氧化锰烟气脱硫的可行性
来源期刊:中国有色金属学报(英文版)2013年第10期
论文作者:叶万奇 李运姣 孔 龙 任苗苗 韩 强
文章页码:3089 - 3094
关键词:热力学平衡图;脱硫;氧化锰;干法脱硫过程
Key words:predominance area diagram; desulfurization; manganese oxides; dry FGD processes
摘 要:为了有效和经济地脱除烟气中的硫,利用文献中的数据绘制不同温度下Mn-S-O系的热力学平衡图,从热力学角度说明使用氧化锰脱硫的可行性。提出最合适的烟气脱硫温度区间为600~800 K,而由于反应的强烈放热使得其能够维持操作的热平衡。天然锰矿由于其孔道结构而具有较大的表面积和较高的化学活性,使其脱硫效率可以保持在很高的水平。Mn-S-O与Fe-S-O系的叠加图中存在MnSO4和Fe2O3的重叠区域,说明可以通过选择性脱硫避免硫酸铁的形成。简单讨论了多级脱硫系统。
Abstract: For the purpose of effective and economic desulfurization of flue-gas, the predominance area diagram of the Mn-S-O system at different temperatures was constructed based on the thermodynamic data obtained from the literatures. It is seen from this figure that flue-gas desulfurization by manganese oxides is feasible from the thermodynamic point of view. Additionally, the most appropriate temperature range for flue-gas desulfurization is between 600 and 800 K, and the reaction is strongly exothermic to maintain the heat balance. The natural manganese ores encompass large tunnels that exhibit large surface areas and highly chemical activity, which can provide a high enough SO2 removing efficiency. From the superposition of the diagrams of Mn-S-O and Fe-S-O systems, it is found that there is a coexistent stability region of MnSO4 and Fe2O3, which provides the possibility of desulfurization by selective sulfation without ferric sulfate forming. A multi-stage desulfurization system has been discussed briefly.
Trans. Nonferrous Met. Soc. China 23(2013) 3089-3094
Wan-qi YE, Yun-jiao LI, Long KONG, Miao-miao REN, Qiang HAN
School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 13 August 2012; accepted 29 November 2012
Abstract: For the purpose of effective and economic desulfurization of flue-gas, the predominance area diagram of the Mn-S-O system at different temperatures was constructed based on the thermodynamic data obtained from the literatures. It is seen from this figure that flue-gas desulfurization by manganese oxides is feasible from the thermodynamic point of view. Additionally, the most appropriate temperature range for flue-gas desulfurization is between 600 and 800 K, and the reaction is strongly exothermic to maintain the heat balance. The natural manganese ores encompass large tunnels that exhibit large surface areas and highly chemical activity, which can provide a high enough SO2 removing efficiency. From the superposition of the diagrams of Mn-S-O and Fe-S-O systems, it is found that there is a coexistent stability region of MnSO4 and Fe2O3, which provides the possibility of desulfurization by selective sulfation without ferric sulfate forming. A multi-stage desulfurization system has been discussed briefly.
Key words: predominance area diagram; desulfurization; manganese oxides; dry FGD processes
1 Introduction
The principal cause of acid rain is generally attributed to the sulfur dioxide (SO2) emitted from coal combustion and other industrial processes. Although the concentration of sulfur dioxide in such gases is small low, the total quantity is very large [1]. Many methods have been developed for removing sulfur dioxide from flue gases [2-4]. Data on worldwide flue gas desulfurization (FGD) applications reveal that wet FGD technologies, in particularly wet limestone FGD processes, have been predominantly chosen from other FGD technologies [5] due to their high efficiency (higher than 90%) of the sulfur dioxide absorption and the use of cheap reactant [2]. In those technologies, SO2-containing flue gases contact with alkaline aqueous slurry which is generally made from either lime or limestone in absorption towers.
Although wet FGD technologies can meet the regulatory requirements for the control of sulfur dioxide emissions, some troublesome problems can’t be ignored. One objection is that the waste products are normally discarded as voluminous liquid slurry in an impoundment and ultimately capped with a clay barrier, which is then covered with topsoil once the slurry is de-watered over time [6]. A still further objection to these processes employing aqueous phase absorption is that such processes usually necessitate cooling the flue gas to about 55 °C [7], which ultimately has a higher density and leads to settle in the vicinity of the stack. As a result, local pollution may become worse, even though the quantities of sulfur compounds emitted to the atmosphere are reduced [3].
Dry FGD technologies, in which SO2-containing flue gases contact with dry sorbents (lime or limestone), are considered to be more suitable for flue gas desulfurization due to their low operating costs, high desulfurization efficiency, and no water consumption. Besides, dry FGD processes are operated at elevated temperature. As a result, the wastes produced by dry FGD processes are easier to dispose than that from wet FGD processes [5].
Manganese oxides (MnOx) have received special emphasis as an absorbent for sulfur dioxide recovery. Both mineral slurry and dry manganese oxides (ores) have been shown to be effective sorbents for sulfur compounds removing [8-11]. In the leaching of manganese ores, sulfur dioxide is shown to be a rapid, effective and sensitive reductant for manganese oxide minerals [8]. However, the formation of dithionate may bring pollution to the follow-up processes [12]. During the absorbing process, the SO2 in the waste gases reacts with the manganese oxide and results in the formation of manganese sulfate, which is then treated to regenerate back to the manganese oxide [6]. By means of such processes, SO2 is removed from the waste gases without cooling it off hence avoiding the undesirable problems of cooling referred to above.
Manganese oxide, which is mentioned above, is quite effective as an absorbent, but it is uneconomical to regenerate since manganese sulfate is too stable to entirely decompose at temperature nearly 1050 °C [10]. What’s more, such equipment and synthesis costs are relatively high, that largely negating the advantage of hot gas cleanup. On the other hand, moving the absorbent from the absorber to the regenerator and back again causes attrition and breakage [13]. Besides, the sorbent material must be durable-resistant to dusting breakage, vapor phase migration, and deactivation due to chemical combination or melting [5]. It’s a big challenge to synthesize an absorbent that meets all the requirements above. Setting manganese oxide (ores) as a raw material while manganese sulfate as the end product may be a better solution for eliminating these drawbacks. A process using manganese ores to remove SO2 and produce manganese sulfate for the recovery of manganese from ores at the same time is a better way economically.
During the investigation of desulfurization, a variety of manganese oxides have been studied [14-17], but there’s no complete thermodynamic diagram shown the feasibility of this method theoretically. In this work, a predominance area diagram of Mn-S-O system is drawn which shows superposition of the diagram of Fe-S-O and Mn-S-O systems, the chemical behavior of several manganese oxides during desulfurization process is analyzed and establish the thermodynamic feasibility of SO2 removal from flue gases is established.
2 Fundamental of diagrams
Sulfur dioxide reacts readily with manganese dioxide to form a manganese sulfate by the reaction below:
MnO2(s)+SO2(g)=MnSO4(s) (1)
The sulfur dioxide equilibrium pressure of a oxide/sulfate mixture can be calculated from the free energy of formation, ΔG, of the corresponding reaction of sulfate formation. For the formation of MnSO4.
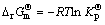 (2)
(2)
with the equilibrium constant
 (3)
(3)
To form a pure sulfate from a pure metal oxide (i.e., for  ), it follows from Eqs. (2) and (3):
), it follows from Eqs. (2) and (3):
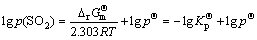 (4)
(4)
where  is the thermodynamic equilibrium constant of the reaction;
is the thermodynamic equilibrium constant of the reaction;  is the equilibrium partial pressure of SO2.
is the equilibrium partial pressure of SO2.
The species that present in Mn-S-O system are S2, O2, SO2, MnS2, MnS, Mn2O3, Mn3O4, MnO, MnO2 and MnSO4 at temperature 600 K. The thermodynamic data of the species were gathered from Ref. [18].
In an analogous manner, the equilibrium reactions and their equilibrium relationships involved between each related two species are listed in Table 1. Equation (2) can be used for calculating equilibrium constant ( ) and obtaining the relationships between
) and obtaining the relationships between  and
and  . Using the equilibrium relationships given in Table 1, a predominance area diagram is constructed, as shown in Fig. 1.
. Using the equilibrium relationships given in Table 1, a predominance area diagram is constructed, as shown in Fig. 1.
3 Results and discussion
3.1 Diagram of Mn-S-O system
Figure 1 shows a phase predominance area diagram for Mn-S-O system as a function of oxygen potential and sulfur dioxide potential at 600 K. The phase equilibriums among the various solid compounds involved are shown as a function of the logarithms of the equilibrium partial pressures of the two components in the gas phase, namely O2 and SO2. In this diagram, each phase is stable within the predominance region, bordered by beelines that represent the coexistence with other phases. The slope of each curve is determined by the stoichiometry of the corresponding reaction [19]. It illustrates the major chemical reactions occurring during flue-gas desulfurization by MnO2 and other manganese oxides. It also reveals the thermodynamic principles embodied in this process, indicating that manganese dioxide can remove sulfur dioxide to the level of less than 1×10-6.
Usually, a typical hot flue gas composition [20] resulting from the oxidation of high sulfur coal or oil can be given by point A, as shown in Fig. 1. It is located in the stability region of MnSO4 at 600 K, hence MnO2 will react with sulfur dioxide according to Eq. (1). As SO2 is removed from the fuel gas, the  will reduce gradually, reflected by the vertical downward line in the figure. The extent of sulfur removal possibly would be low enough to less than 1×10-6, until to the plane of the MnO2-MnSO4 phase boundary, to approach an equilibrium value (10-12.8) of point B, which is shown in Fig. 1. In addition, the low valence manganese oxides are readily converted to MnSO4 even in very slightly oxidizing atmosphere. This implies that MnSO4 is a stable form of manganese for virtually all commercially available coal-combustion flue gases. It is different from the desulfurization of hot reducing gases by Mn-based pellets which loaded at temperature below 800 °C and regeneration accomplished above 900 °C to avoid sulfate formation [14].
will reduce gradually, reflected by the vertical downward line in the figure. The extent of sulfur removal possibly would be low enough to less than 1×10-6, until to the plane of the MnO2-MnSO4 phase boundary, to approach an equilibrium value (10-12.8) of point B, which is shown in Fig. 1. In addition, the low valence manganese oxides are readily converted to MnSO4 even in very slightly oxidizing atmosphere. This implies that MnSO4 is a stable form of manganese for virtually all commercially available coal-combustion flue gases. It is different from the desulfurization of hot reducing gases by Mn-based pellets which loaded at temperature below 800 °C and regeneration accomplished above 900 °C to avoid sulfate formation [14].
Table 1 Equilibrium reactions and relationships for Mn-S-O system at 600 K

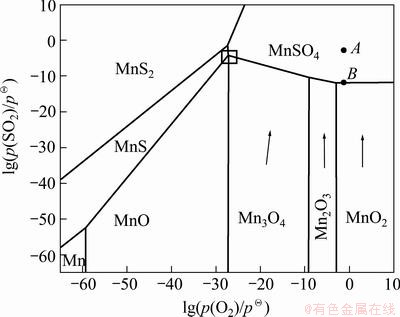
Fig. 1 Predominance area diagram of Mn-S-O system at 600 K
Figure 2 is a scale-up diagram of Fig. 1. Combining with Fig. 1, it can be seen that MnO, Mn3O4 and Mn2O3 are also suitable for desulfurization. Preliminary studies are consistent with the results of this work.

Fig. 2 Scale-up diagram of Fig. 1
INGRAHAM and MARIER [21] tested the rate of SO2 absorption by MnO2, Mn2O3, and Mn3O4 at temperatures between 573 and 723 K. It was found that the rate of reaction decreased rapidly while the mass gain increased almost linearly with time for both Mn2O3 and Mn3O4. The results were confirmed in Ref. [11]. LI et al [15] measured SO2 absorption by various forms of MnO2 and Mn2O3 and found significant effects of solid characteristics on absorption capacity.
There are three known mineral polymorphs of MnO2, among which pyrolusite is the most stable and abundant [22]. But it’s inevitably mixed with some other low-valence oxide. Evidence from a wide variety of investigation indicated that some other forms of manganese ores can also be used for desulfurization [6,11,23]. The deposition of Mn oxides occurs in the oceans as nodules, in which the initial composition is believed to be close to MnO2. Such nodules are also suitable for sulfur removal because of their large surface areas and chemical activity. Sometimes the performance may be even better as it is measured [11].
3.2 Influence of temperature on desulfurization
In the desulfurization of hot flue gases by manganese ores, the extent of desulfurization depends on temperature and gas composition. Figure 3 shows the superposition of the diagrams of Mn-S-O system at temperatures of 600, 800, and 1000 K. With increasing temperature, the equilibrium lines shift upward and turn to the up and right-hand corner, further constricting the stable region of the sulfate. And the manganese ore will lose its capacity to desulfurization at 1000 K.

Fig. 3 Predominance area diagram of Mn-S-O system from 600 K to 1000 K
It is seen from Fig. 3 that the lower the operating temperature is, the fewer the residual sulfur in the cleaned gas becomes. Desulfurization at high temperature can be more efficient than low temperature because of its higher mass transfer rate. A sufficient high temperature must be ensured, especially in the latter stages of desulfurization processes, to eliminate the diffusion control caused by the product clogging in the pore. Of course, the temperature can’t be too high. Specific temperature will be determined by the characteristics of the minerals and the process. BIENSTOCK and FIELD [24] measured different kinds of manganese oxides at 130 and 330 °C by the bench-scale tests, and found that removal of sulfur dioxide by solids from a simulated flue gas at elevated temperatures is possible. KIANG et al [16 ] proposed a nontopochemical model for the kinetics of sulfur dioxide absorption by a porous spherical pellet of chemical grade manganese dioxide, indicating that the reaction was related to the temperature and first ordered with respect to SO2 concentration. Temperature ranging from 600 to 800 K is recommended to obtain an ideal reaction speed and a desulfurization degree of less than 1×10-6 to meet the environmental requirements.
It should be noted that the oxidation reaction is highly exothermic. Reaction 1, for example, has a reaction heat of -252.3 kJ for a mole of MnSO4 reacted at the temperature of 600 K [18]. Accordingly, oxidation temperature can be regulated by providing supernumerary cooling means. The flue gas discharge temperature is about 150 °C [24] with a concentration of SO2 from 0.05% to 0.23% [13], normally. This value will increase with increasing sulfur content in the coal. Hence there is no need for concern over excessive desulfurization temperatures as in prior processes. The excess sensible heat of the oxidation reaction can conveniently supply the heat requirements of the flue gas warm-up.
3.3 Feasibility of selective desulfurization by manganese ore
Manganese ore is a mixture of many substances, including significant amount of silica, iron, calcium, aluminium together with small amount of zinc, phosphorus, and other materials [22]. Iron oxide was also considered to be a very promising high-temperature desulfurization sorbent because of its favorable sulfation thermodynamics from the standpoint of desulfurization efficiency [25]. Ferric oxide in the ores may be transformed to ferric sulfate which is soluble in the water solution. This will bring a lot of troubles to the follow-up separation process.

Fig. 4 Superposition of diagrams of Mn-S-O and Fe-S-O systems
Figure 4 shows a superposition of the predominance area diagrams of Mn-S-O and Fe-S-O systems. As indicated in Fig. 4, the shaded region shows the coexistent stability region of MnSO4 and Fe2O3. Under appropriate conditions, the MnO2 can be sulfated. Besides, the activity of manganese dioxide is much higher than ferric oxide at high temperature [13], which helps to avoid forming ferric sulfate. This provides the possibility of desulfurization by selectively sulfation.
The unusually high adsorption capacities and scavenging capabilities of manganese oxide minerals provide one of the primary controls of heavy metals and other trace elements in soils and aquatic sediments, because of their large tunnels and related structures that exhibit large surface areas and high chemical activity [26]. There are considerable interests in the use of these materials and synthetic analogues as catalysts and sorbent [27,28]. Some entrained solid particles such as fly ash and other heavy metals from waste gas streams can be removed while the reaction occurs to remove the sulfur dioxide at the same time, in order to prevent air pollution and recover a valuable sulfur-containing product economically. Although the efficiency of desulfurization may tend to drop off in such application because of the accumulation of dust and smoke particles on the surfaces of the fragments [29], it won’t be the main reason for efficiency decrease. Because the fly ash and heavy metals are relatively a very small amount compared with manganese ores. More importantly, the clay-like slimes on the surface of the ores can be removed by leaching and attrition instead of regeneration treatment at high temperature. This will be able to avoid the accumulation of heavy metals and other impurities on the adsorbent.
As the mass transfer rate is limited at lower temperature, a multi-stage desulfurization system can be used to achieve the goal of desulfurization and the recovery of manganese. In this system, many adsorption towers are arranged in series. Higher temperature will be utilized in the first desulfurization segment in order to achieve a larger mass transfer rate to meet the demand of the conversion rate of manganese ores, and the temperature will be reduced gradually in the second or third segment, resulting in removal of sulfur dioxide more thoroughly. Additionally, the goal of elevated temperature won’t be a problem with strong exothermic of oxidation reaction at the first segment which removes most of the SO2. By this kind of installation, it is preferred that such towers can be used serially to permit discharging of adsorbed minerals from one tower, and adding with fresh minerals while the other tower is on stream. Besides, manganese sulfate leached after desulfurization can be routed for further processing into marketable products or for distribution and sale as a useful by-product instead of regenerating at higher temperature [30].
Additional insights into the principle of flue-gas desulfurization by manganese ores likely await a more thorough and meticulous understanding of the atomic structures and reaction mechanism. Specific kinetic data are also looking forward to be studied.
4 Conclusions
1) The predominance area diagram of the Mn-S-O system was drawn, and the thermodynamic feasibility of using manganese oxides for deep desulfurization from flue-gas to the level of less than 1 mg/m3 was demonstrated.
2) According to the superposition of the predominance area diagram at different temperatures, the most appropriate temperature range of desulfurization is 600-800 K, and the reaction is strongly exothermic to maintain the heat balance. The superposition of the diagrams of Mn-S-O and Fe-S-O systems shows that both the manganese and iron have the ability to desulfurization.
3) In order to prevent iron ions from dissolving into the liquid phase, a method for selective sulfation was put forward to avoid iron sulfate from forming. The natural manganese ores such as pyrolusite and manganese nodules encompass large tunnels that exhibit large surface areas and high chemical activity. They can produce a high enough removing efficiency without complex synthesis process.
4) By selecting an appropriate temperature and a suitable process, ferric sulfate can be avoided and a valuable sulfur-containing product, such as manganese sulfate, can be economically and efficiently recovered.
References
[1] ZHU X F, SU S J, REN Z L, GUO C X, JIN Y. Study on flue gas desulfurization with pyrolusite pulp [J]. Journal of Sichuan University, 2000, 32(5): 36-39. (in Chinese)
[2] KALLINIKOS L E, FARSARI E I, SPARTINOS D N, PAPAYANNAKOS N G. Simulation of the operation of an industrial wet flue gas desulfurization system [J]. Fuel Processing Technology, 2010, 91(12): 1794-1802.
[3] BAO J Q, TIAN R, ZHAO X X, JIA C X. Researching into microbial-limestone new wet flue gas desulfurization method [J]. In: eds. IEEE, 2011: 677-679.
[4] GAO H L, LI C T, ZENG G M, ZHANG W, SHI L, LI S H, ZENG Y N, FAN X P, WEN Q B, SHU X. Prediction and experimental validation studies of wet flue gas desulphurization with a novel type PCF device based on limestone-gypsum [J]. Energy & Fuels, 2010, 24: 4944-4951.
[5] SRIVASTAVA R K, JOZEWICZ W. Flue gas desulfurization: the state of the art [J]. Journal of the Air & Waste Management Association, 2001, 51(12): 1676-1688.
[6] PAHLMAN J E, CARLTON S C, HUFF R V, HAMMEL C F, BOREN R M, KRONBECK K P, LARSON J E, TUZINSKI P A, AXEN S G. System and process for removal of pollutants from a gas stream: US6579507 B2 [P]. 2003-06-19.
[7] LEE Y J, ROCHELLE G T. Oxidative degradation of organic acid conjugated with sulfite oxidation in flue gas desulfurization: Products, kinetics, and mechanism [J]. Environmental Science & Technology, 1987, 21(3): 266-272.
[8] RAISONI P R, DIXIT S G. Leaching of manganese ore with aqueous sulphur dioxide solutions [J]. Bulletin of Materials Science, 1988, 10(5): 479-483.
[9] LIU Y, SUN J, HU X X, SHU S J, DING S L, YU Z L. Study on flue gas desulfurization with rhodochrosite and pyrolusite pulp [J]. China’s Manganese Industry, 2008, 26(4): 19-23. (in Chinese)
[10] BEN-SLIMANE R, HEPWORTH M T. Desulfurization of hot coal-derived fuel gases with manganese-based regenerable sorbents. 1. Loading (sulfidation) tests [J]. Energy & Fuels, 1994, 8(6): 1175-1183.
[11] van HECKE M C, BARTLETT R W. Kinetics of sulfation of atlantic ocean manganese nodules [J]. Metallurgical and Materials Transactions B, 1973, 4(4): 941-947.
[12] SUN W Y, SU S J, DING S L, JIANG W J, XU Y. Decomposition characteristics of manganese dithionate in absorption solution of flue gas desulfurization and denitration with pyrolusite slurry [J]. Journal of Sichuan University, 2011, 43(3): 166-170. (in Chinese)
[13] SLACK A V. Removing sulfur dioxide from stack gases [J]. Environmental Science & Technology, 1973, 7(2): 110-119.
[14] BIENSTOCK D, FIELD J H, MYERS J G. Process development in removing sulfur dioxide from hot flue gases (in four parts): Bench-scale experimentation [R]. US Dept. of the Interior, Bureau of Mines, 1961.
[15] LI K, ROTHFUS R R, ADEY A H. Effect of macroscopic properties of manganese oxides on absorption of sulfur dioxide [J]. Environmental Science & Technology, 1968, 2(8): 619-621.
[16] KIANG K D, LI K, ROTHFUS R R. Kinetic studies of sulfur dioxide absorption by manganese dioxide [J]. Environmental Science & Technology, 1976, 10(9): 886-893.
[17] SLIMANE R B, STUART R W, HEPWORTH M T. Preparation and testing of value-added sulfur sorbent pellets from in situ mined Minnesota manganese deposits [J]. Energy & Fuels, 1996, 10(6): 1250-1256.
[18] BARIN I. Thermochemical data of pure substances. Part I & II [M]. New York: VCH, 1995.
[19] BEN-SLIMANE R, HEPWORTH M T. Desulfurization of hot coal-derived fuel gases with manganese-based regenerable sorbents. 2. Regeneration and multicycle tests [J]. Energy & Fuels, 1994, 8(6): 1184-1191.
[20] VAMVUKA D, ARVANITIDIS C, ZACHARIADIS D. Flue gas desulfurization at high temperatures: A review [J]. Environmental engineering science, 2004, 21(4): 525-548.
[21] INGRAHAM T R, MARIER P. Kinetics of the formation of MnSO4, from MnO2, Mn2O3, and Mn3O4 and its decomposition to Mn2O3 or Mn3O4 [J]. Trans Metal Soc AIME, 1968, 242: 2039-2043.
[22] ZHANG W S, CHENG C Y. Manganese metallurgy review. Part I: Leaching of ores/secondary materials and recovery of electrolytic/ chemical manganese dioxide [J]. Hydrometallurgy, 2007, 89(3-4): 137-159.
[23] ERICKSON D C. Regenerable manganese oxide hot gas desulfurization process: US, 4283374 [P]. 1981-08-11.
[24] BIENSTOCK D, FIELD F J. Bench-scale investigation on removing sulfur dioxide from flue gases [J]. J Air Pollut Control Assoc, 1960, 10(2): 121-125.
[25] SASAOKA E, SAKAMOTO M, ICHIO T, KASAOKA S, SAKATA Y. Reactivity and durability of iron oxide high temperature desulfurization sorbents [J]. Energy & Fuels, 1993, 7(5): 632-638.
[26] POST J E. Manganese oxide minerals: Crystal structures and economic and environmental significance [J]. Proceedings of the National Academy of Sciences, 1999, 96(7): 841-844.
[27] ANDREOZZI R, INSOLA A, CAPRIO V, MAROTTA R, TUFANO V. The use of manganese dioxide as a heterogeneous catalyst for oxalic acid ozonation in aqueous solution [J]. Applied Catalysis A: General, 1996, 138(1): 75-81.
[28] JENNE E A. Controls on Mn, Fe, Co, Ni, Cu, and Zn concentrations in soils and water: The significant role of hydrous Mn and Fe oxides [J]. Adv Chem Ser, 1968, 73: 337-387.
[29] ZIMMERLEY S R. Use of deep-sea nodules for removing sulfur compounds from gases: US, 3330096 [P]. 1967-07-11.
[30] ZHU E G, SU S J, SUN W Y, ZENG L Q. Study on removal of iron by oxidation from absorption of flue gas simultaneous desulfurization and denitration with pryolusite [J]. Environment Engineering, 2011, 29(1): 72-75. (in Chinese).
叶万奇,李运姣,孔 龙,任苗苗,韩 强
中南大学 冶金与环境学院,长沙 410083
摘 要:为了有效和经济地脱除烟气中的硫,利用文献中的数据绘制不同温度下Mn-S-O系的热力学平衡图,从热力学角度说明使用氧化锰脱硫的可行性。提出最合适的烟气脱硫温度区间为600~800 K,而由于反应的强烈放热使得其能够维持操作的热平衡。天然锰矿由于其孔道结构而具有较大的表面积和较高的化学活性,使其脱硫效率可以保持在很高的水平。Mn-S-O与Fe-S-O系的叠加图中存在MnSO4和Fe2O3的重叠区域,说明可以通过选择性脱硫避免硫酸铁的形成。简单讨论了多级脱硫系统。
关键词:热力学平衡图;脱硫;氧化锰;干法脱硫过程
(Edited by Chao WANG)
Foundation item: Project (51344006) supported by the National Natural Science Foundation of China
Corresponding author: Yun-jiao LI; Tel: +86-731-88830476; Fax: +86-731-88710171; E-mail: yunjiaoli6601@hotmail.com
DOI: 10.1016/S1003-6326(13)62838-1

