
Preparation of soil nutrient amendment using white mud produced in ammonia-soda process and its environmental assessment
SHI Lin(石 林), LUO Han-jin(罗汉金)
College of Environmental Science and Engineering, South China University of Technology,
Guangzhou 510006, China
Received 15 July 2009; accepted 7 September 2009
Abstract: A novel method to prepare soil nutrient amendment by calcining a mixture of white mud and potassium feldspar and its environmental assessment were investigated. Under the optimal conditions of a blending mass ratio of 70?30 for white mud to potassium feldspar, a calcination temperature of 1 000 ℃, a calcination time of 1.5 h and spherulitic diameter of 2.0 cm, the calcined product, as a soil nutrient amendment, could be prepared with the following nutrient composition (mass fraction): K2O 4.16%, CaO 23.43%, MgO 5.04%, SiO2 22.92%, SO42- 3.71%, and Cl- 3.87% in 0.1 mol/L citric acid solution. The concentrations of heavy metals in the calcined product and the emission concentrations of harmful gases from a mixture of white mud and potassium feldspar during calcination process could qualify the National Standards without causing secondary environmental pollution.
Key words: white mud; potassium feldspar; soil nutrient amendment; environmental assessment; preparation
1 Introduction
Distilled waste sludge containing 50%-60% water, commonly known as “white mud”, is discharged from the Ammonia-soda Process in quantity[1]. In 2000, 59% of soda ash was manufactured by this process in the world[2], and 52.5% of soda ash production used this process in China. There was a total domestic capacity of soda ash production of 1.5×107 t in 2006[3]. And annual demand is still increasing by (1.4-1.5)×106 t[4]. In the Ammonia-soda Process, lime milk is added to the residual mother liquor in the presence of CO2-saturated NH4Cl in order to allow ammonium recycling, which results in a great deal of insoluble impurities generated in the form of CaCO3, CaSO4, Ca(OH)2 , dead-burnt CaO, Mg (OH)2 , SiO2 and so on[5-6].
Due to its finer particle size, larger surface area and colloidal property, it is more difficult to deposit this white mud at the bottom of distilled waste to further implement dehydration. In addition, for its higher content of chlorides in the form of CaCl2 and NaCl, it also possesses a high water adsorptivity, hygroscopy and corrosivity. Moreover, the highly alkaline (pH=10-12) of white mud, in combination with the above-mentioned factors, makes it become a serious obstruction for application in the building and cement industry. So far, the common treatment of white mud is still to discard it into waterway or dispose of it in landfills. Besides taking up potentially cultivatable lands, landfill disposal also causes environmental pollution to soil and underground water because the soluble substances will infiltrate into the groundwater and soil in the process of its weathering and leaching. Therefore, development of technology for resource utilization of white mud is necessary and important at present.
In the southern stretches of the Yangtze River, China, most of cultivated lands display acidic soil properties[7-8]. This phenomenon has become increasingly stronger due to the frequent occurrence of acid rain in recent years[9]. The total area occupied by acid soil has now reached 5.333×1011 m2, 5.6% of which is located in Guangdong Province, China. Nutrient elements such as K, Ca, Mg, S are lacking seriously in the soils in these regions. For example, the extent of deficiency of the element K in paddy soil can be as much as 90%, while for the elements Mg, Si, Ca and S it can be 22%, 22%, 50% and 54%[10], respectively, in the South China.
Abundant nutrient elements, including Ca, Mg and S, exist in white mud and its alkalinity can also neutralize the acidity of soil and adjust the physicochemical properties of the soil. However, if the white mud is directly applied as a soil amendment, its high alkalinity, high content of inert CaCO3, the presence of harmful ingredients such as Cl- and its high viscosity all present distinct disadvantages.
However, addition of the mineral, potassium feldspar, could overcome the most of these problems. The elements K and Si in potassium feldspar are disintegrated to water-soluble potassium and citric acid soluble silicon by a chemical reaction occurring between the white mud and the potassium feldspar during calcination process. At the same time, CaCO3 in the white mud will be broken down into CaO and CO2, while the harmful Cl- ion decreases markedly through volatilization. The calcined product could be used as a soil nutrient amendment and contains higher levels of elements such as K, Ca, Mg, Si and S. Its pH value will be also decreased to some extent, making it more suitable for adjusting acid soil in the South China.
White mud consists primarily CaCO3, CaSO4?2H2O, CaCl2, NaCl, Ca(OH)2, dead-burnt CaO, Mg (OH)2 and SiO2. Of these compounds, CaCO3 can be decomposed into CaO and CO2 vapor at 897 ℃, and when white mud is heated at temperatures higher than 950 ℃, no CaCO3 can be detected[11]. Similarly, CaSO4?2H2O is dehydrated to form CaSO4?1/2H2O in the temperature range of 105-180 ℃ and completely loses all crystal water to form Ⅱ-type CaSO4 at about 350 ℃. Ca(OH)2 is also broken down into CaO and water vapor at 580 ℃. Therefore, after calcining with potassium feldspar in the temperature range of 900-1 000 ℃, the raw materials would be expected to transform into a new reaction system consisting of potassium feldspar KAlSi3O8- CaSO4-CaO, with other minor components such as CaCl2, NaCl and MgO.
There has been substantial research into the KAlSi3O8-CaSO4-CaO reaction system by previous researchers and ourselves[12-15]. The reaction equation for this system has been put forward as the following:
2KAlSi3O8+CaSO4+14CaO=
K2SO4+3CaO?Al2O3+6(2CaO?SiO2) (1)
The CaCl2, NaCl and Mg(OH)2 existing in the white mud will lead to a decrease in the melting point of potassium feldspar and therefore will reduce the activation energy of the reaction system[16], allowing a reduction in the reaction temperature required. At the same time, a replacement reaction could also occur between this halide in white mud and potassium feldspar. The soluble potassium chloride will dissociate from potassium feldspar when the temperature is close to 1 000 ℃[17-18], described as following equations:
KAlSi3O8+NaCl=NaAlSi3O8+KCl (2)
2KAlSi3O8+ CaCl2=CaAl2Si2O8+2KCl+4SiO2 (3)
Therefore, the feasibility of preparation of a soil nutrient amendment with the elements K, Ca, Mg, Si and S , by calcining a mixture of white mud with potassium feldspar, is carried out in this study.
2 Experimental
The experimental equipments in the sample preparation and composition determination are listed as follows: drying oven (type: Boxue BGZ-50), muffle furnace (type: SX3-4-13), tube furnace (type: SK2-2-12), X-ray fluorescence spectrometer (type: PANalytical Axios), Na-K flame photometer (type: FP640), inductively coupled plasma atomic emission spectrometer (type: PE-2100), ion chromatograph (type: IC-3000) and X-ray diffractometer (type: D/Max-ШA).
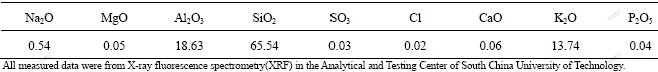
The white mud sample (Table 1) was derived from the South China Soda Ash Manufacturing Co., Ltd, Guangzhou, China. And the potassium feldspar ore (Table 2) was shipped from Shandong Province, China. All of the raw materials, white mud, potassium feldspar, some limestone and additives, were first dried at 105 ℃ in a drying oven until a constant mass was obtained. The dried materials were then finely ground with a mortar and sifted with a sieve of 0.074 μm. The raw materials were then weighed and added into the mixer in reactant proportions described by this reaction equation. If the total molar quantities of CaCO3 and Ca(OH)2 in the white mud were suspected to be insufficient, a bit of limestone was also added. Adequate water at about 40% of total mass of raw materials was also added into the mixer to allow adequate stirring. The mixture was made into spherolite with diameters of 0.5, 2.0, 4.0 and 6.0 cm, respectively, at the semi-plastic stage. The spherolite samples, after being dried, were put into a muffle furnace to calcine for 0.5, 1, 1.5, or 2 h at three different temperatures of 900, 950 or 1 000 ℃. The content of soluble K2O in the calcined product was measured by the Na-K flame photometry. The X-ray diffraction(XRD) patterns about calcined product under different conditions were plotted. The nutritional cations K+, Ca2+, Mg2+, Si4+ and heavy metals in the calcined product were detected systematically by inductively coupled plasma atomic emission spectrometry (ICP-AES ). The anions SO42- and Cl- were determined by ion chromatography (IC). The measurement results were compared with data obtained from the control standards of pollutants in coal fly ash used for agriculture (GB8173—1987) and environmental quality standards in soil (GB15618—1995).
Table 1 Chemical compositions of white mud (mass fraction, %)

Table 2 Chemical compositions of potassium feldspar ore (mass fraction, %)
Under the conditions of a calcination temperature of 1 000 ℃ and a ventilation of 6 L/min, white mud was calcined in a tube furnace for 1.5-2 h. The harmful gaseous pollutants SO2 and HCl generated during calcination were detected by iodimetry and IC, respectively. The gaseous HCl was first absorbed by a caustic solution, and then the anion Cl- in the absorption solution was detected by IC. All measured results were compared with the National Emission Standards for gaseous pollutants.
3 Results and discussion
3.1 Decomposition rate of potassium feldspar
The decomposition rate(η) of potassium feldspar during calcination process is listed in Table 3. Its computing equation is described as follows:
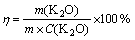 (4)
(4)
where m(K2O) represents mass (g) of soluble K2O in the calcinated product; m and C(K2O) represent mass (g) of K2O and percentage compositions (%) of potassium feldspar in materials, respectively.
Table 3 Decomposition rate (%) of potassium feldspar after calcining mixture of white mud and potassium feldspar

As the calcination temperature increased, the decomposition rates also became increasingly higher and higher. The maximum decomposition rate, about 90%, occurred at 1 000 ℃. The relationship between decomposition rate of potassium feldspar and calcination time also demonstrated a similar temperature trend. Moreover, at a calcination temperature of 1 000 ℃, a higher decomposition rate could be attained for a calcination time of 1.5 h. Higher decomposition rates also occurred with medium spherulitic size. The optimal conditions for decomposition of over 90% of the potassium feldspar were achieved at a calcination temperature of about 1 000 ℃, a calcination time of 1.5 h, and using a spherulitic size of about 2.0 cm as shown in Table 3. The calcined product was off-white with loose porosity, and showed no sintering phenomena. Its XRD pattern is illustrated in Fig.1. The mineral composition of calcined product mainly consisted of dicalcium sillicate, tricalcium aluminate, and at the same time, also contained some other minerals such as K2SO4, KCl, Ca(OH)2 and unreacted potassium feldspar.
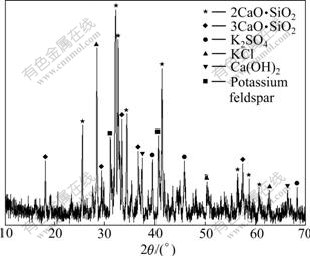
Fig.1 XRD pattern of calcined product under optimal conditions
3.2 Content of soluble nutrient elements and concen- trations of heavy metals in calcined product
Using a blending mass ratio of 70?30 of white mud to potassium feldspar at spherulitic size of 2.0 cm, calcined product was prepared at 1 000 ℃ for 1.5 h. The contents of soluble nutrient elements and concentrations of heavy metals were then detected with ICP-AES and IC, respectively, after being treated with 0.1 mol/L citric acid solution. The measured contents were: K2O 4.16%, CaO 23.43%, MgO 5.04%, SiO2 22.92%, SO42- 3.71%, and Cl- 3.87% (Table 4). The concentrations of heavy metals were presented in Table 5. These data were compared with the control standards for pollutants in coal fly ash used in agriculture (GB8173—1987) and the environmental quality standards in soil (GB15618—1995).
Table 4 Content of soluble nutrients present in calcined product dissolved in 0.1 mol/L citric acid solution (mass fraction, %)
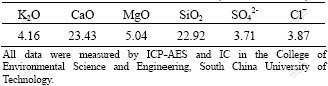
Table 5 Concentrations (mg/kg) of heavy metals present in calcined product dissolved in 0.1 mol/L citric acid solution
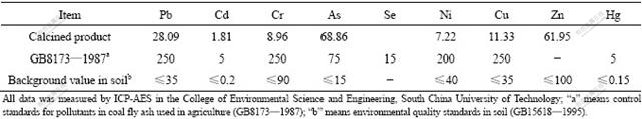
The concentrations of heavy metals in the calcined product are usually lower than the control standard of pollutants in coal fly ash used in agriculture. Most of these concentrations are also lower than environmental quality standard in soil.
3.3 Assessment on harmful gases
The harmful gases emitted from a mixture of white mud and potassium feldspar during calcination process are usually classified into two types. The first type is SO2 gas, which will be given off partly from decomposition of gypsum existing in white mud:
CaSO4=CaO+SO2↑+O2↑, 1 350 ℃≤t≤1 400 ℃ (5)
However, the decomposition temperature of gypsum will further decrease if combustion is kept at a reducing condition due to existence of coal powder during calcination process:
2CaSO4+C=2CaO+2SO2↑+CO2↑ (6)
Another harmful gas is HCl(g), which can escape from CaCl2 in white mud in the temperature range of 727-1 000 ℃[6]:
CaCl2+2H2O=Ca(OH)2+2HCl↑ (7)
CaCl2+H2O=CaO+2HCl↑, t=727 ℃ (8)
CaCl2+H2O+SiO2=CaSiO3+2HCl↑, t=1 000 ℃ (9)
Although pure lime hydrate (Ca(OH)2) is unstable above 577 ℃ (it decomposes to form CaO and H2O), it may still be present at a higher temperature because it is soluble in calcium chloride. The calcined product can contain CaO and Ca(OH)2 depending on the ambient humidity and calcination temperature[19]. The melting point and boiling point of NaCl are 801 ℃ and 1 413 ℃, respectively. But in the presence of CaCl2, they will decrease significantly, depending on molar ratio of NaCl to CaCl2. The previous study showed that NaCl in humid air will be completely dried and dehydrated at 420 ℃[20]. It will not react with water in air until 850 ℃ in a closed vessel. However, it is easily liquefied at 700-800 ℃ and will vaporize at around 1 250 ℃[6].
The measurement method for SO2 gas was iodometry, while HCl gas was measured by IC after HCl vapor was absorbed completely by a caustic solution. The quantity of harmful gases emitted was measured with a gas flow meter at a control rate of 6 L/min. The measurement time was set in 1.5-2 h. The concentrations of SO2 and HCl gases are listed in Table 6.
Table 6 Concentrations (mg/m?) for harmful flue gases emitted from white mud calcined at 1 000 ℃ for 1.5-2 h

The concentration of SO2 gas emitted from white mud under the optimal calcining conditions was 0.97-1.29 mg/m3. It was substantially lower than the SO2 emission standard value of 850 mg/m3 that is the limit for a National Industrial Boiler (GB9078—1996).
HCl, as a gaseous pollutant, is harmful, corrosive and contributes to acid rain[19]. The emission concentrations for HCl gas escaping from white mud under the optimal calcining conditions were in the range of 71-75 mg/m3, which were also lower than the second-class emission standard value of 100 mg/m? that applies to new pollution sources (GB16297—1996). However, although this meets the second-class emission standard for HCl, precautions should be taken that HCl gas emission should be limited to meet first-class emission standard. Further methods should be taken to control HCl emissions during calcination process by considering the use of absorbents with stronger absorption capacities for HCl vapor, such as caustic solution or lime hydrate[21-22]. Dust pollution caused by blending and calcination of raw powder material would be also eliminated by this control process at the same time.
4 Conclusions
A novel kind of soil nutrient amendment containing the nutrient elements K, Ca, Mg, Si and S can be prepared by calcining a mixture of white mud with potassium feldspar. The main conclusions are drawn as following:
1) The highest decomposition rate 96.16% for potassium feldspar can be attained when the blending mass ratio of white mud to potassium feldspar is maintained at 70:30, the calcination temperature is kept at 1 000 ℃, a calcination time is maintained at 1.5 h, and a spheruliric diameter of 2.0 cm is used.
2) A novel soil nutrient amendment is prepared with the following nutrient composition: K2O 4.16%, CaO 23.43%, MgO 5.04%, SiO2 22.92%, SO42- 3.71%, and Cl- 3.87% in 0.1 mol/L citric acid solution.
3) The concentrations of heavy metals in the calcined product are much lower than the limits issued by the National Standards. Most of them are also lower than the background values commonly encountered in soils. Thus, heavy metals released by this process would not be expected to bring about any serious pollution problem for farmlands or crops.
4) The harmful gases are to be released from a mixture of white mud and potassium feldspar during calcination process. The most harmful gaseous pollutant is HCl, but the release levels still meet the National Emission Standards regulated by China. Better methods for HCl removal, including absorption by caustic solution or lime hydrate, could possibly be adopted to eliminate these environmental pollutants.
References
[1] ZHU Mao-xu, LEE Li, QANG Hai-hua, WANG Zheng. Removal of an anionic dye adsorption/precipitation process using alkaline white mud [J]. Journal of Hazardous Materials, 2007, 149(3): 735-741.
[2] GEORG S. Cleaner production in the Solvay process: General strategies and recent developments [J]. Journal of Cleaner Production, 2008, 16(5): 833-841.
[3] YANG Liang-zuo, LI Yong-dan, ZHANG Li-hong. The treatment and utilization of soda slag [J]. Environmental protection Science, 2008, 34(2): 70-73. (in Chinese)
[4] Sumitomo studying soda ash sales in Asia [J]. Focus on Surfactants, 2008, 2008(2): 4.
[5] GAO Can-zhu, DONG Yuan, ZHANG Hong-juan, ZHANG Jia-ming. Utilization of distiller waste and residual mother liquor to prepare precipitated calcium carbonate [J]. Journal of Cleaner Production, 2007, 15(15): 1419-1425.
[6] KUANG Shao-ping, ZHANG Chao-jie, JIANG Zhi-gang, SHI Zhen-ju. Review on comprehensive utilization techniques of alkaline slag in soda ash factory [J]. China Resources Comprehensive Utilization, 2006, 24(3): 20-24. (in Chinese)
[7] FAN Ming-hui, SUN Chuan-min, HE Zheng-wei, WANG Xiao-di. The mineralogical characters of red earth in South China [J]. Journal of Chengdu University of Technology, 1999, 26(3): 313-316. (in Chinese)
[8] DONG Han-ying, QIU Rong-liang, L? Yue-na. Releasing of Si2+ and Al3+ under simulated acid rain in South China [J]. Chinese Journal of Environmental Science, 2000, 21(1): 75-77. (in Chinese)
[9] LARSSEN T, SCHNOOR J L, SEIP H M, ZHAO Da-wei. Evaluation of different approaches for modeling effects of acid rain on soils in China [J]. The Science of the Total Environment, 2000, 246(2/3): 175-193.
[10] HUANG Zhi-hong. The manufacturing technique of soil nutrient amendment with Ca, Mg and other elements using alkaline slag [J]. Soda Industry, 2000(1): 20-22. (in Chinese)
[11] ATAL A, STECIAK J, LEVENDIS Y A. Combustion and SO2-NOx emissions of bituminous coal particles treated with calcium magnesium acetate [J]. Fuel, 1995, 74(4): 495-506.
[12] SAXENA E, DATAR D, ZAHEER S. Extraction of potash from feldspars [J]. Trans Indian Cerama Soc, 1956(19): 12-12.
[13] BAKR M Y, ZATOUT A A, MOUHAMED M A. Orthoclase, gypsum and limestone for production of aluminum salt and potassium salt [J]. Interceram, 1979, 28(1): 34-35.
[14] FANG Wu-wei, MA Hong-wen. Thermodynamic analysis and experiments of thermal decomposition for potassium feldspar at intermediate temperatures [J]. Journal of the Chinese Ceramic Society, 2004, 32(7): 789-799.
[15] SHI Lin. Research on K-feldspar-CaSO4-CaO thermal decomposition system [J]. Journal of Mineralogy and Petrology, 2007, 27(4): 1-7. (in Chinese)
[16] HAN Xiao-zhao, YAO Wei-tang, HU Bo, DENG Zheng-tao. Extraction of potassium from potash feldspar by ion-exchange [J]. Chinese Journal of Applied Chemistry, 2003, 20(4): 373-375. (in Chinese)
[17] PENG Qing-jing, PENG Liang-bin, ZOU Xiao-yong, HUANG Cheng. Study on the extracting potassium from potash feldspar ores with calcium chloride [J]. Journal of Chemical Engineering of Chinese University, 2003, 17(2): 185-189. (in Chinese)
[18] PENG Qing-jing, ZOU Xiao-yong, HUANG Cheng. Extraction of potassium from potash feldspar ores with sodium chloride [J]. The Chinese Journal of Process Engineering, 2002, 2(2): 146-150. (in Chinese)
[19] KONDO H, ASAKI Z, KONDO Y. Hydrolysis of fused calcium chloride at high temperature [J]. Metallurgical and Materials Transactions B, 1978, 9(4): 477-483.
[20] SMITH D M, NEU M P, GARCIA E, MORALES L A. Hydration of plutonium oxide and process salts, NaCl, KCl, CaCl2, MgCl2: Effect of calcination on residual water and dehydration [J]. Waste Management, 2000, 20(7): 479-490.
[21] SHEMWELL B, LEVENDIS Y A, SIMONS G A. Laboratory study on the high-temperature capture of HCl gas by dry-injection of calcium-based sorbents [J]. Chemosphere, 2001, 42(5/7): 785-769.
[22] CHAI Yi, ZHANG Qiang, HOU Xue-jun. Absorption and desorption of hydrogen chloride [J]. Chlor-alkali Industry, 2004(5): 22-24. (in Chinese)
Foundation item: Project(20877026) supported by the National Natural Science Foundation of China
Corresponding author: SHI Lin; Tel: +86-20-33757292; E-mail: celshi@scut.edu.cn
DOI: 10.1016/S1003-6326(08)60454-9
(Edited by YANG Hua)