Effects of nano-AlN on phase transformation of low temperaturevitrified bond during sintering process
来源期刊:中国有色金属学报(英文版)2009年增刊第3期
论文作者:尚勇 侯永改 乔桂英 邹文俊 肖福仁 廖波
文章页码:706 - 710
Key words:low temperature vitrified bond; nano-AlN; sintering; phase transformation
Abstract: The effects of nano-AlN and sintering temperature on bending strength and wear resistance of low temperature vitrified bond for diamond grinding tools were studied. Furthermore, the phase transformation during sintering process was investigated by means of thermo-gravimetric analysis (TG), differential thermal analysis (DTA) and X-ray diffraction (XRD). The results show that the higher bending strength and wear resistance of low temperature vitrified bond are obtained by adding nano-AlN in bonds and sintering at optimum temperature. Nano-AlN added in bonds promotes the crystallization during sintering process and refines the grain sizes of crystalline phase.
基金信息:the Natural Science Foundation of Hebei Province, China

SHANG Yong(尚 勇)1, HOU Yong-gai(侯永改)1,2, QIAO Gui-ying(乔桂英)3, ZOU Wen-jun(邹文俊)2,
XIAO Fu-ren(肖福仁)1, LIAO Bo(廖 波)2
1. Key Laboratory of Metastable Materials Science and Technology, College of Material Science and Engineering, Yanshan University, Qinhuangdao 066004, China;
2. College of Materials Science and Engineering, Henan University of Technology, Zhengzhou 450007, China;
3. School of Environment and Chemical Engineering, Yanshan University, Qinhuangdao 066004, China
Received 10 August 2009; accepted 15 September 2009
Abstract: The effects of nano-AlN and sintering temperature on bending strength and wear resistance of low temperature vitrified bond for diamond grinding tools were studied. Furthermore, the phase transformation during sintering process was investigated by means of thermo-gravimetric analysis (TG), differential thermal analysis (DTA) and X-ray diffraction (XRD). The results show that the higher bending strength and wear resistance of low temperature vitrified bond are obtained by adding nano-AlN in bonds and sintering at optimum temperature. Nano-AlN added in bonds promotes the crystallization during sintering process and refines the grain sizes of crystalline phase.
Key words: low temperature vitrified bond; nano-AlN; sintering; phase transformation
1 Introduction
Superhard grinding tool, such as cubic boron nitride(cBN) and/or diamond tools, are widely applied to grinding for superhard materials, metal materials, ceramics, glass etc, because they have the highest grinding efficiency. The cBN and/or diamond grinding tools are a group of composite materials that are composed of cBN and diamond grits and a bonding matrix. The familiar bonding materials include metal, resin and vitrified glass and electro-plated[1-2]. Vitrified bond cBN and/or diamond grinding tools have inimitable advantage over metal and resin ones, such as higher bond strength than resin bond ones, and more excellent self-dressing capability than metal ones, because of their high elastic modulus and low fracture toughness of the glass bonding materials[3-6].
The mechanical properties of vitrified bond cBN and diamond grinding tools have a strong correlation with the matrix composition and microstructure[4-5, 7]. The researcher’s previous works present that the Na2O-B2O3-SiO2 system glass has lower thermal expansion coefficient and sintering temperature, which is suited to manufacture the high quality vitrified bond superhard grinding tools[3-9]. Nevertheless, in some circumstances, bonds consisting of pre-fritted glass powder can give advantages, such as perfectly formed glass bonds and an absence of adverse reactions, when siliceous ingredients, like clays and mineral feldspars, are heated to high temperature. An example of the latter is the sudden expansion of quartz at its inversion temperature (573 ℃), which causes cracks to form within glass bond bridges and a subsequent loss in bonding strength [4]. Moreover, alkali oxide levels reduce the chemical durability (resistance of the glass bond to attack and degradation by aqueous coolants) [4, 10-11]. In addition, the properties of vitrified bond superhard grinding tools greatly depend on the interfacial characteristics between bond and superhard materials [12-15]. However, the someplace Na2O-B2O3-SiO2 system bond is difficult to obtain satisfying properties including bending strength, fracture mode, heat transfer, chemical durability and interfacial strength, etc. Therefore, some researchers explored the methods by adding alumina, alkali metal oxides, etc in bonds to improve the properties of grinding tools [11, 15].
In this work, the effects of nano-AlN on properties of low temperature vitrified bond of Na2O-B2O3-SiO2 borosilicate glass system were investigated.
2 Experimental
Basic vitrified bond of Na2O-B2O3-SiO2 glass was fired to pre-fritted glass, and crushed, seized to produce a fine, powdered fritted glass. A part of the basic vitrified bond then was mixed with 6%-8% nano-AlN powder (mass fraction). The purity of nano-AlN powder is 99%, and its particle size is about 40 nm. Thermo-gravimetric analysis (TG) and differential thermal analysis (DTA) of as-produced pre-fritted glass with or without nano-AlN vitrified bond and nano-AlN powder were performed using WCT-2 TGA/DTA thermo-gravimetric apparatus at heating rate of 5 K/min in argon atmosphere. The structure of samples after TGA/DTA analysis was also studied by X-ray diffraction (XRD) with PANalytical model X’Pert PRO X-ray diffractometer by CuKa radiation at an acceleration voltage of 40 kV and current of 40 mA.
Green samples were pre-pressed to form two kinds of dimensions 25 mm×6 mm×6 mm and d6 mm×6 mm, respectively. Then, green samples were sintered in an electric furnace at 700, 710 and 730 ℃ in argon atmosphere. The three-point bending tests of sintered samples were carried out on a CMT4504 universal testing machine. Sliding wear tests were performed in air on a pin-on-disc type tribometer under unlubricated conditions. The sample was put as pin in contact with a rotated alumina grinding wheel disc. A series of interrupted tests were conducted at a constant speed loads for 15 min. The samples mass before and after the test were measured, and the mass loss of the samples was employed to present its wear resistance.
3 Results and analysis
3.1 Effect of nano-AlN on bending strength and wear resistance of vitrified bond
Bending strength of vitrified bond samples with and without nano-AlN sintered at different temperatures are shown in Fig.1. It can be seen from Fig.1 that the nano-AlN has great effect on the bending strength of vitrified bond, moreover, it also has effect on the sintering process parameters. For example, from our previous work, the bending strength of vitrified bond without nano-AlN can get the highest value when the sintering temperature is 700 ℃. The result is the same to this work. As the sintering temperature increases, the bending strength of vitrified bond decreases remarkably. However, by adding nano-AlN in frit, the bending strength of vitrified bond increases notably, and the optimal sintering temperature also increases, furthermore, with increasing of the amount of added nano-AlN, the optimal sintering temperature tends to a higher value.

Fig.1 Effect of sintering temperature and nano-AlN on bending strength of vitrified bond
The effects of nano-AlN on wear resistance of vitrified bond have the same tendency, which is clearly shown in Fig.2. From Fig.2, at lower sintering temperature (700 ℃), the mass loss of vitrified bond without nano-AlN is lower. With the sintering temperature increasing, its mass loss rises rapidly. For the samples with nano-AlN, the mass loss get the lowest value. When the sintering temperatures of samples with 6% and 8% nano-AlN are 710 and 730 ℃, respectively. Furthermore, the mass loss of samples with nano-AlN sintered at optimum sintering parameters is remarkably lower than the samples without nano-AlN sintered at optimum sintering parameters. Those indicate that nano-AlN added in bonds can improve the wear resistance of vitrified bonds.
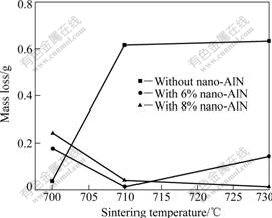
Fig.2 Effect of sintering temperature and nano-AlN on wear resistance of vitrified bond
From those results above, adding nano-AlN in pre-fritted glass not only affects the strength and wear resistance of sintered vitrified bond, but also affects the sintering process parameters. The optimal sintering temperature increases with increasing the amount of added nano-AlN. The results indicate that nano-AlN added in vitrified bond can affect the sintering character of pre-fritted glass, which should be deeply investigated.
3.2 TGA and DTA analyses
Fig.3 shows DTA curves of samples without and with 6% and 8% nano-AlN measured at argon atmosphere. It can be seen from Fig.3 that the DTA curve of sample without nano-AlN has only one thermal effect in temperature range from 150 ℃ to 650 ℃, which is an exothermic effect with peak temperature at about 500 ℃ corresponding to the decomposition of the impurity on nano-AlN particles. The result indicates that the nano-AlN is stable in argon atmosphere at temperature lower than 800 ℃.

Fig.3 DTA curves of fritted glass and samples without and with nano-AlN
For sample of vitrified bond without nano-AlN, when the temperature is below 685 ℃, a small endothermic effect can be observed at 500 ℃, the mass loss presents a rapidly descending tendency corresponding to this thermal effect (see Fig.4). The thermal effect is considered the decomposing of bonds. When the temperature rises over 685 ℃, the DTA curves present an exothermic effect. However, it can be noted that the mass loss corresponding to this thermal effect is notably varied (see Fig.4). The result indicates that the temperature is a beginning melting point of pre-fritted glass. Hence, the vitrified bond is sintered at slightly higher temperature, the higher bending strength of sintered vitrified bond is obtained.
With adding nano-AlN in pre-fritted glass, the DTA curves for all samples have similar patterns when temperature is below 680 ℃. When the temperature is over 650 ℃, an intensive exothermic effect at temperature of 720 ℃ appears in the DTA curves of all samples with nano-AlN. Moreover, when the amount of added nano-AlN increases, the peak value increases, and the temperature of maximal peak value increases, too. The temperature of peak value for samples increases from about 715 ℃ to 722 ℃ with the amount of nano-AlN increases from 6% to 8%. Furthermore, the beginning temperature of this exothermic effect is about 675 ℃. However, the TG curves show that the mass loss corresponding to this thermal effect for sample with 8% nano-AlN notably vary compared with those of pre-fritted glass (see Fig.4). The results imply that two possibilities occur during the heating process, which are chemical reaction between fritted glass and AlN and crystallization of glass phase in pre-fritted glass.
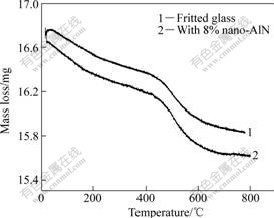
Fig.4 TG curves of fritted glass and samples with 8% nano-AlN
3.3. XRD analysis
The XRD patterns of pre-fritted glass and samples without and with 8% nano-AlN after heat treatment at 750, 730 and 680 or 730 ℃ in argon atmosphere, respectively. It can be seen that the structure of AlN heated at 730 ℃ retains a simple hexagonal structure, and all diffraction peaks are broad, which indicates that the AlN structure remains as nano-structure, and it is steady (Fig.5(a)). Moreover, weak amorphous phase peak exists in the range from 28? to 42?. This indicates that some amorphous phase exists in nano-AlN powder.
For the pre-fritted glass heated at 730 ℃, some crystalline phases and amorphous phase peaks are observed (see Fig.5(b)). The crystalline phases are identified with ICDD files, which are mainly α-SiO2 and β-SiO2 and a little Al2O3.
For the sample with 8% nano-AlN heated at 680 ℃, the XRD pattern has a little variety. It presents the mixed pattern of AlN with pre-fritted glass (see Fig.5(c)). This result indicates that the pre-fritted glass mixed nano-AlN has no any reaction when calcination temperature is below 680 ℃. The result is the same with the DTA result. However, when the heating temperature reaches 730 ℃, the XRD pattern presents a great variety (see Fig.5(d)). The intensity of α-SiO2 and β-SiO2 diffraction peaks increases notably. Meanwhile, the diffraction peaks of AlN disappear, and some diffraction peaks can

Fig.5 XRD patterns of samples after reheating: (a) sample without nano-AlN after reheating at 750 ℃; (b) Fritted glass after sintering at 730 ℃; (c) Bond with 8% nano-AlN after sintering at 680 ℃; (d) Bond with 8% nano-AlN after sintering at 730 ℃
be observed at 2q of 23? and 28?, which can be confirmed to be tridymite phase. Moreover, it can also be found that the intensity of amorphous phase has a little change. This indicates that AlN promotes crystallization of α-SiO2 and tridymite. These phase varieties induce the improvement of properties of vitrified bond.
4 Discussion
The mechanical properties of vitrified bonds for diamond grinding tools have a strong correlation with the bond microstructure, and the microstructure lies on the matrix composition and sintering parameters [3, 5]. In order to ensure higher properties of low temperature vitrified bond, the raw materials, such as clays, feldspars and borax, must be melted at high temperature to prepare pre-fritted glass, and the microstructure of pre-fritted glass should contain some amorphous and crystal phases[7]. The amorphous phase with lower melting temperature can decrease the sintering temperature and increase interphase combining strength of bond with abrasive. While the crystal phase can increase the strength of this bond to improve the properties of vitrified bond grinding tools. For vitrified bond of Na2O-B2O3-SiO2 fritted glass studied in this work, the lower melting temperature of the vitrified bond can be obtained by adding B2O3 and clays. The melting beginning temperature is about 685 ℃ (Fig.4). Moreover, certain amount of crystal phases, such as SiO2 and Al2O3, are also obtained (Fig.5). However, higher content of SiO2 has an adverse reaction, i.e. the latter is the sudden expansion of quartz at its inversion temperature (573 ℃), which causes cracks to form within glass bond bridges and a subsequent loss in bonding strength[4]. Our results of this work are the same as that of the theory (Figs.1, 2 and 5). Yet, this adverse effect of quartz inversion in vitrified bonds is correlative with the content and size of quartz phase[4]. The adverse effect can be reduced by refining the crystal phase, consequently, the strength of vitrified bonds can be improved.
From the results stated above, adding nano-AlN in frit can improve the properties of nano-AlN s (see Figs.1 and 2), because of the effect of AlN on phase transformation during sintering (see Figs.4 and 5). For nano-AlN, three possible main effects on phase variety may include during sintering process. Firstly, higher surface energy is beneficial to decrease the melting temperature (see Fig.4), the melting temperature decreases from 680 to 675 ℃. Secondly, the instable amorphous phases on nano-AlN particles react with fritted glass and bond to form Al2O3. This is confirmed by XRD analysis results. Compared the XRD patterns of vitrified bond without nano-AlN with those of the vitrified bond with 8% nano-AlN (Figs.5(a) and (d)), although 8% nano-AlN is added into the vitrified bond, the diffraction peaks of Al2O3 represent an increasing trend (Fig.5). The increase of amount of Al2O3 can be confirmed coming from the reaction of nano-AlN with frit and/or clays. Thirdly, nano-AlN becomes crystallization nucleus and promotes the crystallization of α-SiO2, β-SiO2 and tridymite, furthermore, refine the microstructure of crystal phase. This can be confirmed by broadening diffraction peaks of α-SiO2 and β-SiO2. Therefore, the microstructures and properties of vitrified bonds are meliorated observably by adding certain amount of nano-AlN in vitrified bonds.
5 Conclusions
1) By adding nano-AlN powder in low temperature vitrified bond for diamond grinding tools, the higher bending strength and wear resistance can be obtained at optimum sintering temperature. Compared with samples without and with nano-AlN, when the amount of added nano-AlN is 6%, the bending strength increases from 20 to 40 MPa and the mass loss decreases from 0.037 4 to 0.013 g.
2) Adding nano-AlN powder in vitrified bonds can decrease the melting temperature and promote the crystallization of a-SiO2, b-SiO2 and tridymite during sintering process and refine the microstructure of crystal phase, furthermore, improve the properties of sintered vitrified bond.
Reference
[1] KOPAC J, KRAJNIK P. High-performance grinding—A review [J]. Journal of Materials Processing Technology, 2006, 175: 278-284.
[2] KLOCKE F, BRINKSMEIER E, EVANA C, HOWES T, INASAKI I, MINKE E, TOENSHOFF H K, WEBSTER J A, STUFF D, High-speed grinding—fundamentals and state of the art in Europe, Japan and the USA [J]. Ann CIRP, 1997, 46(2): 715-724.
[3] JACKSON M J, MILLS B. Materials selection applied to vitrified alumina & CBN grinding wheels [J]. Journal of Materials Processing Technology, 2000, 108: 114-124.
[4] JACKSON M J. Sintering and vitrification heat treatment of cBN grinding wheels [J]. Journal of Materials Processing Technology, 2007, 191: 232-234.
[5] GRABCHENKO A. Role of wheel diamond concentration in grinding polycrystalline of super hard materials [J]. Soveit Journal of Super Hard Materials, 1984, 6(1): 58-62.
[6] WERNERG, RENTER M. Grinding ability of PCD [J]. Industrial Diamond Review, 1989, (1): 120-122.
[7] LI Zhi-hong, YUAN Qi-ming, YANG Zheng-fang. Study on manufacturing technology of vitrified bond cBN grinding tools [J]. Acta Material Composite Sinica, 2003, 20(5): 39-43. (in Chinese)
[8] LIN Kuan-hong, PENG Shih-feng, LIN Shun-tian. Sintering parameters and wear performances of vitrified bond diamond grinding wheels [J]. International Journal of Refractory Metals & Hard Materials, 2007, 25: 25-31.
[9] HOU Yong-gai, PENG Jing, ZOU Wen-jun LU Peng-xian, MA Qiu-hua. Study on vitrified bond diamond tools for grinding PCD cutter [J]. Diamond & Abrasives Engineering, 2007, (6): 51-53, 61. (in Chinese)
[10] WANG Peng-fei, Li Zhi-hong, ZHU Yumei. Effect of CaO on the surface morphology and strength of water soaked Na2O-B2O3-Al2O3-SiO2 vitrified bond [J]. Journal of Non-Crystalline Solids, 2008, 354: 3019-3024.
[11] DAVIS M J, IHINGER P D, LASAGA A C. Influence of water on nucleation kinetics in silicate melt [J]. Journal of Non-Crystalline Solids, 1997, 219: 62-69.
[12] FAN F, TANG W, LIU S, HEI L, LI C, CHEN G, LU F. An effort to enhance adhesion of diamond coatings to cemented carbide substrates by introducing Si onto the interface [J]. Surface and Coatings Technology, 2006, 200(24): 6727-6732.
[13] LIM Y M, SHIN S K, KIM M K. A study on the effect of externally bonded composite plate-concrete interfaces [J]. Composite Structures, 2008, 82(3): 403-412.
[14] MIYAZAKI M, ONOSE H LIDA N, KAZAMA H. Determination of residual double bonds in resin-dentin interface by Raman spectroscopy [J]. Dental Materials, 2003, 19(3): 245-251.
[15] WANG P F, LI Z H, LI J, ZHU Y M. Effect of ZnO on the interfacial bonding between Na2O-B2O3-SiO2 vitrified bond and diamond [J]. Solid State Sciences, 2009, 11(8): 1427-1432.
(Edited by LI Yan-hong)
Foundation item: Project(E2008000834) supported by the Natural Science Foundation of Hebei Province, China
Corresponding author: LIAO Bo; Tel: +86-335-8077110; Fax: +86-335-8077110; E-mail: frxiao@ysu.edu.cn

