
Pyrite depression in marmatite flotation by sodium glycerine-xanthate
HE Ming-fei, QIN Wen-qing, LI Wei-zhong, ZENG Ke
School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China
Received 6 January 2010; accepted 15 March 2010
Abstract: The depression of pyrite in marmatite flotation by sodium glycerine-xanthate (SGX) was investigated through microflotation, zeta potential and adsorption measurements. The flotation tests of mineral show that in the presence of SGX, marmatite can be activated by Cu2+ and shows good flotability, while pyrite cannot be activated and therefore shows poor flotability. At the pH value range from 4 to 11, the flotation selectivity between marmatite and pyrite is obvious when the SGX concentration is below 50 mg/L. The depression mechanism of SGX on sulfide minerals is discussed based on zeta potential and adsorption isotherm. Zeta potential measurement demonstrates that in the presence of Cu2+, SGX can strongly adsorb on the surface of pyrite, while it cannot adsorb on the surface of marmatite. The results of adsorption isotherms show that the adsorption density of SGX on pyrite is greater.
Key words: flotation; sodium glycerine-xanthate; marmatite; pyrite
1 Introduction
For a long time the mostly used depressants in the flotation separation of complex sulfide ores are inorganic reagents, which include lime, cyanide, zinc sulfate, bichromate, sodium sulfide, potassium permanganate and sulfite. But there are many practical problems in the application of inorganic depressants, such as the toxicity of cyanide, the high dosage and incontrollablity of lime and its effects on the recovery of rare metals in the ores. Compared with inorganic depressants, organic depressants have the advantages of better selectivity and multiple structure, and moreover they are environmentally friendly. In recent years, organic depressants have received increasing attention from many scholars. XIONG et al[1] reported that sodium glycerine-xanthate (SGX) exhibits strong depressing reaction on the flotation of arsenopyrite and weak depressing reaction on marmatite with butyl-xanthate as the collector, and the infrared spectrum analysis showed that there are some —OH and —CSS— in sodium glycerine-xanthate molecule, which competes with butyl-xanthate on the mineral surface. NAGARAJ[2] revealed that polyacrylamide (PAM) with different functional groups could be used in the inhibition of sulfide ores. BOULTON et al[3] used low relative molecular mass polyacrylamide(PAM) polymers to separate copper-activated sphalerite from pyrite in the presence of isobutyl xanthate. It was found that all the PAMs depressed pyrite flotation with little or no sphalerite. SUN et al[4] used mercapto organic compound DMPS as depressant in the separation of copper-activated marmatite from pyrrhotite in the presence of butyl xanthate. The flotation tests of single mineral showed that DMPS had strong depressing effect on pyrrhotite in the absence and presence of copper ion. Infrared absorption spectra demonstrated that there are a number of functional groups such as —SH, —SO3 in the molecular structure of DMPS. CHANTURIYA and MATVEEVA[5] found that low molecular organic depressor dimethyldithiocarbamate could boost the separation efficiency of sulfide ores, thus improving the grade of nickel in the separation of copper-nickel concentrates. VALDIVIESO et al[6] found that dextrin is a good depressant for pyrite in flotation with xanthate collectors. However, the depressing effect of dextrin does not result from the reduced xanthate adsorption, while results from enveloping the dixanthogen which is a hydrophobic entity on the surface of pyrite reacted with xanthate, and dextrin depresses pyrite as efficiently as cyanide. The interaction of dextrin with pyrite was investigated by RATH et al[7] through adsorption, flotation and electrokinetic measurements.
Marmatite (Zn,Fe)S, an iron-rich sphalerite containing more than 6% iron is difficult to be separated from pyrite or pyrrhotite in the flotation of lead-zinc ores. The difference between marmatite and sphalerite is the iron, which is present in the form of isomorphism. The marmatite is sensitive to lime and other depressants, and the addition of lime to pH 9.5 in the absence of any other zinc depressants is effective in reducing marmatite flotation[8]. Marmatite can be recovered through collectorless flotation in acidic solution. ZHANG and HU[9] investigated the collector-and-collectorless flotation of marmatite by flotation tests and FTIR, and they reported that in ethyl xanthate solution, marmatite can be floated only in acidic solution, while when marmatite is activated by Cu2+, the floatability would be improved extensively in a pH range from 2 to 12. In this study, marmatite flotation performance obtained is similar to the results reported in Refs.[4, 6, 10].
In light of the difficulty in the flotation separation of marmatite and pyrite, in this work a new organic depressor sodium glycerine-xanthate (SGX) is used and its effects on the two minerals are examined at different pulp pH using butyl xanthate as collector and Cu2+ as activator. By measurements of zeta potential and adsorption isotherms, the depressing mechanism is discussed.
2 Experimental
2.1 Materials
The single mineral samples used in this study were from Mengzi Mining & Metallurgy Corporation, Yunnan, China. Chemical composition analysis of marmatite gave the following chemical compositions: 54.2% Zn, 10.8% Fe, 33.6% S, 1.4% others, and the pyrite chemical composition: 45.5% Fe, 52.05% S, 2.45% others.
2.2 Flotation tests
The flotation tests were carried out in a microflotation cell with a 40 mL effective volume. The amount of sample used for each experiment was 2 g, which was ultrasonically washed for 5 min to remove any possible oxides on the mineral surface. The washing solution was decanted and a fresh solution with a given pH was added before flotation. The flotation time was 4 min. The flotation recovery (R) was calculated from R=[m1/(m1+m2)]×100%, where m1 and m2 are the mass of the floated and unfloated products, respectively.
2.3 Zeta potential measurement
Zeta Reader (DELSA440SX, Made in USA) was used to measure the zeta potential by the measurements of electronic pulse. The pure mineral was ground to <5 μm in the agate mortar. 0.1 g mineral powder was added to a beaker with 100 mL distilled water and the mineral surface was cleaned using ultrasonic generator for 3 min to remove the oxidation film. Then the required reagents were added to the solution and stirred for 2 min with a magnetic stirring apparatus. After that, the solution was ready for the measurement. pH regulators were HCl and NaOH solutions.
2.4 Adsorption isotherms measurement
Adsorption isotherms were measured in a glass conditioning vessel at 25 °C. 10 g pyrite sample was conditioned for 5 min in KCl solution at different pH values. A known concentration of SGX stock solution was introduced to the vessel and continuously stirred until adsorption equilibrium reached 60 min, verified by equilibration study[11]. A sample was obtained from the solution to determine the concentration of SGX remaining in the solution. Another aliquot of concentrated SGX solution was added to the pyrite suspension and conditioned for a further 60 min. The extracted samples were centrifuged twice. Colorimetric techniques are then used to determine the concentration of SGX remaining in the solution. It was assumed that the amount of SGX depleted from the solution had been adsorbed onto the pyrite surface. The amount of SGX in the solution was measured using the Dubois method[12].
3 Results and discussion
3.1 Flotation of marmatite and pyrite
The flotation recovery of marmatite and pyrite as a function of pH value using butyl xanthate as collector is shown in Fig.1. The results demonstrate that the recovery of marmatite drops clearly as pH value increases, and the recovery of pyrite drops markedly in alkaline conditions. When the pH value is below 9, the recovery is about 55%. The result indicates that marmatite and pyrite cannot be separated with butyl xanthate as collector, because the coefficient of Fe atom in the LUMO(0.59) is much greater than that of Zn atom(0.11) for Fe-bearing sphalerite. Hence, the Fe-bearing sphalerite demonstrates reactive characteristics to iron[13-15].

Fig.1 Effects of pH on flotation of marmatite and pyrite in presence of sodium butyl xanthate of 1.0×10-4 mol/L
In order to improve the recovery in the xanthate flotation of sulfide minerals, copper, lead, silver, and other metal ions are used as activators. ZHANG[16] and YU[17] conducted a detailed experimental investigation on the activated flotation of marmatite with xanthate and copper sulfate at pH=5.0. It was found that copper sulfate significantly improves marmatite recovery. In these flotation experiments, the cupric ions directly exchange with Zn2+ on the marmatite surface, which accounts for the improved flotation of marmatite:
Cu2++(Zn, Fe)S=(Cu, Fe)S+Zn2+ (1)
The experiments for the activation of marmatite and pyrite by cupric ions (sulfate) at an initial concentration of 1.0×10-4 mol/L sodium butyl xanthate were carried out and the results are shown in Fig.2. It can be seen that the floatability of marmatite is significantly improved with the addition of copper sulfate. And the flotation recovery is above 90% at pH<11. When the pH value is above 11, the recovery decreases clearly. Pyrite can also be activated by Cu2+ addition, and the recovery increases greatly. It should be noted that the recovery of marmatite is greater than that of pyrite in alkaline solutions, but the flotation selectivity between marmatite and pyrite is lower.
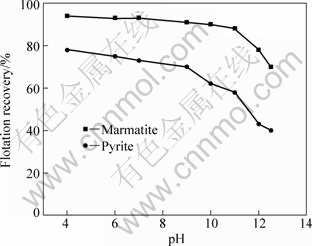
Fig.2 Effects of pH on flotation of marmatite and pyrite in presence of Cu2+ of 1.0×10-4 mol/L and sodium butyl xanthate of 1.0×10-4 mol/L
The flotation results of marmatite and pyrite at different pH values with 1×10-4 mol/L butyl xanthate and 1×10-4 mol/L Cu2+ in the presence of SGX are shown in Figs.3 and 4. As can be seen from Figs.3 and 4, the flotation behavior of marmatite in the presence of SGX has a remarkable difference compared with that of pyrite in pH value range of 4.0-12.5. Marmatite has a good floatability and SGX exhibits little effect on the flotation of marmatite when the SGX concentration is below 50 mg/L. However, the SGX at higher concentration is able to depress marmatite and the flotation recovery of marmatite decreases with the SGX concentration increasing to 100 mg/L, while the floatability of pyrite decreases significantly with the increasing SGX concentration. SGX has a stronger depression effect on pyrite when the SGX concentration is above 100 mg/L, and the flotation is completely inhibited at pH>10. At the pH value range from 4 to 11, the flotation selectivity between marmatite and pyrite is obvious when the SGX concentration is below 50 mg/L. There are —OH and —CSS— in SGX molecule, which competes with butyl xanthate on the mineral surface. Hydrophilic groups are adsorbed on the surfaces of pyrite, thus inhibiting the flotation of pyrite. The results provide a possible way to separate marmatite from pyrite by using SGX as a depressant and Cu2+ as an activator and butyl xanthate as the collector.
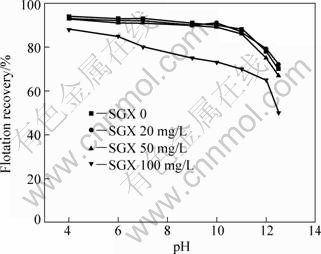
Fig.3 Effects of pH on flotation of marmatite in presence of Cu2+ of 1.0×10-4 mol/L and butyl xanthate of 1.0×10-4 mol/L
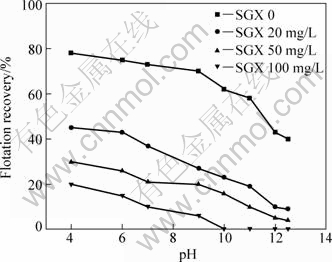
Fig.4 Effects of pH on flotation of pyrite in presence of Cu2+ of 1.0×10-4 mol/L and butyl xanthate of 1.0×10-4 mol/L
3.2 Electrochemical measurements
There are three sets of —OH and one —CSS— in the molecular of anionic depressant SGX. After the depressant is absorbed tightly on the surface of sulfide ores, it can form a hydrophilic film and hinder the absorption of the collector on the surface. At the pH value range from 4 to 11, the flotation selectivity between marmatite and pyrite is greater when the SGX concentration is below 50 mg/L. The evidence of selectivity, i.e. the adsorption of SGX on sulphide minerals is provided in the zeta potential results shown in Figs.5 and 6. Fig.5 shows the zeta potential of marmatite as a function of pH value, in the absence or presence of the SGX. All measurements are performed in 0.01mol/L KCl solution. The zeta potential is related to pH value, which decreases markedly with the increase of pH value in the absence of SGX. The SGX cannot be adsorbed on the surface of marmatite in the presence of Cu2+ at lower SGX concentrations, which explains why zeta potential shows no change at lower SGX concentrations and decreases at higher SGX concentrations (100 mg/L). It is shown in Fig.6 that the zeta potential of pyrite becomes negative and the potential becomes even negative with increasing pH. The decreasing pyrite potential after SGX addition shown in Fig.6 is due to the depressant absorbed tightly on the surface of pyrite.
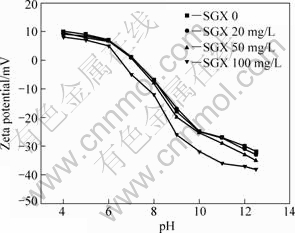
Fig.5 Zeta potential of marmatite as function of pH in presence of 1.0×10-4 mol/L Cu2+ and 1.0×10-4 mol/L butyl xanthate
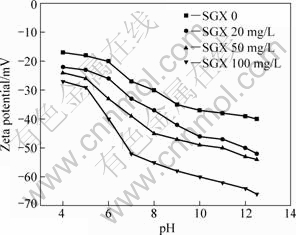
Fig.6 Zeta potential of pyrite as function of pH in presence of 1.0×10-4 mol/L Cu2+ and 1.0×10-4 mol/L butyl xanthate
3.3 Adsorption isotherms
Adsorption of SGX on pyrite as a function of pH value is given in Fig.7 at two distinct SGX concentrations, namely 20 mg/L and 100 mg/L. As noted, the adsorption is pH-dependent. At lower SGX concentration, the adsorption density of SGX is below 4 mg/m2. While at higher SGX concentration, the adsorption density increases quickly and the adsorption density is greater than that at lower SGX concentration. Pyrite depression is therefore largely influenced by solution pH and SGX concentration.
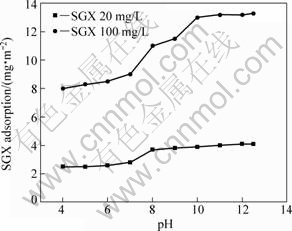
Fig.7 Adsorption density of SGX on pyrite as function of pH at 20 and 100 mg/L initial SGX concentrations
4 Conclusions
1) Sodium glycerine-xanthate(SGX) exhibits strong depressing action on the flotation of pyrite and weak depressing action on marmatite with butyl xanthate as a collector. In the presence of SGX, the flotation of marmatite can be activated by Cu2+ and the flotation of pyrite be inhibited with butyl xanthate as the collector. The results suggest that it is possible to selectively separate marmatite from pyrite by depressing pyrite with SGX. This separation can be achieved over a wide pH range.
2) Zeta potential experiments demonstrate that the SGX can be adsorbed on the surface of pyrite and thus depress pyrite flotation in the presence of Cu2+ and butyl xanthate.
3) At high pH values and SGX concentration, the adsorption density of SGX on pyrite is high. Therefore, pyrite depression is greatly influenced by solution pH and SGX concentration.
References
[1] XIONG Dao-ling, HU Yue-hua, QIN Wen-qin, HE Ming-fei. Synthesis of glycerine-xanthate and its depressing mechanism in separation of marmatite from arsenopyrite [J]. Journal of Central South University of Technology, 2006, 13(6): 678-682.
[2] NAGARAJ D R. Development of new flotation Chemicals [J]. Trans Indian Inst Met, 1997, 50: 355-363.
[3] BOULTON A, FORNASIERO D, RALSTON J. Selective depression of pyrite with polyacrylamide polymers [J]. International Journal of Minerals Processing, 2001, 61: 13-22.
[4] SUN Wei, LIU Run-qing, CAO Xue-feng, HU Yue-hua. Flotation separation of marmatite from pyrrhotite using DMPS as depressant [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(3): 571-675.
[5] CHANTURIYA V A, MATVEEVA T N. Products of sorption of dimethyldithiocarbamate on sulfide minerals [J]. Izdatel’stvo SORAN, 2003(1): 85-91.
[6] VADIVIESO A L, CERVANTES T C, SONG S, CABRERA A R, LASKOWSKI J S. Dextin as a non-toxic depressant for pyrite in flotation with xanthates as collector [J]. Minerals Engineering, 2004, 17: 1001-1006.
[7] RATH R K, SUBRAMANIAN S, PRADEEP T. Surface chemical studies on pyrite in the presence of polysaccharide-based flotation depressants [J]. Journal of Colloid and Interface Science, 2000, 229: 82-91.
[8] QUAST K, HOBART G. Marmatite depression in galena flotation [J]. Minerals Engineering, 2006, 19: 860-869.
[9] ZHANG Qin, HU Yue-hua. Mechanism of Cu2+ ion activation flotation of marmatite in absence and presence of ethyl xanthate [J]. The Chinese Journal of Nonferrous Metal, 2004, 14(4): 676-680. (in Chinese)
[10] WU Bo-zeng, QIU Guan-zhou, QIN Wen-qing. Flotation behavior and electrochemistry of marmatite in butyl xanthate solution [J]. Mining and Metallurgical Engineering, 2004, 24(6): 34-36. (in Chinese)
[11] MORRIS G E. The adsorption characteristics of polymeric depressants at the talc-water interface [D]. Adelaide: University of South Australia, 1996: 40-51.
[12] DUBOIS M, GILLES K A, HAMILTON J K, REDERS R A, SMITH F. Colorimetric method for determination of sugars and related substances [J]. Anal Chem, 1956, 28: 350-355.
[13] SCOGGINS M W, MILLER J D. Determination of water-soluble polymers containing primary amide groups using the starch-triiodide method [J]. Society of Petroleum Engineers Journal, 1979, 19: 151-156.
[14] CHEN Ye, CHEN Jian-hua, GUO Jin. A DFT study on the effect of lattice impurities on the electronic structures and floatability of sphalerite [J]. Minerals Engineering, 2010, 23: 1120-1130.
[15] LI Yu-qiong, LONG Qiu-rong, CHEN Jian-hua. Molecular structures and activity of organic depressants for marmatite, jamesonite and pyrite flotation [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 1993-1999.
[16] ZHANG Qin. The study of electrochemistry flotation behavior and surface adsorption of lead-antimony-zinc-iron sulfides [D]. Changsha: Central South University, 2004: 25-55. (in Chinese)
[17] YU Run-lan. Study on the basic theory of flotation electrochemistry of Pb-Sb-Fe-Zn sulfide minerals [D]. Changsha: Central South University, 2004: 55-56. (in Chinese)
使用抑制剂甘油基黄原酸钠浮选分离铁闪锌矿与黄铁矿
何名飞,覃文庆,黎维中,曾 科
中南大学 资源加工与生物工程学院,长沙 410083
摘 要:研究抑制剂甘油基黄原酸钠(SGX)在铁闪锌矿与黄铁矿浮选分离过程中的作用机理。通过浮选实验考察该抑制剂对硫化矿物的浮选抑制行为。结果表明,用丁黄药作捕收剂,在SGX存在下铁闪锌矿能被Cu2+活化从而具有良好的可浮性,而黄铁矿不能被Cu2+活化;在pH 为 4-11的范围, SGX的用量小于50 mg/L 时,可以实现两种矿物的选择性分离。动电位分析表明,SGX在Cu2+存在的条件下不能阻止丁黄药的阴离子在铁闪锌矿表面的吸附,但能阻止丁黄药的阴离子在黄铁矿表面的吸附。吸附等温测试结果表明,SGX在黄铁矿表面的吸附量远比在铁闪锌表面量大。
关键词:浮选;甘油基黄原酸钠;铁闪锌矿;黄铁矿
(Edited by YUAN Sai-qian)
Foundation item: Project (50774094) supported by the National Natural Science Foundation of China
Corresponding author: HE Ming-fei; Tel: +86-15887733998; E-mail: hemingfei1@126.com
DOI: 10.1016/S1003-6326(11)60837-6