
Oxidation and hot corrosion behavior of
Ti2AlNb-based alloy with and without enamel coating at 800 ℃
ZHENG De-you(郑德有), XIONG Yu-ming(熊玉明), ZHU Sheng-long(朱圣龙),
LI Ming-sheng(李明升), WANG Fu-hui(王福会)
State Key Laboratory for Corrosion and Protection, Institute of Metal Research,
Chinese Academy of Sciences, Shenyang 110016, China
Received 28 July 2006; accepted 15 September 2006
Abstract: The oxidation and hot corrosion behavior of Ti2AlNb-based alloy with and without enamel coating at 800 ℃ was investigated. The results indicated that Ti-22Al-25Nb alloy exhibited poor oxidation resistance at 800 ℃. The constitution of oxide scale had the effect on its oxidation rate. Because of the S and Cl accelerating the corrosion process, Ti-22Al-25Nb alloy suffered severe hot corrosion and exhibited very poor hot corrosion resistance. Enamel coating could remarkably improve the high temperature oxidation resistance of Ti-22Al-25Nb alloy because it had good chemical stability and matched thermal expansion coefficient with the substrate. In (Na,K)2SO4+NaCl molten salts at 800 ℃, chemical reactions between molten salts and enamel coating occurred and complicated products formed on the surface of the enamel coating; Cl- in the molten salts could penetrate through the coating and induced the substrate corrosion, but enamel coating still had good hot corrosion resistance.
Keywords: Ti2AlNb; enamel coating; oxidation; hot corrosion
1 Introduction
The ordered orthorhombic Ti2AlNb-based alloys have been studied recently as one of the potential materials for high temperature applications [1-7]. As high temperature structural materials, it is required that Ti2AlNb-based alloys have not only excellent mechanical properties but also good oxidation and hot corrosion resistance in exacting environments. The mechanical properties of Ti2AlNb-based alloys have been investigated extensively; however, relatively few studies have been carried out on the oxidation behavior of Ti2AlNb alloys [8-9], especially for the hot corrosion behavior of these alloys. Therefore, a better understanding of the oxidation and hot corrosion behavior of Ti2AlNb-based alloys is necessary for their practical applications.
Recent studies have indicated that the enamel coating is a good candidate as high temperature coating for Ti-based alloys [10], Ti3Al-based [11] and TiAl intermetallics [12-13] due to its high thermal stability and better compatibility with the substrate. But the effectiveness of enamel coating towards improving the oxidation and hot corrosion resistance of Ti2AlNb-based alloys has not been studied.
The aim of this study is to further clarify the oxidation and hot corrosion behavior of Ti2AlNb-based alloy and to develop effective method to protect this alloy from high temperature corrosion. In this paper, the oxidation and hot corrosion behavior of Ti-22Al-25Nb (mole fraction, %) at 800 ℃ was investigated. Further- more, an attempt was made to improve the oxidation and hot corrosion resistance of this alloy by applying an enamel coating. The influence of the enamel coating on the oxidation and hot corrosion resistance of Ti-22Al-25Nb alloy was also discussed.
2 Experimental
The Ti2AlNb samples were cut to form approximately 20 mm×10 mm×3 mm sized specimens. The specimens were polished with SiC-paper to 800 grit and ultrasonically cleaned in acetone. The nominal composition of enamel frit is shown in Table 1 [11]. The enamel frit was prepared using the mixture of mineral materials followed by melting at 1 450 ℃, water quenching and grinding. The specimens were firstly sand blasted. Enamel frit mixed with ethanol was air-sprayed on the coarse surface of these alloys, dried at 100 ℃ and then fired in air at 900 ℃ for 30 min to form enamel coating.
Table 1 Nominal composition of enamel frit(mass fraction, %)

Oxidation tests were performed at 800 ℃ in static air. The specimens were placed in alumina crucibles, and cooled to room temperature at regular time intervals for mass measurement. Hot corrosion tests were carried out with 75%(mass fraction) (Na2SO4+K2SO4)+25%(mass fraction) NaCl molten salts at 800 ℃ for 300 h. The coating with uniform area density of 2-3 mg/cm2 of mixed salts was applied with brush on preheated specimens. Due to the molten salts film consuming, these specimens were brushed with salts again every 20 h in order to supply enough salts deposition. The samples were withdrawn at regular intervals of time and the mass change was measured. The sensitivity of the balance used in this study was 10-4 g.
After tests, the oxidation and hot corrosion products on the surface of exposed samples were identified by X-ray diffraction (XRD). The morphologies of exposed samples and corresponding composition were examined by scanning electron microscopy (SEM) with X-ray energy dispersive spectroscopy (EDS).
3 Results and discussion
3.1 Microstructure of enamel coating
Fig.1 shows the microstructure of enamel coating on Ti-22Al-25Nb alloy after firing at 900 ℃ for 30 min. The enamel coating seemed to consist of a gray matrix with fine particles. From Fig.1, it can be seen that the enamel coating is very dense, uniform and adherent to the substrate alloy, which consists of O+α2+B2 phases [1].
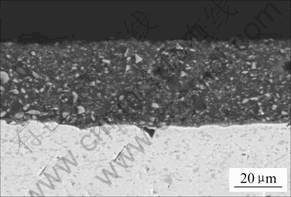
Fig.1 Cross sectional microstructure of enamel coating on Ti- 22Al-25Nb alloy after firing at 900 ℃ for 30 min
3.2 Isothermal oxidation
Fig.2 represents the oxidation kinetics of Ti-22Al-25Nb alloy with and without enamel coating at 800 ℃ for 300 h. Because the scale formed on the bare alloy tended to spall, the mass gain of specimen was determined together with alumina crucible. The bare alloy had non parabolic and rather complex oxidation kinetics, which accorded with other report [9]. The oxidation rate of bare alloy was relatively low at the early stage of exposure, and then increased after about 50 h. Further, severe spallation of the oxide scale was observed on uncoated sample. During the whole exposure time, the enamel coated specimen showed much lower oxidation rate than that of bare alloy, and no obvious spallation occurred. The cross sectional microstructures of specimens oxidized at 800 ℃ for 300 h are shown in Fig.3. After 300 h oxidation at 800 ℃, mixed oxide scale formed on the top of Ti-22Al-25Nb alloy, which was mainly composed of TiO2,AlNbO4 and Nb2O5 in terms of XRD/EDS analysis. Moreover, cracks took place during cooling. It can be seen that the Ni-plating has penetrated into the oxide scale, which is evidence of severe scale cracking (Fig.3(a)). The enamel coating still kept intact, and no obvious change was observed on coating itself except for some reaction products forming at the interface between enamel coating and substrate. The result of EDS indicated that these products were rich in elements of Ti, Al, Nb, Si and O (Fig.3(b)).
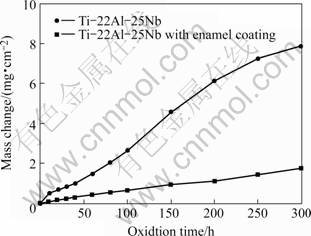
Fig.2 Oxidation kinetics of Ti-22Al-25Nb alloy with and with- out enamel coating at 800 ℃
In order to further clarify the oxidation behavior of Ti-22Al-25Nb alloy, the XRD analysis of samples oxidized at 800 ℃ for shorter time was carried out, as illustrated in Fig.4. After 20 h oxidation, TiO2, Al2O3, NbO2 and Nb2O5 were formed on the surface of Ti-22Al-25Nb alloy, which indicated that all elements in the substrate alloy could form corresponding oxides simultaneously during the initial period of oxidation. With the time increasing, metastable NbO2 would transform into stable Nb2O5. Moreover, the formation of Nb2O5 was reported to proceed quickly at high temperature [14]. Such fast growing Nb2O5 could react with Al2O3 that formed at the early stage of oxidation to form AlNbO4, which was confirmed by the result of XRD analysis (Fig.4). From Fig.4, it can be seen that AlNbO4 has already formed besides TiO2 and Nb2O5 on the Ti-22Al-25Nb alloy oxidized at 800 ℃ after 50 h, while Al2O3 could hardly be detected. Furthermore, the acceleration of oxidation rate at about 50 h might result from the losing of Al2O3 in the scale due to the reaction between Al2O3 and Nb2O5. It was reported that the formation of AlNbO4 oxide phase could decrease the oxidation resistance of alloy [15]. Therefore, Ti-22Al-25Nb alloy exhibited poor oxidation resistance (Figs.2 and 3(a)).

Fig.3 Cross sectional microstructures of Ti-22Al-25Nb alloy without (a) and with enamel coating (b) after 300 h oxidation at 800 ℃ (The Ni-Plating was electrolessly plated to protect the oxide scale from flaking off during preparation of cross-section for SEM.)
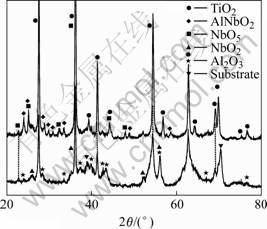
Fig. 4 XRD patterns of Ti-22Al-25Nb alloy oxidized at 800 ℃for different times
After firing at 900 ℃, the enamel frit could become a very dense, uniform coating on Ti-22Al-25Nb alloy. Such dense structure could effectively hinder oxygen from migrating into substrate alloy; as a result, the oxidation rate of enamel coated sample was very low (Fig.2). Moreover, the enamel coating had high thermal stability and good matching of its thermal expansion coefficient (TEC) with the substrate, therefore, no obvious spallation occurred during tests and the enamel coating still kept intact after oxidation at 800 ℃ even up to 300 h (Fig.3(b)). From the above discussion, it can be concluded that enamel coating could significantly improve the oxidation resistance of Ti-22Al-25Nb alloy.
3.3 Hot corrosion
Fig.5 compares the mass change per unit area up to 300 h of the Ti-22Al-25Nb alloy with and without enamel coating corroded with 75% (Na2SO4+K2SO4) +25%(mass fraction) NaCl molten salts at 800 ℃. The bare Ti-22Al-25Nb alloy had very large mass change, which indicated that catastrophic corrosion occurred. During test, scale spallation was observed on bare alloy when the specimens were cooled in air. Lots of spallation also occurred when these specimens were cleaned in boiling water for removing residual salts. However, the enamel coating had much lower mass gain than that of uncoated alloy and effectively decreased corrosion rate of the Ti-22Al-25Nb alloy in molten salts at 800 ℃.
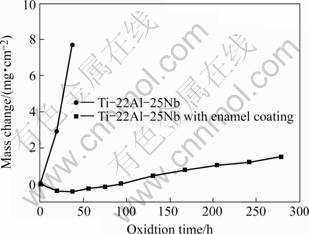
Fig.5 Hot corrosion kinetics of Ti-22Al-25Nb alloy with and without enamel coating at 800 ℃
Figs.6 and 7 show surface morphology and cross sectional microstructure of Ti-22Al-25Nb alloy without and with enamel coating after hot corrosion with 75% (Na2SO4+K2SO4) +25%(mass fraction) NaCl molten salts at 800 ℃ for 300 h, respectively. Severe spallation was observed on the surface of bare alloy, as can be seen from Fig.6(a). The result of XRD analysis for these spallation products indicated that they were mainly composed of TiO2 and Al2O3. From the corresponding cross sectional microstructure, there were still very thick and loose corrosion products left on it (Fig.6(b)). According to the EDS result, these products were also mixed with TiO2 and Al2O3. For the enamel coated alloy, complicated corrosion products formed on the surface of sample (Fig.7(a)). Based on the XRD/EDS result, however, it was difficult to determine their phases and constitution accurately due to complicated reaction taking place between the enamel coating and molten salts at 800 ℃. From Fig.7(a), it could also be seen that some corrosion products like cauliflower formed on the surface of enamel coating. In terms of EDS analysis, they mainly consisted of TiO2 and Al2O3. After corrosion for 300 h, the enamel coating still kept intact and adherent to substrate, and some reactions were also observed to form at the coating/substrate interface (Fig.7(b)).
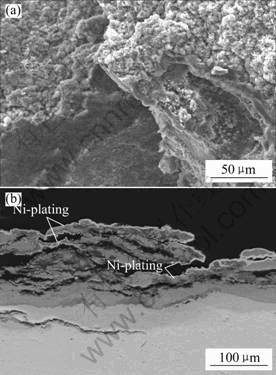
Fig.6 Surface morphology (a) and cross sectional micro- structure (b) of Ti-22Al-25Nb alloy after hot corrosion at 800 ℃ for 300 h
In the potential aerospace applications of Ti2AlNb-based alloy, sulfate or chloride salts may deposit on the surface of materials resulting in the accelerated degradation of alloy. TANG et al had investigated the hot corrosion behavior of TiAl-based intermetallics in molten salts containing S and Cl. The results showed that the existence of S and Cl in molten salts could induce self-sustaining corrosion process, and accelerate the corrosion of TiAl-based alloy [16]. Based on this mechanism, S and Cl in molten salts induced self-sustaining corrosion process to occur for Ti and Al in Ti-22Al-25Nb alloy, as a result, severe spallation took place during the test and very thick and loose corrosion products mainly composed of TiO2 and Al2O3 formed on the surface of uncoated alloy (Fig.6).
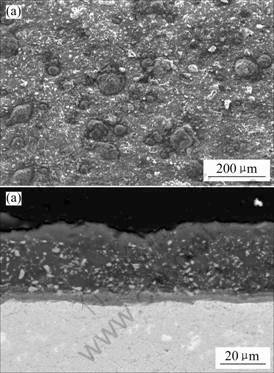
Fig.7 Surface morphology (a) and cross sectional micro- structure (b) of Ti-22Al-25Nb alloy with enamel coating after hot corrosion at 800 ℃ for 300 h
In this study, the enamel coating, as a compound of lots of oxides shown in Table 1, had complex chemical composition. During the test, a series of chemical reactions would take place between some oxides in enamel coating and molten salts to form complicated corrosion products when corresponding thermodynamic conditions were satisfied at 800 ℃. In the meantime, Cl- in the molten salts could penetrate through the enamel coating [17], and react with Ti and Al in substrate alloy to form volatile chlorides of TiCl2 and AlCl3, respectively. At the surface of enamel coating, where the oxygen potential was high, the chlorides might reoxidize to form mixed TiO2 and Al2O3 oxides on the surface of enamel coating (Fig.7(a)). In addition, the evaporation of these chlorides plus some oxides of coating components dissolved into the molten salt might cause the mass loss of enamel coating at the early stage of corrosion [17]. It should be noted that enamel coating still kept intact and adherent to substrate (Fig.7(b)). The enamel coating could provide good protection for bare alloy and effectively improve the hot corrosion resistance of Ti-22Al-25Nb alloy at 800 ℃.
4 Conclusions
1) At 800 ℃, Ti-22Al-25Nb alloy exhibited poor oxidation resistance due to the formation of TiO2, AlNbO4 and Nb2O5 mixed scales. The constitution of oxide scale had the effect on its oxidation rate. In (Na,K)2SO4+NaCl molten salts at 800 ℃, Ti-22Al-25Nb alloy suffered severe hot corrosion and exhibited very poor hot corrosion resistance.
2) Enamel coating could remarkably improve the high temperature oxidation and hot corrosion resistance of Ti-22Al-25Nb alloy because it had dense structure, high thermal stability and good matching thermal expansion coefficient with the substrate.
Acknowledgements
The authors would like to thank Prof. LI Shi-qiong (Central Iron & Steel Research Institute, China) for affording the alloy.
References
[1] GERMANN L, BANERJEE D, GU?DOU J Y, STRUDEL J L. Effect of composition on the mechanical properties of newly developed Ti2AlNb-based titanium aluminide [J]. Intermetallics, 2005, 13: 920-924.
[2] YANG S J, NAM S W, HAGWARA M. Investigation of creep deformation mechanisms and environmental effects on creep resistance in a Ti2AlNb based intermetallic alloy [J]. Intermetallics, 2004, 12: 261-274.
[3] YANG S J, EMURA S, HAGIWARA M, NAM S W. The role of TiB particulate reinforcement in Ti2AlNb based composite under high cycle fatigue [J]. Scripta Mater, 2003, 49: 897-902.
[4] TANG F, NAKAZAWA S, HAGIWARA M. The effect of quaternary additions on the microstructures and mechanical properties of orthorhombic Ti2AlNb-based alloys [J]. Mater Sci Eng A, 2002, 329-331: 492-498.
[5] WU B, SHEN J Y, CHU M Y, SHANG S L, ZHANG Z, PENG D L, LIU S Q. The ordering behavior of the O phase in Ti2AlNb-based alloys [J]. Intermetallics, 2002, 10: 979-984.
[6] PENG J H, MAO Y, LI S Q, SUN X F. Microstructure controlling by heat treatment and complex processing for Ti2AlNb based alloys [J]. Mater Sci Eng A, 2001, 299: 75-80.
[7] NANDY T K, BANERJEE D. Creep of the orthorhombic phase based on the intermetallic Ti2AlNb [J]. Intermetallics, 2000, 8: 915-928.
[8] RALISON A, DETTENWANGER F, SCH?TZE M. Oxidation of orthorhombic Ti2AlNb alloys in the temperature range 550-1000 degrees C in air [J]. Mater High Temp, 2003, 20: 607-629.
[9] RALISON A, DETTENWANGER F, SCH?TZE M. Oxidation of orthorhombic Ti2AlNb alloys at 800 ℃ in air [J]. Mater Corros, 2000, 51: 317-328.
[10] XIONG Y M, ZHU S L, WANG F H. The oxidation behavior and mechanical performance of Ti60 alloy with enamel coating [J]. Surf Coat Technol, 2005, 190: 195-199.
[11] ZHENG D Y, XIONG Y M, ZHU S L, WANG F H. Effect of enamel coating on long-term oxidation and hot corrosion behavior of Ti-24Al-17Nb-0.5Mo alloys [J]. Trans Nonferrous Met Soc China, 2004, 14(Suppl): 359-363.
[12] TANG Z L, WANG F H, WU W T. Effect of Al2O3 and enamel coatings on 900 ℃ oxidation and hot corrosion behaviors of gamma-TiAl [J]. Mater Sci Eng A, 2000, 276: 70-75.
[13] XIONG Y M, ZHU S L, WANG F H. The oxidation behavior of TiAlNb intermetallics with coatings at 800 ℃ [J]. Surf Coat Technol, 2005, 197: 322-326.
[14] JHA S K, KHANNA A S, HARENDRANATH C S. Oxidation characteristics of Ti3Al-Nb alloys and improvement in the oxidation resistance by pack aluminizing [J]. Oxid Met, 1997, 47: 465-493.
[15] JIANG H, HIROHASI M, LU Y, IMANARI H. Effect of Nb on the high temperature oxidation of Ti-(0-50%)Al [J]. Scripta Mater, 2002, 46: 639-643.
[16] TANG Z L, WANG F H, WU W T. Hot-corrosion behavior of TiAl-base intermetallics in molten salts [J]. Oxid Met, 1999, 51: 235-250.
[17] GUAN C H, TANG Z L, WANG F H, WU W T. Effect of enamel coating on oxidation and hot corrosion resistance of Ti-24Al-14Nb-3V [J]. Chinese J Mater Res, 2000, 14: 75-80.
(Edited by PENG Chao-qun)
Foundation item: Projects supported by the National High Technology Research & Development Program of China; and the Key Program of the Chinese Academy of Sciences
Corresponding author: ZHENG De-you; Tel: +86-24-23904856; E-mail: dyzheng@imr.ac.cn