文章编号:1004-0609(2008)09-1732-04
高精度恒温环境电化学量热系统的建立及
Fe(CN)63?/Fe(CN)64?体系的热电化学
张正华1,方 正1,王少芬2,毛 英1,陈坤汉2
(1. 中南大学 化学化工学院,长沙 410083;
2. 长沙理工大学 化学与环境工程学院,长沙 410077)
摘 要:以SRC?100溶解反应量热仪恒温系统、温度传感器和数据采集软件,结合CHI660B电化学工作站建立了控温精度为±0.001 K的高精度恒温环境下电化学量热系统。采用恒电流极化方法对5组不同浓度的Fe(CN)63?/Fe(CN)64?体系进行热电化学测试,获得该半电池反应电极电压—电流—温差—时间4维数据,根据热电化学基本方程计算得该电对偶反应“热电化学表观焓变”和绝对标度下标准氢电极熵变分别为?80.16 kJ/mol和87.57 J/(K?mol)。
关键词:热电化学;熵变;绝对标度;溶解反应量热仪
中图分类号:O 642.3;O 646 文献标识码:A
Establishment of electrochemistry calorimetrical system with high precision temperature-constant condition and thermo-electrochemistry of Fe(CN)63?/Fe(CN)64? system
ZHANG Zheng-hua1, FANG Zheng1, WANG Shao-fen2, MAO Ying1, CHEN Kun-han2
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. College of Chemistry and Environmental Engineering, Changsha University of Science and Technology,
Changsha 410083, China)
Abstract: A precision system for thermo-electrochemical measurements with controlled temperature of ±0.001 K was set up based on the SRC?100 solution reaction isoperibol calorimeter and an electrochemical workstation. The data of electric potential and temperature difference against time for equal molar Fe(CN)63?/Fe(CN)64? couple with 5 sets of different concentrations at 298.15 K were obtained under the condition of various constant-current polarizations. According to the basic equation for thermo-electrochemistry (TEC), the thermo-electrochemical apparent enthalpy change for the studied couple and the entropy change for the standard electrode reaction on the absolute scale are determined as ?80.16 kJ/mol and 87.57 J/(K·mol), respectively, being close to those in literatures.
Key words: thermo-electrochemistry; entropy change; absolute scale; solution-reaction isoperibol calorimeter
热电化学方法是结合热化学和电化学测试的一种物理化学研究方法,它同时测量过程中电压?电流?热流?时间4维信息,并基于热力学、动力学及电化学原理分析实验数据。目前已有不少学者采用简单的可逆体系对热电化学理论和实验方法进行研究[1?5]。使用测温元件测量电极表面温度变化能得到反应瞬时热效应,并易于实现对单个电极反应的热电化学测试。但目前还没有一套能独立承担测温的热电测试仪器。以往自组装建立热电测试系统结构复杂,由于温度控制欠稳定以及数据采集等问题,使用不便[6?10]。
SRC?100溶解反应量热仪[11?12]恒温能力为 ±0.001 K,远高于普通超级恒温水浴槽±0.1 K的恒温环境,并配有易操作的数据采集软件SRCS-Data Acquisition,数据能以约4.6个/s的速度自动纪录温度变化。通过对SRC?100溶解反应量热仪的改造,使得该仪器可用于测定电极表面温度变化,通过与CHI660B电化学工作站结合,建立了性能稳定的热电化学测试系统, 并对Fe(CN)63?/Fe(CN)64?(以下简写为Fe(CN)63?/4?)体系进行热电化学测试。
1 热电化学理论
对半电池反应,有[13?14]

2 实验
采用电导率≤0.3 μs/cm的去离子水将等摩尔分析纯K3Fe(CN)6和K4Fe(CN)6 配制成5种浓度的溶液, 加入1 mol/L的KCl作支持电解质。
将溶解反应量热仪热敏电阻取出贴在工作铂电极表面,用环氧树脂固定制成工作电极,该热敏电阻与仪器中另外3个精密电阻组成电桥。反应热效应通过热敏电阻阻值变化,转化为电势信号并由软件SRCS-Data Acquisition采集。实验恒温环境由SRC?100配套的超级恒温水浴提供,控温精度为 0.001 K(在3 min内稳定控温可达
0.001 K(在3 min内稳定控温可达 0.000 4 K)。电解槽采用自制H型电解槽,它保持电解液与水浴本体较好的热传导,以保证电解液温度稳定,使得每次电极过程有相同的起始条件。辅助电极为铂电极,参比电极为饱和甘汞电极。
0.000 4 K)。电解槽采用自制H型电解槽,它保持电解液与水浴本体较好的热传导,以保证电解液温度稳定,使得每次电极过程有相同的起始条件。辅助电极为铂电极,参比电极为饱和甘汞电极。
启动量热仪使其恒温水浴温度充分稳定,实验开始前调节测温电桥电位,使仪器信号处于零点,对不同浓度Fe(CN)63?/ 4?体系进行低电流下的恒电流扫描,数据采集软件分别记录电化学过程的电势和热电势信号。
3 结果与讨论
3.1 电极表面温度变化与输出电势间的关系
调节水浴温度,标定电极表面温度变化与输出电势之间的关系。记录时间200 s,以此时间内输出平均电势作为该温度下的输出热电势。以25 ℃为零点,分别计算出不同温差下对应的电势差,结果如图1所示。
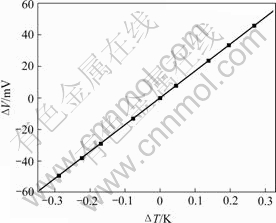
图1 温度变化与电桥输出电势的关系
Fig.1 Relationship between temperature change of platinum electrode and potential output of bridge
由图1可知,?T与V呈很好的线性关系,即
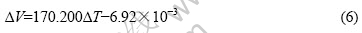
3.2 Fe(CN)63?/4?体系热电化学测试
图2所示为浓度0.2 mol/L Fe(CN)63?/4?体系热电势?θ—t曲线和电极电势V—t曲线,其他浓度时的曲线也示于图中。图3所示为不同浓度下Fe(CN)63?/4?体系
KX和Y与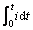 的关系。
的关系。
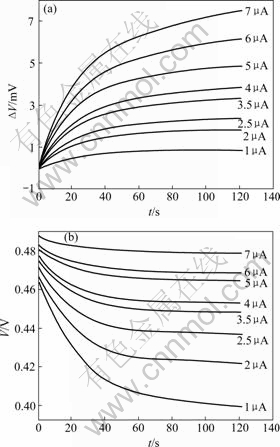
图2 0.2 mol/L Fe(CN)63?/4?体系在不同恒流下热信号与时间的关系及极化电势与时间的关系
Fig.2 Relationships between heat signal and time(a) and potential and time(b) at different currents for 0.2 mol/L Fe(CN)63?/4? system
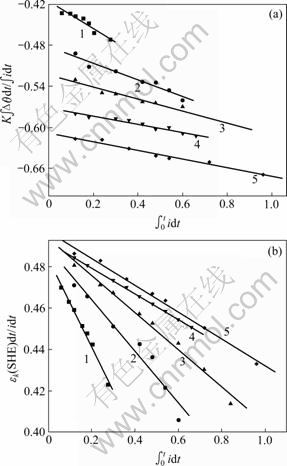
图3 不同浓度下Fe(CN)63?/4?体系的KX(a)及Y(b)与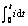 的关系
的关系
Fig.3 Plots of KX vs 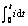 (a) and Y vs
(a) and Y vs 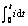 (b) for Fe(CN)63?/4? system with various concentrations: 1—0.075 mol/L; 2—0.15 mol/L; 3—0.20 mol/L; 4—0.25 mol/L; 5—0.3 mol/L
(b) for Fe(CN)63?/4? system with various concentrations: 1—0.075 mol/L; 2—0.15 mol/L; 3—0.20 mol/L; 4—0.25 mol/L; 5—0.3 mol/L
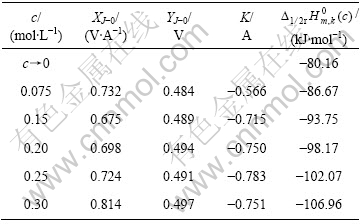
由上所述,可得K值(见表1)。将各浓度下K值
代入式(4),获得不同浓度下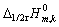 (c)。以
(c)。以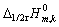 (c)对浓度c作图,结果如图4所示。由图 可知,不同浓度时的
(c)对浓度c作图,结果如图4所示。由图 可知,不同浓度时的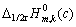 与浓度c呈线性关系,当外推到c→0,得到
与浓度c呈线性关系,当外推到c→0,得到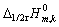 (c→0) = ?80.16 kJ/mol。
(c→0) = ?80.16 kJ/mol。
图4 Fe(CN)63?/4?体系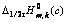 与浓度c的关系
与浓度c的关系
Fig.4 Relationship between 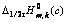 and concentration c for Fe(CN)63?/4? system
and concentration c for Fe(CN)63?/4? system
表1 Fe(CN)63?/4?体系的X,Y,K,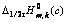 与浓度的关系
与浓度的关系
Table 1 Changes in X, Y, K and 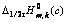 with concentration c
with concentration c
由文献[15]提供的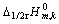 值,结合式(5)求出 绝对标度标氢电极熵变为87.57 J/(K·mol),与87.8 J/(K·mol)[16]吻合。
值,结合式(5)求出 绝对标度标氢电极熵变为87.57 J/(K·mol),与87.8 J/(K·mol)[16]吻合。
4 结论
1) 建立了以SRC?100为主体的热电化学测试系统,并对等摩尔浓度Fe(CN)63?/4?进行测定。
2) 测得Fe(CN)63?/4?体系在298.15 K下的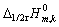 为?80.16 J/mol,绝对标度下标氢电极熵变为87.57 J/(K·mol)。
为?80.16 J/mol,绝对标度下标氢电极熵变为87.57 J/(K·mol)。
REFERENCES
[1] REITA T. The electrochemical Peltier effect observed with electrode reactions of Fe(Ⅱ)/Fe(Ⅲ) redox couples at a gold electrode[J]. J Electroanal Chem,1975, 65(1): 263?273.
[2] BOUDEVILLE P. Thermometric determination of electrochemical Peltier heat (thermal effect associated with electron transfer) of some redox couples[J]. Inorg Chim Acta, 1994, 226(1/2): 69?78.
[3] DECKER F, FRACASTORO-DECKER M, CELLA N, VARGAS H. Acoustic detection of the electrochemical Peltier effect[J]. Electrochim Acta,1990, 35(1): 25?26.
[4] DONEPUDI V S, CONWAY B E. Electrichemical calorimetry of individual electrode processes: application to the reactions in the zinc bromine battery[J]. Electroanal Chem, 1984, 131: 1447?1453.
[5] HIRONORI N, TOSHIYUKI N, YASUHIKO I. The single electrode Peltier heats of Li/Li+, H2/H– and Li+/Pd-Li couples in molten LiCl-KCl systems[J]. Electrochim Acta, 2004, 49: 4987?4991.
[6] REITA T. An experimental study of the electrochemical Peltier heat[J]. J Electroanal Chem,1973, 45(3): 500?503.
[7] GRAVES B B. Differential voltammetric scanning thermometry of tenth formal formaldehyde solution in formal perchloric acid thermo-electro-chemical study for Cu2+/Cu electrode in HClO4 solution[J]. Anal Chem, 1972, 44(6): 993?1002.
[8] BOUDEVILLE P, TALLEC A. Electrochemistry and calorimetry. Ⅳ: Determination of electrochemical Peltier heat[J]. Thermochim Acta, 1988, 126(15): 221?234.
[9] WANG Hui-xiang, WANG Dian-zuo, LI Bai-dan, SUN Shui-yu. Improved methods to determine the electrochemical Peltier heat using a thermistor (Ⅰ): Improved heat-sensor electrodes and lumped-heat-capacity analysis[J]. J Electronal Chem,1995, 392(1): 13?19.
[10] JIANG Zhi-yu, ZHANG Jie, DONG Liang-jun, ZHUANG Ji-hua. Determination of the entropy change of the electrode reaction by an AC electrochemical–thermal method[J]. J Electroanal Chem,1999, 469(1): 1?10.
[11] 汪存信, 宋昭华, 熊文高, 屈松生. 具体恒定温度环境的反应热量计的研制[J]. 物理化学学报, 1991, 7(5): 586?588.
WANG Chun-xin, SONG Zhao-hua, XIAO Wen-gao, QU Song-sheng. Development of isoperibol reaction calorimeter[J]. Acta Physico-Chim Sin, 1991, 7(5): 586?588.
[12] YU Hua-guang, LIU Yi, TAN Zhi-cheng, DONG Jia-xing. A solution reaction isoperibol calorimeter and standard molar enthalpies of formation of Ln(hq)2Ac (Ln=La, Pr)[J]. Thermochim Acta, 2003, 401: 217?224.
[13] FANG Z, ZHANG H Z, ZHANG P M. Basic equations for thermoelectrochemistry and the entropy change of the standard hydrogen electrode reaction[J]. Acta Metall Sin, 1996, 9: 189?191.
[14] FANG Z, ZHANG Q R, ZHANG H Z. Thermoelectrochemistry and its application to metallurgical research[J]. J Mater Sci Technol, 2001, 17(1): 20?24.
[15] DEAN J A. LANGE’S handbook of chemistry thirteenth edition[M]. New York: McGraw-Hill Book Company, 1985: 1481?1482.
[16] 黄子卿. 电解质溶液理论导论[M]. 北京: 科学出版社, 1983: 53?54.
HUANG Zi-qing. Introduction to theory of electrolyte solution[M]. Beijing: Science Press, 1983: 53?54.
基金项目:国家自然科学基金资助项目(50374077)
收稿日期:2007-11-20;修订日期:2008-05-26
通讯作者:方 正,教授,博士;电话:0731-8660356;E-mail: zfang@csu.edu.cn
(编辑 龙怀中)