
Slurry electrolysis of ocean polymetallic nodule
WANG Cheng-yan(王成彦), QIU Ding-fan(邱定蕃), YIN Fei(尹 飞),
WANG Han-yuan(王含渊), CHEN Yong-qiang(陈永强)
Beijing General Research Institute of Mining and Metallurgy, Beijing 100044, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract: The ocean poly-metallic nodule was leached by using slurry electrolysis process in HCl-NaCl medium. The leaching rates of Mn, Co, Cu and Ni in the ocean poly-metallic nodule are all above 97%. Meanwhile, the high purity of electrolytic MnO2 is also obtained as an anode product. The effects of electrolysis electric quantity, acidity, temperature, slurry density, grain size and iron ions concentration were studied. The results show that the ocean poly-metallic nodule can be treated economically in the slurry electrolysis process.
Key words: ocean poly-metallic nodule; slurry electrolysis process; high purity MnO2
____________________________________________________________________________________________
1 Introduction
The copper-, cobalt- and nickel-rich ocean poly-metallic nodule has attracted much attention for a long time[1-2]. Since 1960s a large number of works on exploration, mining, and metallurgy for the ocean nodule have been done, and great progress has been made[3-5]. The main technologies have been developed in metallurgy including reducing smelting, vulcanizing smelting, reducing roasting, direct acid leaching, reducing acid leaching, bioprocessing and reducing ammonia leaching, etc[6-8]. Due to high water content of nodule (about 30%), high energy consumption is needed for pre-dehydration of the nodule before smelting or roasting[9-10]. By contrast, traditional hydrometallurgy technology can treat the nodule without any pre-dehydration although chemical reagent consumption is relatively high[11]. A new clean hydrometallurgy process with high efficiency and low cost is thus necessary.
Slurry electrolysis (SE) is a hydrometallurgical process which has been developed for over twenty years[12-17]. It combines traditional hydrometallurgical technique, such as leaching, solution purification and electrowinning into one step. It can simultaneously leach sulfide ore on the anode by oxidation and reduce oxide ore in slurry on the cathode. It therefore transfers the high-energy-consuming anode or cathode reaction into efficient leaching process of metal from the slurry, decreasing the cell voltage of electrolysis and electricity consumption. And the hydrometallurgical flow-sheet is simplified.
The SE technology can also utilize low-price direct current to leach nodule in cathode, producing the MnO2 in anode directly at the same time. It was observed that the valence of the manganese in the nodule had not changed during the SE. Only the migration of MnO2 occurred in the electric field. The MnO2 was leached in cathode and produced again in anode. As a result, the SE technology consumes neither reagent nor electric energy. It consists of a reverse reaction of MnO2 on the anode and the cathode. The electric energy is needed for overcoming the resistance of solution and side reaction on electrode. The unchanged valence of the manganese in nodule is the cardinal difference between the SE and traditional hydrometallurgical technologies. As a new hydrometallurgical technology, the slurry electrolysis process can realize the economic treatment of ocean nodule.
2 Experimental
Chemical composition of the experimental nodule is as follows (mass fraction, %): Mn 25.19, Ni 1.12, Cu 0.72, Co 0.25, and Fe 8.43.
All experiments were conducted in a 5 L electrolyser. Ti/MnO2 strip and graphite stick were used as anode and cathode, respectively. A percolating diaphragm was installed to separate the electrolyser into anode zone and cathode zone. In the cathode zone, slurry was agitated by a JJ-90 type electric agitator. The electrolyser was maintained in a thermostatic water bath to keep the temperature constant.
3 Results and discussion
3.1 Leaching mechanism of ocean nodule in SE
In HCl-NaCl medium, the leaching process of the ocean poly-metallic nodule in SE is very complicated. In cathode zone, the leaching reactions take place:
Cathode reduction:
MnO2+2e-+4H+=Mn2++2H2O (1)
Fe2+ reduction:
MnO2+2Fe2++4H+=2Fe3++Mn2++2H2O (2)
And Fe3+ ion is then reduced on the cathode again:
Fe3++e-=Fe2+ (3)
Hydrochloric acid reduction:
MnO2+4HCl=MnCl2+2H2O+2Cl2↑ (4)
With the leaching of manganese on the cathode, the copper, cobalt and nickel stored in nodule are accordingly leached in anode zone. The dissolved manganese ion will deposit on the anode in the form of MnO2:
Mn2++2H2O=MnO2+2e-+4H+ (5)
It can be seen that SE consists of the reducing leaching of nodule in cathode zone, the oxidizing deposition of Mn2+ on anode and the reducing regeneration of Fe2+ on the cathode. The reduction reactions on the cathode are correlated closely with the oxidation reactions on the anode.
3.2 Effect of electrolysis electric quantity
The electrolysis electric quantity in SE is a decisive factor in the leaching of the nodule. Effect of electrolysis electric quantity on the leaching rates of Mn, Co, Cu and Ni is shown in Fig.1. The leaching rates of manganese could reach about 97% under the electric quantity of 0.8 times the theoretical one of manganese in nodule. The leaching rates of cobalt, nickel, and copper were all about 98%. The leaching rate of copper dropped above the theoretical electric quantity of manganese. It was mainly caused by the fact that the copper was reduced into copper powder again and deposited into residue.

Fig.1 Effect of electric quantity on leaching rates of nodule (Leaching conditions: NaCl 120 g/L, Mn 51.83 g/L, 70 ℃, 650-750 r/min, J+=100 A/m2, J-=140 A/m2, L/S=15:1, pH= 0.5-2)
3.3 Effect of acidity
The acidity of the solution influences the reduction potential of MnO2 remarkably. It will enlarge the polarization of cathode by reducing the concentration of hydrochloric acid, which will decrease the dissolution of the nodule. Fig.2 shows the influence of the acidity on the leaching rates of the nodule. When the pH of the cathode solution was greater than 2, the leaching rates of Mn and Co dropped rapidly.
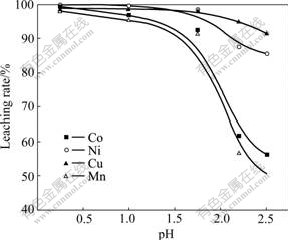
Fig.2 Effect of pH on leaching of nodule (Leaching conditions: NaCl 120 g/L, Mn 51.83 g/L, 70 ℃, 650-750 r/min, J+=100 A/m2, J-=140 A/m2, L/S=15:1, 200 min)
3.4 Effect of temperature
As it is well known, the effect of temperature on the leaching rates of metal elements in minerals is great. It not only influences the moving speed of ions in solution and the reaction speed of chemical leaching, but also influences the electrochemical reaction speed on the surface of electrode. The variation of the leaching rate of Mn versus time at difference temperatures is shown in Fig.3. The nodule could be leached easily. The leaching rate of manganese in the nodule was higher than 95% when the temperature was greater than 50 ℃ by giving the theoretical electric quantity of manganese.

Fig.3 Effect of temperature on leaching of nodule (Leaching conditions: NaCl 120 g/L, Mn 0 g/L, HCl 20 g/L, Fe 1 g/L, <0.074 mm 86.9%, 750 r/min, J-=140 A/m2, L/S=50:1)
3.5 Effect of iron ion concentration
The iron ions acted as the main reducing agents in the SE of the nodule. They reacted with the nodule directly by changing the reaction ways on the cathode and the transformation of reaction electron, decreasing the cell voltage significantly. The variation of the leaching rate of Mn versus time under different concentrations of iron is shown in Fig.4.
Fig.4 indicates that the iron ions concentration exerts a remarkable influence on the leaching rate of the nodule. The initial leaching speed of manganese was nearly two times higher with Fe 5 g/L in the solution than that without iron ions. The change of the leaching speed was minor when the iron ion concentration was greater than 1 g/L. With electric quantity less than 0.8 times the theoretical one of manganese, almost all of the manganese was leached.
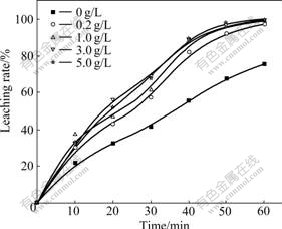
Fig.4 Effect of iron ion concentration on leaching of nodule (Leaching conditions: NaCl 120 g/L, Mn 0 g/L, HCl 5 g/L, 70 ℃, <0.074 mm 86.9%, 750 r/min, J-=100 A/m2, L/S= 200:1)
3.6 Effect of slurry density
The test results of different slurry density are shown in Fig.5. The leaching rates of Co, Ni, Cu and Mn began to drop lightly when the L/S was smaller than 5. When the L/S was greater than 8, the effect of slurry density was not obvious.

Fig.5 Effect of slurry density on leaching of nodule (Leaching conditions: NaCl 120 g/L, Mn 51.83 g/L, 70 ℃, 650-750 r/min, J+=100 A/m2, J-=140 A/m2, pH=0.5-1.5)
3.7 Effect of grain size
Fig.6 shows the effect of the grain size on the leaching. It is indicated that the grain size has great influence on the leaching rates of cobalt and manganese, but has little effect on the leaching rates of the copper and nickel. It is reasonable to control the grain size of the nodule with 80%-90% passing through 0.074 mm.
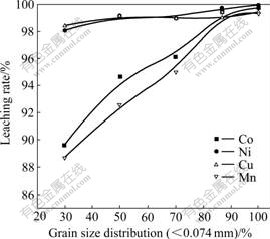
Fig.6 Effect of grain size of nodule on leaching (Leaching conditions: NaCl 120 g/L, Mn 51.83 g/L, 70 ℃, 650-750 r/min, J-=140 A/m2, L/S=15:1, pH =0.5-1.5)
3.8 Comprehensive experiments
The optimum conditions of the leaching of the nodule on the cathode and the electrolysis of MnO2 on the anode were taken into account on the basis of the above study. The comprehensive experiments were conducted by choosing the temperature of 70, 80 and 90 ℃, respectively. The other experiment conditions are listed in Table 1.
Table 1 Results of comprehensive tests

The results of comprehensive experiments proved that the leaching rates of manganese, cobalt, nickel and copper can exceed 97% by giving 0.8 times theoretical electric amount for manganese in the nodule with acidity of 0.8. The contents of manganese, cobalt, nickel and copper in leached residue are Mn<3%, Co<0.02%, Ni<0.05%, Cu<0.04%, respectively. The current efficiency of the anode is about 70%. The content of manganese in electrolysis MnO2 is greater than 58%, in which the content of the cobalt, nickel and copper are all smaller than 0.005%, and the content of the iron is smaller than 0.02%.
4 Conclusions
1) It is feasible to adopt the slurry electrolysis to treat the ocean poly-metallic nodule in HCl-NaCl medium. The optimum conditions are: NaCl 120 g/L, Mn 40-70 g/L, temperature 70 ℃, pH 0.5-1.5, cathode current density 200 A/m2, L/S=(6-10):1, and 0.8 times the theoretical electric quantity of the manganese. The leaching rates of all the main metal elements, i.e. manganese, cobalt, nickel and copper all exceed 97%.
2) The average voltage is 2.6 V. The power consumption per ton ore is 700-900 kW?h. The HCl (37%) consumption per ton ore is about 1.6 t. Meanwhile, high quality electrolytic MnO2 is produced with an output per ton ore of about 280 kg.
3) Under optimum conditions, the contents of manganese, cobalt, nickel and copper in leached residue are Mn<3%, Co<0.02%, Ni<0.05%, Cu<0.04%, respectively. The current efficiency of the anode is about 70%. The content of manganese in electrolysis MnO2 is greater than 58%, in which the contents of the cobalt, nickel and copper are all smaller than 0.005%, and the content of the iron is smaller than 0.02%.
References
[1] WANG Yun-shan, LI Zuo-hu, LI Hao-ran. Kinetics of oxidative leaching of ocean polymetallic nodules in molten potassium hydroxide medium [J]. Transactions of Nonferrous Metals Society of China, 2005, 15(3): 697-701.
[2] KUMARI A, NATARAJAN K A. Electroleaching of polymetallic ocean nodules to recover copper, nickel and cobalt [J]. Minerals Engineering, 2001, 14(8): 877-886.
[3] QIU Ding-fan. Slurry electrolysis [M]. Beijing: Metallurgical Industry Press, 1999: 12-26. (in Chinese)
[4] QIU Ding-fan. Chemical process of slurry electrolysis on chalcopyrite [J]. Nonferrous Metals (Metallurgy Section), 1996(5): 1-3. (in Chinese)
[5] WANG Cheng-yan, QIU Ding-fan, ZHANG Yin-sheng, JIANG Pei-hai. Mechanism of leaching bismuthinite in slurry electrolysis process [J]. Nonferrous Metals (Quarterly), 1995(2): 54-59, 73. (in Chinese)
[6] MUKHERJEE A, RAICHUR A M, MODAK J M, NATARAJAN K A. Bioprocessing of polymetallic Indian ocean nodules using a marine isolate [J]. Hydrometallurgy, 2004, 73(3/4): 205-213.
[7] JIANG Kai-xi, JIANG Xun-xiong, WANG Sheng-dong, LI Zhen-you, FAN Yan-qing, ZHAO Lei, WANG Hai-bei. Study on ammonia leaching of ocean polymetallic nodules using carbon monoxide as a reducing agent [C]// Proceedings of the Sixth ISOPE Ocean Mining Symposium. Changsha: The International Society of Offshore and Polar Engineers. 2005: 218-222.
[8] CHEN Ling, YANG Xian-wan, SI Yun-sen, ZHANG Ying-jie. Technological condition in slurry electrolysis of high-silver galena concentrate [J]. Transactions of Nonferrous Metals Society of China, 2002, 12(2): 344-348.
[9] WANG Cheng-yan, QIU Ding-fan, ZHANG Yin-sheng. Study on slurry electrolysis process for leaching bismuthinite [J]. Nonferrous Metals (Quarterly), 1995, 47(3): 52-56. (in Chinese)
[10] YANG Xian-wan, ZHANG Ying-jie. Mechanism of slurry electrolysis [M]. Beijing: Metallurgical Industry Press, 2000: 62-90. (in Chinese)
[11] SHEN Qing-feng, YANG Xian-wan, SI Yun-sen, XIE Ke-qiang. Cathode process of pyrolusite in slurry electrolysis [J]. Proceedings of the China Association for Science and Technology, 2008, 4(3): 153-156.
[12] MISRA V N, HALBE D, SPOTTISWOOD D J. Extractive metallurgy of gold and base metals [C]// Proceedings of the International Conference on Extractive Metallurgy of Gold and Base Metals. Kalgoorlie, 1992: 38-46.
[13] ZHANG Y J, YANG X W, DENG L H, YAO G. Leaching mechanism of sulfide ores in slurry electrolysis [J]. Transactions of Nonferrous Metals Society of China, 2000, 10(1):105-108.
[14] WANG Cheng-yan, QIU Ding-fan, JIANG Pei-hai. Technological research on complicated antimony-lead concentrate slurry electrolysis [J]. Nonferrous Metals (Quarterly), 2002, 54(3): 42-46. (in Chinese)
[15] WANG Cheng-yan, QIU Ding-fan, JIANG Pei-hai. Leaching mechanism of complicated antimony-lead concentrate in slurry electrolysis [J]. Nonferrous Metals (Quarterly), 2002, 54(4): 42-47. (in Chinese)
[16] WANG Y, LI Z, LI H. A new process for leaching metal values from ocean polymetallic nodules [J]. Minerals Engineering, 2005, 18(11): 1093-1098.
[17] ARSLAN F, DUBY P F. Electro-oxidation of pyrite in sodium chloride solutions [J]. Hydrometallurgy, 1997, 46: 132-138.
_______________________
Foundation item: Projects(50674014, 50734005) supported by the National Natural Science Foundation of China
Corresponding author: WANG Cheng-yan; Tel: +86-10-88399551; Fax: +86-10-88377374; E-mail: chywang@yeah.net
(Edited by YANG Bing)