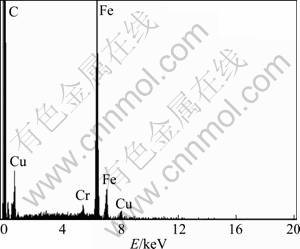
Fig. 9 EDS analysis of worn surface of steel ball lubricated with liquid paraffin containing 0.9% surface-capped Cu nanoparticles
4 Conclusions
1) Two-phase extraction method can be readily adopted to prepare environmentally friendly Cu nanoparticles surface-modified by DTC8. As-prepared Cu nanoparticles have a small particle size and narrow size distribution. The synthesis approach is simple and does not need extra protective gases, showing promising application for large-scale production of Cu nanoparticles.
朗读显示对应的拉丁字符的拼音字典 - 查看字典详细内容
2) The as-synthesized surface-modified Cu nanoparticles as a lubricant additive in liquid paraffin possess excellent antiwear performance. This is because surface-capped Cu nanoparticles with a low melting point are able to deposit on sliding steel surface to form a good protective film; also because surface-capped Cu nanoparticles can fill up micro-pits on the rubbing steel surface and exert self-repairing function.
References
[1] GAO Yong-jian, CHEN Guo-xu, OLI Ya, ZHANG Zhi-jun, XUE Qun-ji. Study on tribological properties of oleic acid-modified TiO2 nanoparticle in water [J]. Wear, 2002, 252(5-6): 454-458.
[2] ZHOU Jing-fang, WU Zhi-shen, ZHANG Zhi-jun, LIU Wei-min, DANG Hong-xin. Study on an antiwear and extreme pressure additive of surface coated LaF3 nanoparticles in liquid paraffin [J]. Wear, 2001, 249(5-6): 333-337.
[3] CHEN Shuang, LIU Wei-min. Characterization and anti-wear ability of non-coated ZnS nanoparticles and DDP-coated ZnS nanoparticles [J]. Materials Research Bulletin, 2001, 36(1-2): 137-143.
[4] RAPOPORT L, FLEISCHER N, TENNE R. Fullerene-like WS2 nanoparticles: Superior lubricants for harsh conditions [J]. Advanced Materials, 2003, 15(7-8): 651-655.
[5] ZHANG Yi-dong, YAN Jia-sheng, SUN Lei, YANG Guang-bin, ZHANG Zhi-jun, ZHANG Ping-yu. Friction reducing anti-wear and self-repairing properties of nano-Cu additive in lubricating oil [J]. Journal of Mechanical Engineering, 2010, 46(5): 74-79. (in Chinese)
[6] ZHANG Ming, WANG Xiao-bo, LIU Wei-min, FU Xi-sheng. Performance and anti-wear mechanism of Cu nanoparticles as lubricating oil additives [J]. Industrial Lubrication and Tribology, 2009, 61(6): 311-318.
[7] YU He-long, XU Yi, SHI Pei-jing, XU Bin-shi, WANG Xiao-li, LIU Qian. Tribological properties and lubricating mechanisms of Cu nanoparticles in lubricant [J]. Transactions of Nonferrous Metals Society of China, 2008, 18(3): 636-641.
[8] CHOI Y, LEE C, HWANG Y, PARK M, LEE J, CHOI C, JUNG M. Tribological behavior of copper nanoparticles as additives in oil [J]. Current Applied Physics, 2009, 9(2): 124-127.
[9] SUN Lei, ZHOU Jing-fang, ZHANG Zhi-jun, DANG Hong-xin. Synthesis and tribological behavior of surface modified (NH4)3PMo12O40 nanoparticles [J]. Wear, 2004, 256(1-2): 176-181.
[10] MOTT D, MAI N T, THUY N, MAEDA Y, LINH T, KOYANO M, MAENOSONO S. Bismuth, antimony and tellurium alloy nanoparticles with controllable shape and composition for efficient thermoelectric devices [J]. Phys Status Solidi A, 2011, 208(1): 52-58.
[11] WANG Y, CHEN J, CHEN L, CHEN Y B, WU L M. Shape- controlled solventless syntheses of nano Bi disks and spheres [J]. Crystal Growth and Design, 2010, 10: 1578-1584.
[12] SAMIM M, KAUSHIK N K, MAITRA A. Effect of size of copper nanoparticles on its catalytic behaviour in Ullman reaction [J]. Bulletin of Material Science, 2007, 30: 535-540.
[13] ZHANG Bao-sen, XU Bin-shi, XU Yi, GAO Fei, SHI Pei-jing, WU Yi-xiong. Cu nanoparticles effect on the tribological properties of hydrosilicate powders as lubricant additive for steel–steel contacts [J]. Tribology International, 2011, 44: 878-886.
[14] ZHANG Yi-dong, YAN Jia-sheng, YU Lai-gui, ZHANG Ping-yu. Effect of nano-Cu lubrication additive on the contact fatigue behavior of steel [J]. Tribololy Letters, 2010, 37: 203-207.
[15] MA Jian-qi, WANG Xiao-bo, FU Xing-guo, LIU Wei-min, CUI Ruo-mei. Investigation of the tribological properties of oil-soluble Cu nanoparticles as additive in CD 15W/40 diesel engine oil [J]. Tribology, 2004, 24(2): 134-138. (in Chinese)
[16] ZHOU Jing-fang, ZHANG Zhi-jun, WANG Xiao-bo, LIU Wei-min, XUE Qun-ji. Investigation of the tribological behavior of oil-soluble Cu nanoparticles as additive in liquid paraffin [J]. Tribology, 2000, 20(2): 123-126. (in Chinese)
[17] ZHANG Ling, CHEN Lei, ZHOU Hui-di, CHEN Jian-min. Synthesis and tribological behavior of surface coated Cu nanoparticles in liquid paraffin [J]. China Surface Engineering, 2009, 22(3): 62-66. (in Chinese)
[18] ZHANG H, SIEGERT U, LIU R, CAI W. Facile fabrication of ultrafine copper nanoparticles in organic solvent [J]. Nanoscale Res Lett, 2009, 4: 705-708.
[19] TONG M, CHEN W, SUN J, GHOSH D, CHEN S. Dithiocarbamate-capped silver nanoparticles [J]. J Phys Chem B, 2006, 110: 19238-19242.
[20] YANG Jun, CHEN Shuang. Preparation of oil-soluble Cu nanoparticles by a two-phase method [J]. Acta Chimica Sinica, 2007, 65(20): 2243-2248. (in Chinese)
[21] SUN Lei, ZHOU Jing-fang, ZHANG Zhi-jun, DANG Hong-xin. Synthesis and tribological behavior of surface modified (NH4)3PMo12O40 nanoparticles [J]. Wear, 2004, 256: 176-181.
[22] TARASOV S, KOLUBAEV A, BELYAEV S, LERNER M, TEPPER F. Study of friction reduction by nanocopper additives to motor oil [J]. Wear, 2002, 252: 63-69.
[23] WU Y Y, TSUI W C, LIU T C. Experimental analysis of tribological properties of lubricating oils with nanoparticle additives [J]. Wear, 2007, 262: 819-825.
[24] YIN Han-dong, ZHANG Ru-fen, MA Chun-lin. Study on tribenzyltin dithiocarbamicacid complexes by IR spectroscopy [J]. Chinese Journal of Spectroscopy Laboratory, 1998, 15 (5): 10–13. (in Chinese)
[25] MARTIN J M, OHMAE N. Nanolubricants [M]. England: John Wiley & Sons Ltd, 2008.
[26] FAN K, LI J, MA H, WU H, REN T, KASRAI M, BANCROFT G M. Tribological characteristics of ashless dithiocarbamate derivatives and their combinations with ZDDP as additives in mineral oil [J]. Tribology International, 2008, 41: 1226-1231.
[27] ZENG X, LI J, WU X, REN T, LIU W. The tribological behaviors of hydroxyl-containing dithiocarbamate-triazine derivatives as additives in rapeseed oil [J]. Tribology International, 2007, 40: 560-566.
表面修饰Cu纳米微粒的制备及摩擦学性能
杨广彬, 柴单淘, 熊秀娟, 张晟卯, 余来贵, 张平余
河南大学 特种功能材料教育部重点实验室,开封 475004
摘 要:利用两相萃取法合成了二辛基胺二硫代氨基甲酸(DTC8)表面修饰铜纳米微粒;采用X射线衍射仪、透射电子显微镜、红外光谱仪表征了铜纳米颗粒的尺寸、形貌和结构,采用四球摩擦磨损试验机评价了纳米铜添加剂在液体石蜡中的摩擦学性能,并采用扫描电子显微镜观察了磨斑形貌。结果表明:DTC8修饰铜纳米微粒的粒径较小,粒径分布较窄。与此同时,表面修饰纳米铜作为润滑油添加剂具有优异的抗磨性能,这可能是由于熔点低且易变形的纳米铜可填充磨损表面微坑而起到自修复作用所致。
关键词:铜纳米微粒;表面修饰;摩擦学性能;添加剂;制备
(Edited by LI Xiang-qun)
Foundation item: Project (2007CB607606) supported by the Ministry of Science and Technology of China; Project (50975077) supported by the National Natural Science Foundation of China
Corresponding author: ZHANG Ping-yu; Tel: +86-378-3886397; Fax: +86-378-3881358; E-mail: pingyu@henu.edu.cn
DOI: 10.1016/S1003-6326(11)61185-0