氰化浸出、氨预处理及氨氰浸出法处理富铜金矿
来源期刊:中国有色金属学报(英文版)2015年第2期
论文作者:A. D. BAS E. KOC Y. E. YAZICI H. DEVECI
文章页码:597 - 607
Key words:copper-gold ores; ammoniacal cyanide leaching; ammonia leaching; pretreatment; cyanide leaching
摘 要:采用直接氰化浸出、氨预处理及氨氰浸出等方法处理含硫化铜的金矿。研究这些浸出体系中金和铜的溶解行为。当NaCN含量小于5 g/L 时,金的氰化浸出受到所含硫化铜的严重干扰,这与组分平衡计算结果一致。氨预处理可以消除铜的干扰,在后续的氰化浸出过程中,即使在剂量较小的情况下,金也可以获得较高的提取率。采用Box-Behnken试验设计研究了氨氰体系中NH3、NaCN和Pb(NO3)2的主要影响以及其相互作用。其中NH3和NaCN浓度是影响金提取率的主要因素,而Pb(NO3)2的影响有限。增加NH3的浓度可以提高金提取的选择性和提取率,同时降低氰化物的用量。统计研究结果表明试剂间的相互作用对金提取无重大影响。氨预处理和氨氰浸出法在含硫化铜的金矿的处理中具有广阔的应用前景。
Abstract: The treatment of a copper sulphide-bearing gold ore by direct cyanide leaching, ammonia pretreatment and ammoniacal cyanide leaching was investigated. Dissolution behaviour of gold and copper in these leaching systems was demonstrated. Severe interference by the copper containing sulphides with cyanide leaching of gold is observed at ρ(NaCN)≤5 g/L. This is consistent with speciation calculations. Ammonia pretreatment is shown to readily eliminate the copper interference, allowing almost complete extraction of gold with concomitantly low reagent consumption in subsequent cyanide leaching. In ammoniacal cyanide system, Box-Behnken experimental design shows the main and interaction effects of NH3, NaCN and Pb(NO3)2. The concentrations of NH3 and NaCN are statistically confirmed to be significant factors affecting extraction of gold while the effect of Pb(NO3)2 is limited. Increasing the concentration of NH3 improves the selectivity and extent of gold extraction and reduces the cyanide consumption. The contribution of reagent interactions to gold extraction is statistically insignificant. These findings highlight that ammonia pretreatment and ammonia-cyanide leaching are promising approaches for the treatment of gold ores with high copper sulphide content.
Trans. Nonferrous Met. Soc. China 25(2015) 597-607
A. D. BAS, E. KOC, Y. E. YAZICI, H. DEVECI
Hydromet-B&PM Group, Division of Mineral & Coal Processing, Department of Mining Engineering,
Karadeniz Technical University, Trabzon 61080, Turkey
Received 28 March 2014; accepted 1 September 2014
Abstract: The treatment of a copper sulphide-bearing gold ore by direct cyanide leaching, ammonia pretreatment and ammoniacal cyanide leaching was investigated. Dissolution behaviour of gold and copper in these leaching systems was demonstrated. Severe interference by the copper containing sulphides with cyanide leaching of gold is observed at ρ(NaCN)≤5 g/L. This is consistent with speciation calculations. Ammonia pretreatment is shown to readily eliminate the copper interference, allowing almost complete extraction of gold with concomitantly low reagent consumption in subsequent cyanide leaching. In ammoniacal cyanide system, Box-Behnken experimental design shows the main and interaction effects of NH3, NaCN and Pb(NO3)2. The concentrations of NH3 and NaCN are statistically confirmed to be significant factors affecting extraction of gold while the effect of Pb(NO3)2 is limited. Increasing the concentration of NH3 improves the selectivity and extent of gold extraction and reduces the cyanide consumption. The contribution of reagent interactions to gold extraction is statistically insignificant. These findings highlight that ammonia pretreatment and ammonia-cyanide leaching are promising approaches for the treatment of gold ores with high copper sulphide content.
Key words: copper-gold ores; ammoniacal cyanide leaching; ammonia leaching; pretreatment; cyanide leaching
1 Introduction
Cyanide with its competency to form a strong complex with gold, is the most preferable reagent for leaching of gold from ores. Cyanide leaching is not inherently a selective process since many other metals/minerals present in the ore are also readily soluble under cyanide leaching conditions [1]. In this regard, most copper minerals are highly soluble in cyanide solutions (Table 1) although chalcopyrite and tetrahedrite are relatively stable. The presence of these soluble minerals may interfere with cyanide leaching and downstream processes for gold recovery [3-5].
The detrimental effect of copper is associated with the excessive consumption of cyanide (Eqs. (1)-(5)) through the formation of highly stable copper cyanide complexes leading to poor gold extractions when sufficient level of cyanide is not provided [6,7]. MUIR [4] reported that every 1% reactive copper present in the ore consumes 30 kg/t NaCN, which adversely affects the process economics. Cyanide consumption can be even higher (up to 51.5 kg/t for every 1% of copper contained) due to the formation of thiocyanate (Eqs. (3) and (5)) and cyanate (Eq. (4)) in addition to copper cyanide complexes when copper sulphides such as covellite are present [3].
Cu2++2CN–→Cu(CN)2 (1)
Cu2O+6CN–+H2O→2Cu(CN)32–+2OH– (2)
Cu2S+7CN–+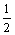 O2+H2O→2Cu(CN)32–+2OH– +CNS– (3)
O2+H2O→2Cu(CN)32–+2OH– +CNS– (3)
2CuO+7CN–+H2O→2Cu(CN)32–+2OH–+CNO– (4)
2CuS+8CN–+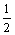 O2+H2O→2Cu(CN)32–+2OH– +2CNS- (5)
O2+H2O→2Cu(CN)32–+2OH– +2CNS- (5)
MUIR et al [8] pointed out that 0.5% Cu is often considered the threshold for cyanide leaching of gold ores. Development of alternative processes to cyanide leaching is, therefore, required for the treatment of copper-rich gold ores. In this regard, flotation [9,10], alternative lixiviant systems such as thiosulphate or ammoniacal cyanide [11,12], leaching of copper prior to cyanidation [2,6,8], cyanide recovery from effluents (e.g. SART process) [5], preaeration prior to cyanidation [6,13,14], and addition of lead nitrate [14,15] have been tested for the treatment of copper-rich gold ores.
Table 1 Solubility of copper minerals in 0.1% NaCN solution [1,2]

Sulphide ion (S2-) that is released from sulphide phases including copper sulphides present in the ore can also lead to the passivation of gold surface in addition to its consumption of cyanide and oxygen [1]. Pre-aeration of ore prior to cyanidation and/or addition of lead nitrate can be beneficial for mitigating problems associated with sulphide minerals during cyanidation of gold [13,14,16].
Preleaching of ore can be undertaken to remove reactive copper minerals prior to cyanide leaching of gold. SCERESINI and STAUNTON [17] proposed the removal of copper by cyanide leaching without aeration prior to gold extraction and then adsorption of copper on activated carbon. Ammonia and sulphuric acid are often regarded as suitable leaching systems for preleaching of copper from gold ores [6,8,18-20]. Compared with acid leaching, ammonia leaching has the merits of high selectivity over gang minerals, low corrosivity and application in alkaline conditions [8]. MUIR et al [8] demonstrated the removal of copper (up to 95%) from an oxidised tailings via ammonia leaching, which resulted in a substantially reduced reagent consumption (from 30 kg/t to 6 kg/t NaCN) in the subsequent cyanide leaching. Despite its potential benefits for cyanide leaching, downstream gold recovery and effluent treatment, ammonia leaching of copper ahead of cyanidation of gold have received very limited interest [7,8].
Having been firstly proposed and patented by HUNT [21], ammonia can be used as a modifying agent in cyanide leaching to mitigate the copper interference, allowing substantial reduction in cyanide consumption (by up to 90%) and selective leaching of gold with only limited dissolution of copper [3,4,22,23]. Ammoniacal cyanide leaching was successfully demonstrated for oxidised ores/tailings with selective extraction of 70%-90% Au [8,18,19,22,24]. However, MUIR [4] noted that copper sulphide bearing gold ores yielded poor gold extraction and demanded high reagent dosages for their treatment. MUIR et al [8] reported that the ammoniacal cyanide leaching was an effective system for the recovery of gold from a copper-rich tailings (1.2% Cu, 5 g/t Au). They noted that the cyanide consumption was substantially reduced from 30 kg/t (in the absence of ammonia) to only 1.6 kg/t NaCN (and 1.1 kg/t NH3 in the presence of 0.3 kg/t Cu2+). The beneficial effect of ammonia was attributed to the stabilization of Cu2+ (Eq. (6)) as Cu(NH3)42+, which also acted as oxidant [4,24]. The selectivity of ammoniacal cyanide leaching for gold appeared to be linked with the precipitation of copper presumably as Cu3(NH3)3(CN)4 [23,25]. pH is an important parameter controlling free NH3 and leaching of gold and copper, which tends to be enhanced with increasing pH [24]. The optimum pH was reported to lie between 10.5 and 11.5 with the gold extraction being more selective at low pH values [3]. DESCHENES et al [14] also pointed out the importance of dissolved oxygen (DO2) concentration in ammoniacal cyanide leaching of gold from a synthetic copper bearing ore containing djurleite and bornite. They observed that the extraction of gold improved with increasing DO2 concentration from 8 mg/L to 20 mg/L.
Cu2++4NH3→Cu(NH3)42+ (6)
It can be inferred from these earlier studies that the chemistry of ammoniacal cyanide leaching is rather complex with intimate interactions among the species of ammonia, copper and cyanide. The presence of copper as sulphides increases the complexity of the system. Therefore, ammoniacal cyanide leaching of a copper sulphide-rich, high grade gold ore was investigated using Box-Behnken experimental design to demonstrate the significance of main and interaction effects of concentrations of NH3, NaCN and Pb(NO3)2 on the extraction of gold and copper, selectivity and cyanide consumption. The addition of Pb(NO3)2 into ammoniacal cyanide leaching was also tested to reduce the adverse effect of S2- since copper is present predominantly as sulphides in the ore. In addition, ammonia leaching as a pretreament process ahead of cyanidation was examined. Meanwhile, direct cyanide leaching of gold at elevated levels of cyanide was carried out for comparison. The findings were analysed and discussed based on the theoretical evaluation of the experimental data.
2 Experimental
2.1 Ore Sample
The ore sample was obtained from the sulphide-rich zones of Mastra Gold Deposit located in 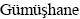 , Turkey. The sample was reduced in size to ≤4 mm by using laboratory type jaw and roll crushers. The crushed ore sample was then rod-milled to 80% passing 75 μm (p80) and then riffled to 120 g portions prior to use in experiments [6,26].
, Turkey. The sample was reduced in size to ≤4 mm by using laboratory type jaw and roll crushers. The crushed ore sample was then rod-milled to 80% passing 75 μm (p80) and then riffled to 120 g portions prior to use in experiments [6,26].
Chemical analysis of the ore sample (Table 2) using XRF for whole rock, ICP-MS for trace elements and AAS for Au, Ag and Cu after digestion with hot aqua regia containing HF and HClO4 has shown that the sample is rich in gold and copper with its content of 56 g/t Au and 1.1% Cu. The ore sample consists predominantly of quartz (85% SiO2) and, to a small extent, sulphide minerals (3.14% S in total) (Fig. 1, Table 2). SEM studies were also undertaken on the ore sample and the flotation concentrate produced from the ore. Accordingly, copper was identified to be present as sulphides including chalcopyrite, covellite and chalcocite in the ore (Figs. 1 and 2). Pyrite and sphalerite were also observed as the other sulphide phases (Fig. 2). High copper content renders the ore refractory in character due to the low gold extraction in direct cyanidation (1.5 g/L NaCN) [6,12].
Table 2 Chemical and mineralogical compositions of ore and bulk concentrate samples
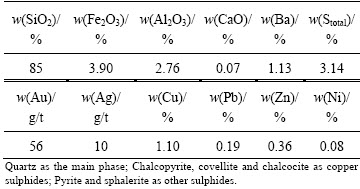

Fig. 1 XRD profile of copper-rich gold ore sample

Fig. 2 Sulphides of copper and other metals identified by SEM analysis of flotation concentrate produced from ore for mineralogical analysis
2.2 Leaching tests
Leaching tests were conducted in baffled glass reactors (1000 mL in nominal capacity) into which leach solutions (480 mL) and the ore (120 g, 25%, mass fraction) were added. Leach solutions were prepared using deionised-distilled water at the prescribed concentration of reagents. In direct cyanide leaching tests, the effect of cyanide concentration in the range of 1.5-7.5 g/L NaCN was studied. In the ammoniacal cyanide leaching tests, main and interaction effects of concentrations of sodium cyanide (0.5-2.5 g/L NaCN), ammonia (0-2 mol/L NH3) and lead nitrate (0-1000 g/t (Pb(NO3)2) were examined. Ammonia leaching for the removal of copper was performed at 1 mol/L NH3 and the cyanide leaching of the residue was carried out at 1.5 g/L NaCN. The reactors were agitated by overhead stirrers at a constant stirring speed of 600 r/min. Air was supplied to the reactors at a flow rate of 2 L/min. The top of the beakers was kept covered during the experiments and pH was controlled at 10.5-11.0 in cyanide leaching and at 10-10.5 in ammonia leaching by the addition of 1 mol/L NaOH. The reactors were sampled at predetermined intervals and the samples were centrifuged to obtain clear supernatants for analysis of free cyanide and/or metals. The concentration of free cyanide was determined by titration with silver nitrate using p-dimethylamino-benzal-rhodanine (0.02% in acetone, mass fraction) as the indicator. It is pertinent to note that the titration method used herein overestimates free cyanide level as copper cyanides also contributes somewhat to the measured concentration [27]. In addition to cyanide, the concentration of dissolved oxygen (DO2) was also monitored using a Lovibond Oxi200 oxygen meter during the leaching tests.
On the termination of leaching tests after 24 h, residues were collected by filtration and then dried in an oven at 105 °C. Dried residues were digested in hot aqua-regia for analysis of metals (gold, silver and copper using an atomic absorption spectrometer (Perkin Elmer AAnalyst 400). Metal extraction was calculated based on the amount of metal remained undissolved in the residue.
2.3 Experimental design
Response surface methodology is one of the most preferred techniques to determine the contribution of several independent variables on the response. A Box- Behnken design (BBD), which has three levels (-1, 0, 1, where 0 corresponds to center level) with equally spaced intervals, is a kind of statistical technique in response surface methodology [28]. In the current work, the experiments were designed by using Box-Behnken approach to investigate the effects of concentration of sodium cyanide (0.5-2.5 mol/L NaCN), ammonia (0-2 mol/L NH3) and lead nitrate (0-1000 g/t Pb(NO3)2) on the extraction of gold. The range of concentration of sodium cyanide was determined according to the preliminary tests. Table 3 gives the experimental design layout with coded/actual levels of the factors.
After deriving a regression model using the results (e.g., Au extraction at 24 h) as a response, analysis of variance (ANOVA) was performed to evaluate the statistical significance of the model and parameters where P-values were used for the significance test. Simply, a P-value of <0.05 suggested the rejection of the null hypothesis, i.e., the significance of a parameter/term, at a confidence level of 95% (α=0.05) [28,29]. To perform statistical evaluation of the experimental data and construction of response surface plots, DESIGN- EXPERT and MINITAB softwares were used.
3 Results and discussion
3.1 Effect of NaCN concentration on direct cyanidation of gold
Previous studies have reported consistently low gold extractions (<12%Au) from the ore in direct cyanide leaching at 1.5 g/L NaCN [6,7,12]. These studies indicated that low gold extractions are linked with high copper content of the ore, i.e., excessively high consumption of cyanide. Theoretical analysis of the leaching system through speciation calculations was performed as shown in Fig. 3, which illustrates the speciation of copper (assuming complete dissolution of copper from the ore) as a function of cyanide concentration covering the experimental conditions. Copper would be completely soluble only at c(CN-)T>100 mmol/L, i.e., w(NaCN)T>19.6 kg/t and free CN- would be available at c(CN-)T>125 mmol/L, i.e., w(NaCN)T>24.5 kg/t (ρ(NaCN)>6.13 g/L NaCN). However, in the leaching of ore, much higher levels of cyanide is required in the view of the consumption of cyanide by other metals, sulphide minerals via the formation of thiocyanate (Eqs. (3) and (5)), reduction of Cu2+ (released from Cu2+-sulphides), catalytic oxidation of cyanide by air in the presence of Cu2+ and volatilisation as HCN via aeration/acidification [3,22,30].
Table 3 Experimental design layout with actual and coded levels of factors
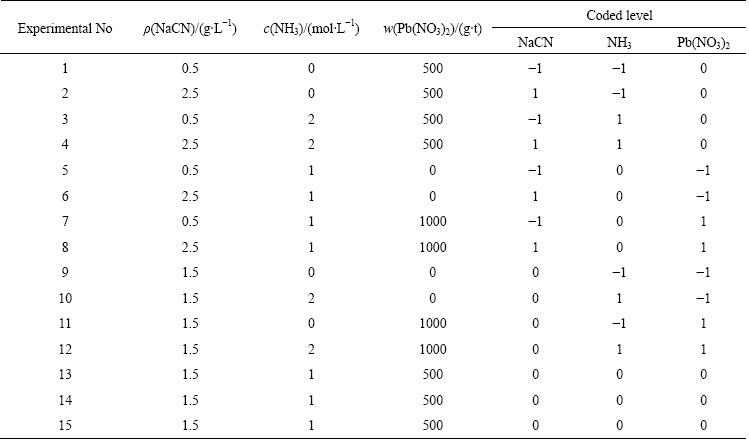
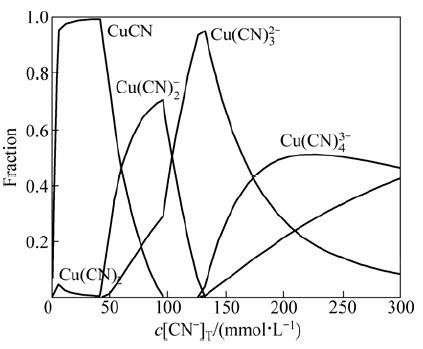
Fig. 3 Speciation of copper(I) depending on concentration of total cyanide (c(Cu)=43.3 mmol/L, 25 °C and pH 10.5) [35]
Accordingly, the effect of high concentrations of cyanide (1.5-7.5 g/L NaCN) on the extraction of gold was tested. Figure 4 illustrates that the extraction of gold substantially improves with increasing the concentration of NaCN from 1.5 to 7.5 g/L. Consistent with the speciation calculations, high gold extractions (≥88% Au) are possible only at the initial cyanide concentrations of ρ(NaCN)≥4 g/L at which the total addition of cyanide amounted to w(NaCN)≥48.2 kg/t. Over 97% extraction of gold is achieved by maintaining an initial cyanide level of ρ(NaCN)≥5 g/L. These findings suggest that the interference of copper sulphides with gold leaching is alleviated by increasing the concentration of NaCN. In addition to their excessive cyanide consumption, the adverse effect of copper sulphides on gold extraction could be also attributed to their consumption of oxygen (Eqs. (3) and (5)), the passivating effect of sulphide ion released from them on gold and their pregrobbing characteristics [3,31]. It has been reported that a molar ratio of cyanide to copper of >4 is often required to achieve an acceptable extraction of gold [1,3,8].
During the initial two hours of leaching, a minimal extraction of gold is observed to occur at all the concentrations of cyanide tested (Fig. 4). On the other hand, the dissolution of copper occurs most rapidly and extensively at the onset (within an hour) of the leaching process (Fig. 5). Thereafter, it remarkably slows down as the leaching process progresses. Increasing the concentration of cyanide improves the leaching of copper with the highest dissolution of 78.5% Cu at 7.5 g/L NaCN. The dissolution behaviour of copper is apparently linked with ore mineralogy, i.e., copper sulphides (Fig. 2). In this regard, the initial rapid dissolution of copper can be attributed to the presence of more readily soluble copper phases (Cu2S) (Table 1). Similarly, the slow rate of dissolution of copper after these initial periods of fast leaching is apparently linked with the copper phases such as chalcopyrite recalcitrant to cyanide leaching. These findings indicate that the dissolution of copper is faster than gold and, in other words, copper appears to protract the release of gold. This in turn suggests the selective dissolution of copper over gold.

Fig. 4 Effect of NaCN concentration on extraction of gold from ore

Fig. 5 Effect of NaCN concentration on dissolution of copper from ore
3.2 Ammonia pretreatment
Figure 6 illustrates that the pretreatment by ammonia leaching renders the ore amenable to high extraction of gold even at low levels of cyanide (1.5 g/L NaCN). In effect, the extraction of gold from the ore substantially improves from 6.7% to 99.8% after the ammonia pretreatment. This improvement appears to be linked with the elimination of copper interference by ammonia leaching. Over 24 h, 73% of copper is leached from the ore in this pretreatment stage. The remaining copper could be associated with chalcopyrite present in the ore. MUIR et al [8] reported that copper dissolution from sulphides in ammonia is a slow process (Eqs. (7)-(9)) with relatively low copper extractions at low temperatures. They also reported that chalcopyrite is less reactive than chalcocite in ammonia as well as in cyanide solutions. It can be inferred that ammonia leaching as pretreatment allows the removal of reactive copper from ore.

Fig. 6 Effect of ammonia leaching (1 mol/L NH3, pH 10.0-10.5) as pretreatment on cyanide leaching of gold (1.5 g/L NaCN, pH 10.5-11.0)
2Cu2S+O2+8NH3+2H2O→2CuS+2[Cu(NH3)4]2++4OH– (7)
CuS+2O2+4NH3→[Cu(NH3)4]2++SO42– (8)
4CuFeS2+17O2+24NH3+4H2O→4[Cu(NH3)4]2++2Fe2O3+8SO42–+8NH4+ (9)
The removal of copper from the ore by ammonia leaching is also observed to culminate in a substantial reduction from 3.06 to 0.076 kg/t per 1% Au extraction in cyanide consumption. MUIR et al [8] reported 90% extraction of gold from Telfer tailings containing 1.15% Cu as oxides and sulphides at the expense of excessively high consumption of cyanide (30 kg/t). After ammonia pretreatment of the tailings with 0.3 mol/L NH3 + 0.1 mol/L NH4+, which resulted in a copper extraction of 82%, they found 80% gold extraction in subsequent cyanide leaching at a NaCN consumption of 6 kg/t. High gold extractions coupled with considerably reduced levels of cyanide consumption suggest that ammonia leaching as a pretreatment process prior to cyanidation can be suitably used for copper-bearing refractory gold ores.
In ammonia leaching, pH and free NH3 concentration are important parameters controlling the dissolution process in which Cu(NH3)42+ is stable at pH 7.9-10.6 (optimum pH 9.3) under the conditions tested in the current work (Fig. 7). Considering the dependence of leaching of sulphides on free NH3 concentration (Eqs. (7)-(9)), pH should be also controlled at 9.3-10.5 to maintain high concentrations of free NH3 in solution and to prevent the precipitation of copper at pH>10.5 (Fig. 7). It was reported that there is a critical level of free NH3 concentration below which copper leaching does not occur [8]. EK et al [32] demonstrated that the ammonia leaching of copper sulphides such as bornite and chalcocite is slowed down by the formation of a copper oxide in connection with the release of OH- (e.g., Eq. (7)), i.e., the increase in pH beyond the stability region of Cu(NH3)42+. They suggested addition of an ammonium salt to control pH. In the current work, the speciation calculations [33] suggest that the critical concentration of NH3 is 0.44 mol/L, indicating that the dissolution of copper is not limited by the availability of NH3 in the leaching test.

Fig. 7 pH-dependent stability of copper species in ammonia solution under experimental conditions (1 mol/L NH3 and 1.1% Cu in ore at 25% pulp density) [33]
3.3 Ammoniacal cyanide leaching
Ammoniacal cyanide leaching has been proposed for the treatment of gold ores that contain reactive copper in appreciable quantities [4,5,8,34]. Compared with the traditional cyanide leaching, ammonia cyanide system allows selective leaching of gold from copper-gold ores with remarkably reduced consumption of cyanide [18]. It is a rather complex system intimately controlled by the reagent interactions within the system [3]. Therefore, based on the preliminary studies [6,12], leaching tests were designed using Box-Behnken design to evaluate main and interaction effects of concentrations of NaCN, NH3 and Pb(NO3)2 on the selective extraction of gold and consumption of NaCN. Table 4 shows gold and copper extractions as well as NaCN consumption (kg/t per 1% Au) over 24 h. Figure 8 is also presented to illustrate the time-dependent leaching profile for gold under the influence of increasing concentrations of NaCN and NH3.
Table 4 Metal extractions and consumption of NaCN over 24 h of leaching under different conditions


Fig. 8 Leaching kinetics showing effects of concentrations of NaCN and NH3
Using the results for the extraction of gold (24 h) as the response (Y), a second order regression model with a coefficient of multiple determinations of (R2) 96% (Eq. (10)) was established. Using Eq. (10), an unknown response (Y) can be calculated at any coded level of concentrations of NaCN (A), NH3 (B) and Pb(NO3)2 (C). Based on the model, the response surface plots for main and interaction effects of two parameters at a fixed level of third parameter were generated as shown in Fig. 9.
Y=19.5+9.8A+23.5B-4.9C+1.5AB+0.7AC-10.3BC+14.38A2+11.1B2-2.75C2 (10)
To determine the linear/quadratic/interaction effects of parameters on the response (i.e., extraction of gold at 24 h), the analysis of variance (ANOVA) was used (Table 5). The regression model is determined to be statistically significant at a confidence interval of α=0.05. Linear (main) and square (quadratic) effects are also found to be statistically important at the same confidence interval with their contributions of 73% and 16.5%, respectively to the response (gold extraction). On the other hand, the contribution of interactions is statistically insignificant (Table 5). Statistical significance of the model terms, i.e., the effects of linear and quadratic terms, is also tested (Table 6). Accordingly, the effects of concentrations of NaCN and NH3 on the extraction of gold are confirmed to be statistically significant at 95% confidence level (α=0.05) whilst the effect of concentration of Pb(NO3)2 is insignificant. The P-values for all the interaction terms (Table 6) are high (>0.05), suggesting their insignificance, consistent with the results in Table 5.
The coefficients of linear and quadratic terms in the model (Table 6) also reflect the mode and magnitude of the effect of factors on the gold extraction. Accordingly, NaCN and NH3 have a positive influence on the gold extraction, which tends to improve with increasing their concentrations, and NH3 has a stronger effect than NaCN (Table 4, Fig. 9). In fact, the maximum gold extraction (86.7%) is achieved at the highest concentration of NaCN and NH3. Based on these findings, further studies by the authors (unpublished data) suggest that gold extraction can be further improved to >97% by further increasing the concentration of NH3 (3-4 mol/L). MUIR et al [25] also reported that, at a fixed c(NH3)/c(NaCN) ratio, increasing the concentrations of NH3 and NaCN resulted in an increase in the extraction of copper and gold, but the latter arrived at a maximum prior to a reverse trend of decline at w(NaCN)=1.5-1.7 kg/t. Furthermore, these investigators recorded no improvement in gold extraction at w(NH3)>2.25 kg/t despite a significant increase in the dissolution of copper.

Fig. 9 Surface plots showing effects of factors
Table 5 Analysis of variance (ANOVA) of regression model
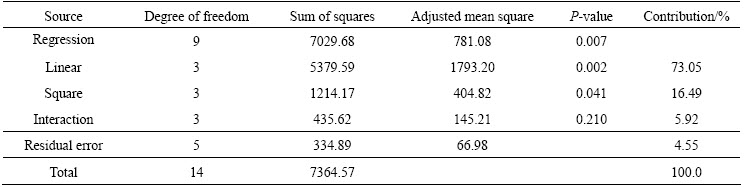
Table 6 Regression coefficients and statistical significance test of model terms

The interaction between the concentrations of NH3 and Pb(NO3)2 was detected by the statistical analysis to be significant only at 90% confidence level (Table 6). It is interesting to note that an adverse effect of increasing concentration of Pb(NO3)2 on the extraction of gold was observed at high concentration of NH3 despite its negligible influence at low NH3 level. An earlier study on the ore showed that the addition of Pb(NO3)2 (up to 1000 kg/t) had no beneficial effect on gold extraction (in the absence of NH3) despite the reduction (by up to 19%) in cyanide consumption [7]. In cyanide leaching, the adverse effect of Pb(NO3)2 could be attributed to the formation of PbS or Pb(OH)2 layer on gold surface [14,35]. In contrast to the current findings, SCERESINI [3] and DESCHENES et al [14] reported the enhancing effect of Pb(NO3)2 on ammoniacal cyanide leaching of gold from a copper-gold ore and a gold ore in the presence of bornite.
The evaluation of the experimental data using dissolution of copper as the response shows that increasing the concentration of NaCN and NH3 has a positive synergistic effect on the dissolution of copper similar to the results for gold extraction (Fig. 9) with almost equal magnitudes for linear (main) terms. No significant linear/quadratic/interaction effect is detected for Pb(NO3)2. In this work, a selectivity index was defined using the extractions of Au and Cu, i.e., Au/Cu ratio (Table 4) and the experimental data were further evaluated based on these selectivity values. It is statistically corroborated that the selectivity of gold extraction increases linearly with increasing the concentration of NH3, i.e., the dissolution of copper is suppressed. The selectivity of gold leaching in ammoniacal cyanide leaching is ascribed to the precipitation of copper presumably as Cu3(NH3)3(CN)4 [23,25]. DAWSON et al [23] demonstrated that the dissolution of copper from metallic copper, chalcopyrite and chalcocite in ammoniacal cyanide solutions occurs and the copper concentration in solution reaches a maximum with the formation of a dark coloured precipitate as leaching progresses.
Furthermore, increasing the concentration of NH3 is shown to consistently reduce the consumption of NaCN (Table 4). MUIR et al [8] provided data for comparison of cyanide and ammoniacal cyanide leaching of six different oxidized copper-gold ores, revealing a substantial reduction (by up to 95%) in cyanide consumption by the addition of NH3. The current findings (Table 4) also show up to 92% decrease in cyanide consumption per 1% of gold extraction. It is interesting to note that the addition of Pb(NO3)2 leads to an increase in NaCN consumption when NH3 is present in the system whilst it results in a decrease in NaCN consumption in the absence of NH3 (Table 4).
4 Conclusions
1) The treatment of a high grade gold ore with a high copper content by cyanide leaching, ammonia leaching as a pretreatment prior to cyanide leaching and ammoniacal cyanide leaching is demonstrated. Cyanide leaching tests show strong interference effect of the contained copper sulphides with leaching of gold. Copper interference appears to be linked with readier dissolution of reactive copper sulphides than gold and, hence, depletion of cyanide available by copper. Consistent with the speciation calculations, the maintenance of prohibitively high levels of cyanide (≥5 g/L NaCN) is required for achieving high extractions (>97%) for gold at the expense of dissolution of up to 79% copper. Pretreatment of the ore by ammonia leaching is found to remove reactive copper (73%), leading to ready extraction of gold (99.8%) in subsequent cyanide leaching even at low cyanide levels (1.5 g/L NaCN).
2) Ammoniacal cyanide leaching is shown to suppress the dissolution of copper and significantly enhance the leaching of gold with a reduced consumption of cyanide. Notwithstanding this, the highest gold extraction is determined to be 86.7%, which is relatively low compared with that after ammonia treatment.
3) The statistical analysis of data has verified that the concentration of NH3 is the most influential factor controlling selectivity and extent of gold extraction. Cyanide consumption and gold extraction tend to improve with increasing the concentration of NH3. The effect of addition of Pb(NO3)2 on gold extraction is statistically confirmed to be insignificant. However, the addition of Pb(NO3)2 increases NaCN consumption in the presence of NH3 whilst a reverse trend is observed in the absence of NH3.
Acknowledgements
The authors would like to acknowledge Mastra Gold Mine (Koza Gold Operations) for kindly providing ore samples, Associate Prof. Dr. Ibrahim ALP and Assistant Prof. Dr. Oktay CELEP for their valuable contributions during sample preparation and leaching tests, and Mr. Fatih ERDEMIR (Department of Metallurgical & Materials Engineering, KTU) for SEM-EDS analysis. The authors also appreciate The Scientific and Technological Research Council of Turkey (TUBITAK) for providing financial support via a S&T research project (Project No. 213M492).
References
[1] MARSDEN J O, HOUSE C I. Chemistry of gold extraction [M]. 2nd ed. Littleton, Colorado: Society for Mining, Metallurgy, and Exploration (SME), 2006: 651.
[2] HEDLEY N, TABACHNICK H. Chemistry of cyanide [M]//Mineral Dressing Notes. Vol. 23. American Cyanamid, 1958.
[3] SCERESINI B. Gold-copper ores [M]//ADAMS A D. Advances in Gold Ore Processing, Developments in Mineral Processing, Vol 15. Amsterdam, The Netherlands: Elsevier Publishers, 2005: 789-824.
[4] MUIR D M. A review of the selective leaching of gold from oxidised copper–gold ores with ammonia–cyanide and new insights for plant control and operation [J]. Minerals Engineering, 2011, 24: 576-582.
[5] DAI X, SIMONS A, BREUER P. A review of copper cyanide recovery technologies for the cyanidation of copper containing gold ores [J]. Minerals Engineering, 2012, 25 (1): 1-13.
[6] BAS A D, KUCUK A, YAZICI E Y, DEVECI H. Assesment of ammoniacal ammonium sulphate leaching as a pretreatment process for copper bearing gold ores [C]//Proceedings of the XIIIth International Mineral Processing Symposium (IMPS). Bodrum, Turkey, 2012: 563-569.
[7] BAS A D, YAZICI, E Y, DEVECI H. Treatment of copper rich gold ores by ammonia assisted cyanide leaching [C]//Proceedings of the XXVI International Mineral Processing Congress (IMPC). New Delhi, India, 2012: 356-365.
[8] MUIR D M, LA BROOY S R, FENTON K. Processing copper–gold ores with ammonia or ammonia–cyanide solutions [C]//Proceedings of the World Gold ’91 Conference. Cairns: Australasian Inst Min Metall/SME, Littleton, Co., 1991: 145-150.
[9] BULATOVIC S M. Flotation behaviour of gold during processing of porphyry copper-gold ores and refractory gold-bearing sulphides [J]. Minerals Engineering, 1998, 10 (9): 895-908.
[10] FORREST K, YAN D, DUNNE R. Optimisation of gold recovery by selective gold flotation for copper-gold-pyrite ores [J]. Minerals Engineering, 2001, 4(2): 227-241.
[11] FENG D, van DEVENTER J S J. Ammoniacal thiosulphate leaching of gold in the presence of pyrite [J]. Hydrometallurgy, 2006, 82(3-4): 126-132.
[12] BAS A D, OZDEMIR E, YAZICI E Y, CELEP O, DEVECI H. Ammoniacal thiosulphate leaching of a copper-rich gold ore [C]//Proceedings of the 15th International Conference on Environmental and Mineral Processing (EaMP). Ostrava, Czech Republic, 2011: 83-90.
[13] KONDOS P D, DESCHENES G, MORRISON R M. Process optimization studies in gold cyanidation [J]. Hydrometallurgy, 1995, 39: 235-250.
[14] DESCHENES G, GU H, XIA C, PRATT A, FULTON M, CHOI Y, PRICE J. A study of the effect of djurliete, bornite and chalcopyrite during the dissolution of gold with a solution of ammonia-cyanide [J]. Minerals, 2012(2): 459-472.
[15] DESCHENES G., PRUD’HOMME P J H. Cyanidation of a copper-gold ore [J]. International Journal of Mineral Processing, 1997, 50(3): 127-141.
[16] RAJALA J, DESCHENES G. Extraction of gold and silver at the Kupol Mill using CELP [C]//Proceedings of the World Gold Conference. The Southern African Institute of Mining and Metallurgy, 2009: 35-42.
[17] SCERESINI B, STAUNTON W P. Copper/cyanide in the treatment of high copper gold ores [C]//Proceedings of the 5th Extractive Metallurgy Conference. Melbourne: The Australasian Institute of Mining and Metallurgy, 1991: 123-125.
[18] MUIR D M, LA BROOY S R, CAO C. Recovery of gold from copper-bearing ores [C]//Proceedings of the World Gold ’89, Littleton, Colorado, USA: SME, 1989: 363-374.
[19] MENDES F D, MARTINS A H. Sulfuric acid leaching of lgarape Bahia gold-copper ore for copper extraction-An ore pretreatment for gold recovery by cyanidation [J]. Minerals & Metallurgical Processing, 2002, 19(3): 165-168.
[20] SERBEST V. Effect of secondary copper minerals on cyanide leaching of gold ores [D]. Trabzon, Turkey, Karadeniz Technical University, 2010. (In Turkish)
[21] HUNT B. Process of precipitating and recovering precious metals from their solutions. US Patent 689. 190: 1901[P].
[22] HAYES G A, CORRANS I J. Leaching of gold-copper ores using ammoniacal cyanide [C]//Proceedings of International Conference on Extractive Metallurgy of Gold and Base Metals. Melbourne: Australasian Inst Min Metall, 1992: 349-353.
[23] DAWSON J N, LA BROOY S R, RITCHIE I M. Copper-gold ore leaching: A kinetic study on the ammoniacal cyanidation of copper, chalcocite and chalcopyrite [C]//Proceedings of The AusIMM Annual Conference. Ballarat, 1997: 291-297.
[24] MUIR D M, la BROOY S R, DENG T, SING P. The mechanism of the ammonia–cyanide system for leaching copper–gold ores [C]// HISKEY J B, WARREN G W. Hydrometallurgy: Fundamentals, Technology and Innovations. Proceedings of the 4th International Symposium on Hydrometallurgy. Littleton, USA: SME, 1993: 191-204.
[25] MUIR D M, VUKCEVIC S, SHUTTLEWORTH J. Optimising the ammonia–cyanide process for copper–gold ores [C]//Proceedings of Randol Gold Forum. Perth: Randol International, Golden, Co., 1995: 225-231.
[26] KOC E. Extraction of gold from Mastra gold mine by ammoniacal cyanide leaching [D]. Trabzon, Turkey: Karadeniz Technical University, 2012. (in Turkish)
[27] BREUER P L, SUTCLIFFE C A, MEAKIN R L. Cyanide measurement by silver nitrate titration: Comparison of rhodanine and potentiometric end-points [J]. Hydrometallurgy, 2011, 106(3-4): 135-140.
[28] MONTGOMERY D C. Design and analysis of experiments [M]. 5th ed. New York: John Wiley & Sons Inc., 2001.
[29] MATHEWS P G. Design of experiments with MINITAB [M]. Milwaukee: ASQ Quality Press, 2005.
[30] YAZICI E Y, DEVECI H, ALP I. Treatment of cyanide effluents by oxidation and adsorption in batch and column studies [J]. Journal of Hazardous Materials, 2009, 166(2-3): 1362-1366.
[31] BAS A D, ALTINKAYA P, YAZICI E Y, DEVECI H. Preg-robbing potential of sulphide-bearing gold ores [C]//Proceedings of the XIIIth International Mineral Processing Symposium (IMPS). Bodrum, Turkey, 2012: 613-618.
[32] EK C, FRENAY J, HERMAN J C. Oxidized copper phase precipitation in ammoniacal leaching—The influence of ammonium salt additions [J]. Hydrometallurgy, 1982, 8: 17-26.
[33] MEDUSA. Software for chemical equilibrium diagrams [M]. Sweden: Royal Institute of Technology, 2004.
[34] COSTELLO M C, RITCHIE I C, LUNT D J. Use of the ammonia cyanide leach system for copper gold ores with reference to the retreatment of the Torco tailings [J]. Minerals Engineering, 1992, 5(10-12): 1421-1429.
[35] JEFFREY M I, BREUER P L. The cyanide leaching of gold in solutions containing sulfide [J]. Minerals Engineering, 2000, 13(10-11): 1097-1106.
A. D. BAS, E. KOC, Y. E. YAZICI, H. DEVECI
Hydromet-B&PM Group, Division of Mineral Processing, Department of Mining Engineering,
Karadeniz Technical University, Trabzon 61080, Turkey
摘 要:采用直接氰化浸出、氨预处理及氨氰浸出等方法处理含硫化铜的金矿。研究这些浸出体系中金和铜的溶解行为。当NaCN含量小于5 g/L 时,金的氰化浸出受到所含硫化铜的严重干扰,这与组分平衡计算结果一致。氨预处理可以消除铜的干扰,在后续的氰化浸出过程中,即使在剂量较小的情况下,金也可以获得较高的提取率。采用Box-Behnken试验设计研究了氨氰体系中NH3、NaCN和Pb(NO3)2的主要影响以及其相互作用。其中NH3和NaCN浓度是影响金提取率的主要因素,而Pb(NO3)2的影响有限。增加NH3的浓度可以提高金提取的选择性和提取率,同时降低氰化物的用量。统计研究结果表明试剂间的相互作用对金提取无重大影响。氨预处理和氨氰浸出法在含硫化铜的金矿的处理中具有广阔的应用前景。
关键词:铜-金矿;氨氰浸出;氨浸出;预处理;氰化浸出
(Edited by Yun-bin HE)
Corresponding author: H. DEVECI; Tel: +90-462-377-3681, Fax: +90-462-325-7405; E-mail: hdeveci@ktu.edu.tr
DOI: 10.1016/S1003-6326(15)63642-1

