J. Cent. South Univ. Technol. (2008) 15: 503-507
DOI: 10.1007/s11771-008-0095-7
Bioleaching of sphalerite by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans cultured in 9K medium modified with pyrrhotite
CHEN Song(陈 松), QIU Guan-zhou(邱冠周), QIN Wen-qing(覃文庆), LAN Zhuo-yue(蓝卓越)
(School of Resource Processing and Bioengineering, Central South University, Changsha 410083, China)
Abstract: Elective culture of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans in 9K medium modified with pyrrhotite was studied. Bioleaching of flotation concentrate of sphalerite by the selected bacteria was carried out. The results show that the microorganisms cultured by pyrrhotite are a mixture of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans, of which the capability to oxidize ferrous to ferric irons is enhanced by the high mass ratio of Fe to S in pyrrhotite. Three pyrrhotite samples were separated into various parts with corresponding S/Fe ratios by magnetic separation and were used to culture the elective bacteria as the substrate. The association of the cultures could provide a more rapid and complete oxidation of sphalerite than that of bacteria cultivated by conventional methods.
Key words: bioleaching; pyrrhotite; elective culture; sphalerite
1 Introduction
Bioleaching has been widely used in the commercial extraction of uranium, copper and gold from ore, and it is being exploited in the extraction of other base metals and rare noble metals, such as zinc, cobalt, nickel, molybdenum, gallium, germanium etc. In some cases it has been in the phase of pilot scale experiment[1-2]. It presents more advantages over the conventional metallurgical methods in treating low-grade and complicated ore. The metal sulfides are oxidized by some special bacteria, such as Thiobacillus ferrooxidans, Thiobacillus thiooxidans and Leptospirillum ferrooxidans, to form soluble metal sulfates, elemental sulfur and sulfuric acid[3-4]. Although some special bacteria have the potential to dissolve a number of sulfide minerals containing copper, their widespread commercial acceptance still remains some restrictions resulted from slower growth rate and lower cell density, both leading to poor leaching kinetics compared with the hydrometallurgy alternatives. Hence, to enhance the leaching rate, many studies have been done in the aspects of microbiology, electro- chemistry, metallurgy etc. One of the solutions proposed by researchers is to cultivate effective microorganisms used in bioleaching[5]. There have been more than 20 strains of microorganisms used in the bioleaching of sulfide ore; the most familiar and widely used are Thiobacillus ferrooxidans, Thiobacillus thiooxidans and Thermoacido philic achaebacterium. In the natural environment, the microorganisms with the capacity to dissolve minerals are a mixed culture of different bacterial strains. Being cultivated with the conventional methods of domestication, mutation, crossbreed, cell syncretizing and gene engineering, some capabilities of the microorganism such as the activity, the tolerance of heavy metal ions and the adaptability to sulfide ore were improved into some extent. However, the microorganisms derived from the above methods usually are a single strain, and the improved capability shown in bench scale test may degenerate in industrial cases, so their effect on bioleaching is limited. The bacteria which inhabit sulfide deposits can use sulfide minerals as energy source by dissolving the minerals and oxidizing ferrous iron and elemental sulfur. According to literature reports, the role of bacteria in bioleaching includes the direct attack on minerals, and the oxidation of Fe2+ and S[1-5]. Therefore, we supposed to cultivate bacteria with an appropriate mineral as a selective culture, which can enrich strains of microorganisms and improve their integral capability on bioleaching of the similar mineral. Pyrite (FeS2) and pyrrhotite (Fe1-xS) are the familiar components of sulfide deposit, of which Fe and S are the essential elements to cultivate bacteria, so both are the perfect substrates for selective culture of bacterial used in bioleaching. Considering that pyrrhotite is dissolved more easily than pyrite, and has various mass ratios of Fe to S, experiments were carried out to explore the elective culture of bacteria using pyrrhotite as the substrate.
Sphalerite is the most important resource of zinc ore in China and it comes from different regions and has different physicochemical futures due to the difference metallogeny, the content of iron and other impurities. Although some investigations on bioleaching of zinc sulfide have been carried out, the mechanism for the biooxidation of zinc sulfides is not completely recognized[6-10]. In this paper, the elective culture of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans in 9K medium modified with pyrrhotite was studied, and bioleaching of flotation concentrate of sphalerite by the selected bacteria was carried out.
2 Materials and methods
2.1 Mineral and ore
The chemical components of pyrrhotite are not fixed and the structural formula could be illustrated as Fe1-xS, where x=0-0.223. And when x=0, the iron atoms are perfect in the crystal[11-12]. With the increase of the mass ratio of S to Fe, the magnetism of pyrrhotite increases. Therefore, pyrrhotite can be separated into various parts by magnetic separation.
Table 1 lists the results of magnetic separation of a pyrrhotite collected from Dachang Mine, Guangxi Province, China. The crystal form of the pyrrhotite is of monocline.
The sphalerite concentrate and zinc ore containing sphalerite were obtained from Gaofeng Mine in Guangxi Province, China. The chemical compositions of sphalerite concentrate are listed in Table 2. Microscopic examination in thin slides of the groundmass shows that the zinc minerals are mainly sphalerite.
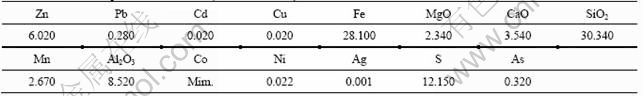
The chemical compositions of raw ore are given in Table 3. Microscopic examination in thin slides of the groundmass shows that the zinc minerals are mainly sphalerite (ZnS), pyrrhotite (FeS1.17) and pyrite (FeS2), and gangue minerals are quartz, gypsum, dickite and sericite.
2.2 Elective culture of bacteria on pyrrhotite
The original microorganisms were collected from Guangxi Dachang Mine, and they were grown in the modified 9K nutrient medium, which was adopted with adding 6% (the ratio of mass (g) to volume (mL)) the pyrrhotite samples No.1-No.3, respectively, replacing 10% iron (Fe2+) as the only energy source. The flasks were placed on an orbital shaker (170 r/min) and incubated at 30 ℃. The pH value was measured periodically, and when it dropped below 1.80, it was adjusted to 1.80 with 5 mol/L sulfuric acid. The number of bacterial cells in solution was counted with a hemacytometer under a biomicroscope with a magnification of 1 000. Repeatedly, when the bacteria reached an exponential growth phase, one-fourth of the culture volume was transferred to the next incubation.
After three times of transfer, the suspended solution of the culture was filtrated through a millipore filter, the bacteria enriched on the membrane were washed with sulfuric acid solution (pH=1.80) to reduce iron contamination. The concentration of bacterial cells was diluted with iron-free 9K nutrient solution to the density of 107 /mL, which was used as the inoculum of the next experiments.
Table 1 Pyrrhotite samples with various mass ratios of S to Fe
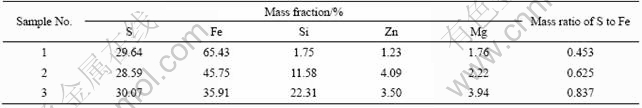
Table 2 Chemical compositions of sphalerite concentrate (mass fraction, %)
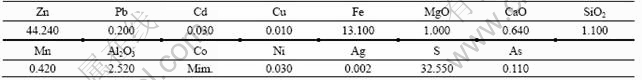
Table 3 Chemical compositions of zinc ore (mass fraction, %)
2.3 Bacterial leaching experiments
Leaching experiments were performed in 250 mL shake flasks at a pulp density of 6% pyrrhotite sample + 90 mL 9K nutrient medium without iron + 10 mL inoculums of cultures. The initial density of bacterial cells in the liquor phase was about 107 /mL. Incubation was performed at 30 ℃ and an initial pH of 2.0 on an orbital shaker at 160 r/min to determine the contribution from chemical leaching of zinc.
The solution samples were withdrawn at the same intervals and the concentration of Zn2+ was determined by EDTA titration[13], and the concentration of ferrous iron and total iron were determined by colourimetry using the phenanthroline method[14]. The pH value in leaching solution was measured with a pH meter. The pH value was monitored and a solution of 4.0 mol/L H2SO4 was used to maintain it at the initial value during the leaching process.
3 Results and discussion
3.1 Mechanism of elective culture on pyrrhotite
Bioleaching of pyrrhotite bearing nickel or gold to extract nickel or gold was reported[15]. Oxidation of pyrrhotite can be illustrated as follows[16].
1) Acid leaching
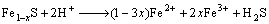 (1)
(1)
2) Bacterial catalyzing
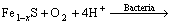
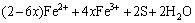 (2)
(2)
 (3)
(3)
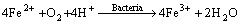 (4)
(4)
3) Oxidizing action of Fe3+
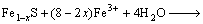
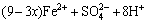 (5)
(5)
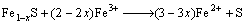 (6)
(6)
4) Hydrolyzation of Fe3+
 (7)
(7)
If the bacteria are cultivated purposefully to accelerate the chemical oxidation of sulfide minerals by ferric ion or the biooxidation of sulfides and elemental sulfur to sulfate, the rate of bioleaching will be increased. A mixed culture can be obtained from the cultivation of bacteria on pyrrhotite with various S/Fe ratios; accordingly, the mixed culture has a strengthened capability to oxidize ferrous ion or sulfides and elemental sulfur, and the adaptability to minerals is also improved, which can accelerate the bioleaching rate of some sulfides.
From Fig.1 the acid consumption of the three samples of pyrrhotite from high to low is: No.1>No.2>No.3. With increasing time, the pH value has a decreasing tendency. The bacterial density is above 2×107 cell/mL in the third day. In sample No.1, the high concentration of iron ion leads to precipitation, so the bacteria are transferred to the next incubation with fresh pyrrhotite.
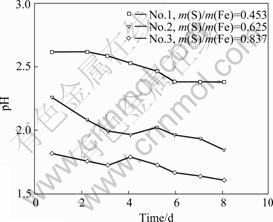
Fig.1 Variation of pH in cultivation of bacteria on pyrrhotite with various mass ratios of S to Fe
3.2 Characteristics of selected bacteria
With the microscopic examination and observation of solid culture, the bacteria cultured in 9K medium modified with pyrrhotite were characterized; the main strains were Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. In sample No.1, Acidithiobacillus ferrooxidan is dominant; Acidithiobacillus thiooxidans is in the majority in sample No.2; and in sample No.3, the two strains of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans are approximately equiponderant. It can be seen here that the bacteria obtained from pyrrhotite cultivation are a mixed culture, in which some strains are dominant in numbers and in oxidizing capacity corresponding to the variation of the S/Fe ratio.
Fig.2 shows the oxidation of ferrous ion by bacteria in 9K medium (the original ferrous concentration was 9 g/L, the inoculation was 3 mL bacterial medium respectively). It can be seen that bacterium No.1 exhibits a fast oxidizing rate, and ferrous ions are completely oxidized to ferric ions after 40 h. The time for oxidizing the ferrous ion for bacteria No.3 and No.2 is 80 and 100 h, respectively. The results are consistent with the bacterial characterization above, in which Acidithiobacillus ferrooxidans is dominant and has the highest capacity of ferrous oxidation. Correspondingly, a higher capacity of elemental sulfur oxidation was observed when the Acidithiobacillus thiooxidans dominate. Comparing the bacterial culture with the different S/Fe ratio, it can be concluded that ferrous oxidizing bacteria are dominant in the pyrrhotite culture with low S and high Fe mass ratio. On the contrary, the sulfur oxidizing bacteria are dominant in the culture with high S and low Fe mass ratio.
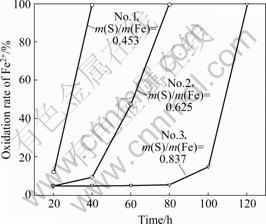
Fig.2 Ferrous ion oxidation of three bacterial cultures
3.3 Bioleaching of flotation concentrate of sphalerite
Bioleaching of sphalerite can be represented as follows.
1) Biochemical reactions
Direct mechanism:
 (8)
(8)
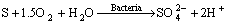
Biooxidation:
 (9)
(9)
 (10)
(10)
2) Chemical reactions
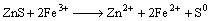 (11)
(11)
 (12)
(12)
The results of bioleaching of sphalerite concentrate are shown in Fig.3. It can be seen that bacterium No.1 has the highest leaching rate. The leaching rate of zinc used with bacterium No.1 is about 84.3%. The leaching rate of zinc used with bacteria No.2 and No.3 are 82.0% and 80.3%, respectively. Thus, the bacteria with the high capacity of ferrous oxidation or sulfur oxidation have better effect on bioleaching of sphalerite concentrate. These results are consistent with the literature report, that the indirect mechanism is dominant in bacterial leaching of sphalerite[17-18].
Bacterium No.1 has a high capacity of oxidizing
ferrous ion to ferric ion, and the generation of ferric ion accelerates sphalerite dissolving, and the products of the dissolving reaction are ferrous ion and elemental sulfur. With increasing elemental sulfur, the transfer of reactant and reaction product is suppressed in some extent if the elemental sulfur is not eliminated in time, which decreases the leaching rate.
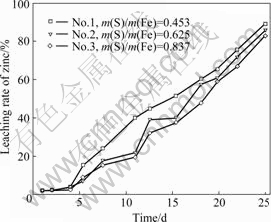
Fig.3 Effect of three bacteria samples obtained from pyrrhotite culture on bioleaching of sphalerite flotation concentrate
The results of bioleaching of sphalerite concentrate with a mixed culture obtained from conventional induction mutation are shown in Fig.4. Compared with a mixed culture of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans obtained from conventional induction mutation, all the three bacteria samples have more positive effects on bioleaching of sphalerite flotation concentrate. Though the bacteria also have the high capacity of ferrous ion oxidation and sulfur oxidation respectively, the leaching rate of zinc extraction of the mixed culture is 85% after 25 d.
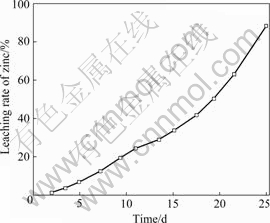
Fig.4 Bioleaching of flotation concentrate of sphalerite with mixed culture obtained from conventional induction mutation
The results of bioleaching of raw ore containing sphalerite are quite different from those of sphalerite concentrate (Fig.5). The bacteria No.2 and No.3, which show the lowest leaching rate of zinc in bioleaching of sphalerite concentrate, have the highest leaching rate of zinc of 90% and 82% respectively in 9 d, while the leaching rate of zinc of bacterium No.1 is 70%. The curves do not pass through the original point because portions of zinc are released during pH adjustment before inoculation.

Fig.5 Effect of three bacteria samples obtained from pyrrhotite culture on bioleaching of raw ore of zinc sulfide
It can be seen that a mixed culture of Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans being approximately equiponderant has a better effect on bioleaching of raw ore of zinc sulfide. The iron in the ore is more likely to be released than that in the concentrate, because portion of the pyrrhotite and pyrite are dissolved by chemical or biochemical process. The iron concentration reaches about 5.0 g/L after the 9th day in bioleaching of zinc sulfide ore, and in the bioleaching of sphalerite concentrate that is about 1.8 g/L after 25 d. With the galvanic reaction and the high concentration of iron in the leached solution, the leaching rate of sulfide ore is faster than that of the concentrate. The sulfur product does not become the baffle of leaching because of low sulfur content in zinc sulfide ores.
4 Conclusions
1) The elective bacteria culture on pyrrhotite is a mixed culture of mainly Thiobacillus ferrooxidans, Thiobacillus thiooxidans and Leptospirillum ferrooxidans etc. The leaching capacity of the culture is improved remarkably.
2) The capability of bacteria cultured on pyrrhotite varies with the mass ratio of S to Fe. With the low S and high Fe content, the ferrous oxidizing bacteria are dominant; on the contrary, the sulfur oxidizing bacteria are dominant.
3) Bacteria with a high capacity of ferrous oxidization or sulfur oxidization adapt to bioleaching flotation concentrate of sphalerite. A mixed culture of equivalent Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans adapt to bioleaching of zinc sulfide ore. The role of bacteria in bioleaching of sphalerite is primarily to oxidize the chemical reaction products of ferrous ion and elemental sulfur, thus the dominant mechanism is the indirect one.
References
[1] MILLER P C, RHODES M K, WINBY R. Commercialization of bioleaching for base-metal extraction [J]. Minerals & Metallurgical Processing, 1999, 16(4): 42-50.
[2] EHRLICH H L. Past, present and future of bioleaching [J]. Hydrometallurgy, 2001, 59(2/3): 127-134.
[3] BOON M, BRASSER H J, HANSFORD G. Comparison of the oxidation kinetics of different pyrite in the presence of Thiobacillus ferrooxidans or Leptospirillum ferrooxidans [J]. Hydrometallurgy, 1999, 53(1): 57-72.
[4] SAND W, RHODE K, SOBOTKE B. Evaluation of Leptospirillum ferrooxidants for leaching [J]. Appl Environ Microbiol, 1992, 58(1): 85-92.
[5] LUO Lian-ming, WANG Jun, XU Jing. Study on methods of improving bacterial leaching velocity of gold ores [J]. Conservation and Utilization of Mineral Resources, 1994(4): 40-43. (in Chinese)
[6] GARCIA J O, MIGHAM B J, TUOVIMEN O. Sphalerite oxidation by Thiobacillus ferrooxidans and thiobacillus thiooxidans [J]. Can J Microbiol, 1995, 41: 578-584.
[7] KONISHI Y, KUBO H, ASIA S. Bioleaching of zinc sulfide concentration by Thiobacillus ferrooxidants [J]. Biotechnol Bioeng, 1992, 39: 66-74.
[8] CHOI W K, TORMA A E, OHILINE R. Electrochemical aspects of zinc sulphide leaching by Thiobacillus ferrooxidans [J]. Hydrometallurgy, 1993, 33(1/2): 137-152.
[9] LAN Zhuo-yue, HU Yue-hua, LIU Jian-she, WANG Jun. Solvent extraction of copper and zinc from bioleaching solutions with LIX984 and D2E [J]. Journal of Central South University of Technology, 2005, 12(1): 45-49.
[10] QIN Wen-qing, LAN Zhuo-yue, LI Wei-zhong. Recovery of zinc from low-grade zinc oxide ores by solvent extraction [J]. Journal of Central South University of Technology, 2003, 10(2): 98-102.
[11] LIANG Dong-yun, HE Guo-wei, ZOU Ni. The isomeromorphism of pyrrhotite and its treatment feature differentia [J]. Journal of Guangdong Nonferrous Metals, 1997, 7(1): 1-5. (in Chinese)
[12] XIA Xue-hui. A kind of pyrrhotite from pyrite deposits in east Liaoning rift mineralogical implications [J]. Geology of Chemical Minerals, 1996, 18(4): 263-270. (in Chinese)
[13] JI S, SHANG Z C. The determination of Zn2+ in compound of Zn2+ and Fe2+ by EDTA titration [J]. Shandong Chem Ind, 2000, 29: 40-41. (in Chinese)
[14] KARAMANEV D G, NIKOLOV L N, MAMATARKOVA V. Rapid simultaneous quantitative determination of ferric and ferrous ions in drainage waters and similar solutions [J]. Mineral Engineering, 2002, 15(5): 341-346.
[15] VEGLI? F, BEOLCHINI F, NARDINI A, TORO L. Bioleaching of a pyrrhotite ore by a sulfooxidans strains: Kinetic analysis [J]. Chemical Engineering Science, 2000, 55(4): 783-795.
[16] LI Hong-mei. Bioleaching of pyrrhotite bearing nickel—A review [J]. Hydrometallurgy, 1999(3): 8-12. (in Chinese)
[17] FOWLER T A, CRUNDWELL F K. Leaching of zinc sulfide by thiobacillus ferrooxidans: Experiments with a controlled redox potential indicate no direct bacterial mechanism [J]. Appl Environ Microbiol, 1998, 64(10): 3570-3575.
[18] FOWLER T A, CRUNDWELL F K. Leaching of zinc sulfide by thiobacillus ferrooxidans: Bacterial oxidation of the sulfur product layer increases the rate of zinc sulfide dissolution at high concentration of ferrous ions [J]. Appl Environ Microbiol, 1999, 65(12): 5285-5292.
Foundation item: Project(50621063) supported by the National Natural Science Foundation of China; Project(2004CD619205) supported by the Major State Basic Research Development Program of China
Received date: 2008-10-15; Accepted date: 2008-03-25
Corresponding author: QIN Wen-qing, Professor, PhD; Tel/Fax: +86-731-8879815; E-mail: csuqwq@hotmail.com
(Edited by YANG Hua)