
Recovery of nickel from mixed solution containing light metals by PSt/MA resin
QI Xin-hua(齐新华), JIA Xue-qing(贾学庆), YANG Ying(杨 瑛), NIU Li-e(牛丽娥), HOU Li-ping(侯立平)
School of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract: The construction procedure and structure of Ni2+ macromolecular complexes with series of poly styrene and maleic anhydride (PSt/MA) were studied. Both the copolymers and the complexes (Ni-PSt/MA) were characterized by means of FTIR, elemental analysis, gel permeation chromatography (GPC) and atomic adsorption spectrometer (AAS). A further study using PSt/MA as chelating resin to recover Ni2+ was performed. The adsorption behavior for Ni2+ and various relating parameters of PSt/MA in the separation process were determined. The results indicate that the adsorption capability varies with pH values and the PSt/MA can recover Ni2+ in higher adsorption rate and higher selective coefficient from a mixed solution containing light metals such as Ca2+ and Mg2+ impurities.
Key words: nickel; macromolecular complexes; recovery; separation; adsorption
____________________________________________________________________________________________
1 Introduction
Nickel is one of the most valuable metals and plays an important role in many fields such as steel making, electroplating and ceramic industry, battery and accumulator manufacturing[1]. However, it always exists as mixture rather than an individual mineral in nature. New and cost-effective methods for metal recovery are currently developed[2-4]. The recovery of Ni2+ from aqueous solution includes membrane filtration, adsorption, precipitation or ion exchange[5-7]. With recent rapid developments in technology, a great deal of attention has been paid to polymer materials[8-11], which can be used repeatedly as chelating resin and liquid membrane extractant for recovery of metals from impurities contained system. These polymers are capable of coordinating to different metal ions through reactive functional groups containing O, N, S, and P as donor atoms[12-13] to form metal macromolecular complexes (MMC). While synthesizing chelating resin, the choice of ligands always plays an important role in achieving selectivity. Among the ligands, the synthetic poly styrene and maleic anhydride (PSt/MA) has attracted special attention due to the advantage of its convenient preparation and solubility of alkali salt in water, as well as it is a spaghetti-shaped linear polymer with varying lengths (that is to say that the distribution of relative molecular mass can be adjusted).
In previous work, the synthesis of MMC containing rare earth metals with polymeric ligands and their applications as florescent materials were reported[14-15]. As part of our continuing investigation into the development of MMC involving nickel, herein the procedure to form Ni-PSt/MA with series polymeric ligands of PSt/MA was presented, and a detailed research on the structure and property characterization for both the copolymers and Ni-PSt/MA complexes was performed. The results indicate that the PSt/MA resin can be used as a potential chelating adsorbent to recover nickel from complicated system. For the purpose of developing practical approach for recovery of nickel in industry, the adsorption behavior of PSt/MA for Ni2+ was investigated. In addition, the adsorption isotherm and parameters in the procedure were determined.
2 Experimental
2.1 Instruments
Vibration spectra from 4 000 to 400 cm-1 were recorded on Thermo Nicolect Mattson 2110 FTIR spectrometer (KBr discs). Element analyses for C and H were carried out on Elemental Vario-El elemental analyzer. Metal contents were determined on Hitachi 180-80 polarized Zeeman atomic adsorption spectrometer (AAS). The average relative molecular mass of PSt/MA was estimated by SN-01A Gel permeation chromatography (GPC), using THF as fluent.
2.2 Materials
All the chemicals used in this work were of analytical reagent grade. Styrene was washed with 10% aqueous sodium hydroxide to remove the inhibitor, and followed by washing with water until it was neutral. The solution of Ni2+ containing light metal ions such as Ca2+ and Mg2+ were prepared by dissolving the appropriate amount of analytical grade sulfates in water.
2.3 Preparation of PSt/MA resin
A mixture of different molar ratio of styrene and maleic anhydride (about 15 g) was added dropwise to 100 mL toluene at (75±1) ℃ under stirring for 6 h with benzoyl peroxide(1% of monomer) as an initiator. The precipitate generated was washed repeatedly with toluene and acetone respectively to remove the unreacted monomers and initiator, and then dried in a vacuum oven at 40 ℃ to a constant mass. The resulting resin is white, amorphous solid and the average relative molecular mass evaluated by GPC method is about 20 000. The synthetic route of the PSt/MA resin and the Ni-PSt/MA are described in Fig.1 and Fig.2.
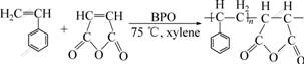
Fig.1 Synthetic route of PSt/MA resin
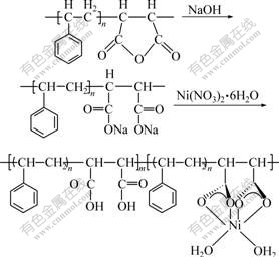
Fig.2 Structure unit of complexes Ni-PSt/MA
2.4 Adsorption experiments
2.4.1 Adsorption capacity
About 1 g dry PSt/MA resin was put into dilute NaOH (0.1 mol/L) solution; the mixture was stirred at room temperature to dissolve completely. The resulting polymeric sodium carboxylates (Na-PSt/MA) solution was adjusted to the pH range of 6-7, and then a sulfate solution containing 1.02 g/L Ni2+ was added. The mixtures were stirred continuously and the concentration of metals was determined by atomic adsorption spectrometry. The formed precipitates (Ni-PSt/MA) were isolated from the solution by centrifugation, then washed with water to remove Ni2+ and any another impurities, and finally washed with ethanol and dried in a vacuum desiccator. The Ni-PSt/MA precipitates cannot be dissolved in water, alcohol and other normal organic solvents, but can be easily dissolved by inorganic acid to release Ni2+, and the protonated insoluble resin of PSt/MA is regenerated. The adsorption capability Q (mg/g) is calculated according to:
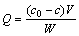 (1)
(1)
Where c0 and c are the initial concentration of Ni2+ and the equilibrium one of Ni2+ in supernatant after sorption; V is the volume of solution used for sorption; and W is the mass of the resin.
2.4.2 Adsorption selectivity
To determine the adsorption selectivity of PSt/MA under competitive condition, the resin was contacted with a binary mixture, in which the concentration of each metal ion was approximately equal. Thus, accurate amount of sodium PSt/MA solution was stirred with a solution of binary mixture. Then, the resin was separated by filtration and the concentrations of the metal ions in the binary mixture were determined by AAS.
2.4.3 Adsorption isotherm
The adsorption isotherm studies were carried out by different volume of sodium PSt/MA solution stirred with 30 mL metal-ion solutions of Ni2+. Then the solutions were filtered, and the concentrations of the metals were determined by AAS. The adsorption data for Ni2+ were analyzed by a regression analysis to fit the Freundlich isotherm model. These data were plotted as a function of the amount of Ni2+ bonded to the resin at equilibrium versus the Ni2+ valuable metal concentration of the solution at equilibrium. The coefficients of this model were computed with linear least-square fitting.
3 Results and discussion
3.1 Characterization of Ni2+ macromolecular complexes (Ni-PSt/MA)
3.1.1 Elemental analysis
The elemental analysis results of the PSt/MA resin and Ni-PSt/MA are shown in Tables 1 and 2.
Table 1 Elemental analysis results of PSt/MA resin
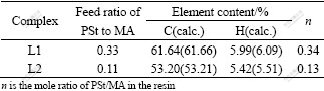
Table 2 Elemental analyses results of complexes Ni-PSt/MA
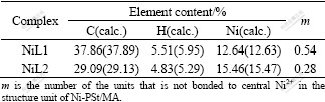
It could be seen that the composition of the resin is slightly different from that of two monomers as starting materials. This may be caused by the different reactivity ratio of two monomers[16]. According to the results of composition determination, the structural unit of Ni-PSt/MA was shown in Fig.2, in which each Ni2+ is bonded to two carboxylates in the chain of the polymer (not necessarily in only one chain), forming stable and repeatable building blocks.
3.1.2 Infrared spectra analysis
The FTIR spectra of the Ni-PSt/MA are obviously different from that of the PSt/MA resin, but they resemble each other. The FTIR spectra data of PSt/MA and the Ni-PSt/MA are listed in Table 3 and the FTIR spectra of the L1 resin and the corresponding Ni-L1 are shown in Fig.3.

Fig.3 FTIR spectra of L1 resin (a) and Ni-L1 (b)
Table 3 FTIR spectra data of PSt/MA resin and complexes Ni-PSt/MA cm-1
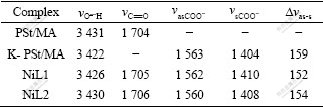
The results show that all the Ni-PSt/MA still have a weaker absorption band at 1 700-1 706 cm-1 ascribed to νC=O of the uncoordinated carboxylic group; the absorption intensity is apparently lower than that of the PSt/MA resin. Two strong absorption bands at 1 408- 1 414 cm-1 and 1 560-1 563 cm-1 are observed in Ni-PSt/MA, which are assigned to the symmetric vibration absorption  and asymmetric vibration absorption
and asymmetric vibration absorption of the carboxylic group,
of the carboxylic group,
respectively. The vC=O absorption band at about 1 700 cm-1 in Ni-PSt/MA does not disappear completely. This is due to a higher hindrance in polymer chains of PSt/MA, which makes the complexes Ni-PSt/MA exist in non-stoichiometric form. The determined Δv (Δv= -
-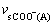 ) for the Ni-PSt/MA is far smaller than that for K-PSt/MA (159 cm-1), which shows that the symmetry of the carboxylic group in the resin is C2v, the same as the free ion. This clearly shows that the carboxylate acting as a bidentate chelate coordinates to the Ni2+ in the resins[17].
) for the Ni-PSt/MA is far smaller than that for K-PSt/MA (159 cm-1), which shows that the symmetry of the carboxylic group in the resin is C2v, the same as the free ion. This clearly shows that the carboxylate acting as a bidentate chelate coordinates to the Ni2+ in the resins[17].
3.2 Results of adsorption experiments
3.2.1 Dependence of pH values
The chelating of Ni2+ with the resin is highly dependent on pH. The effect of pH values on the chelation of Ni2+ is illustrated in Fig.4.
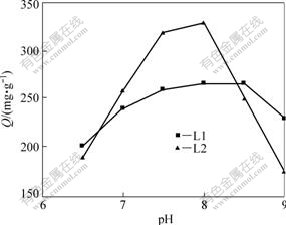
Fig.4 Relationship between adsorption capability and pH values
At lower pH values, carboxylates (COO-) in PSt/MA are in the form of protonation, which induces an electrostatic repulsion of Ni2+. Therefore, competition exists between protons and metal ions for adsorption sites and decreases the adsorption capacity. Thus, the adsorption capability cannot reach the maximum. These results indicate that metal ions can compete with hydrogen ions in adsorption by the carboxylate ions of PSt/MA[18]. On the contrary, under the circumstances of existing excessive chelating resin, especially at higher pH values, the adsorption capability decreases. This phenomenon can be illustrated as the strong tendency that Ni2+ form coordinated anion with carboxylate, which causes the Ni-PSt/MA dissolving in water. The results indicate that the maximum adsorption capability is 338.12 mg/g for L2 resin in the pH value of 8.
3.2.2 Adsorption selectivity of PSt/MA
The ability to selectively remove some particular metal ions from aqueous solution under competitive conditions is of utmost importance in designing chelating resins. The adsorption selectivity of PSt/MA for Ni2+ in a binary component system was investigated. The selective coefficient (K) is calculated as:
 (3)
(3)
where cA1 and cB1 stand for molar fraction of the metal in the solution after adsorption, cA2 and cB2 stand for molar fraction of the metal in the phase of the resin[19] (A=Ni2+1.02 g/L, B=Ca2+0.92 g/L and Mg2+ 1.10 g/L). The experimental results are listed in Table 4.
Table 4 Selective coefficient (K) of PSt/MA resin
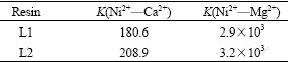
It can be seen that Ni2+ is prior to be adsorbed, and Ca2+, Mg2+ is not adsorbed completely by PSt/MA. All the data imply that the PSt/MA resin can recover Ni2+ in industry.
3.2.3 Adsorption isotherm
A well known model for the adsorption process, particularly for chemisorption process, is presented by Freundlich equation:
lg Qe=lg K+1/nlg ce (4)
where Qe(mg/g) is the amount of metal adsorbed at equilibrium; n is the constant; K is the binding energy constant which re?ects the af?nity of chelating material to metal ions. A linear plot of lg Qe vs lg Ce shows the applicability of the Freundlich model (Fig.5). The Freundlich constant K and n are calculated and presented in Table 5, in which R2 reprents the linear correlation coefficient.
Table 5 Freundlich model for Ni2+

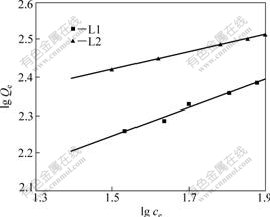
Fig.5 Freundlich isotherms for adsorption of Ni2+
The values 1<n<10 indicate the favorable adsorption of metal ions onto the chelating material.
3.2.4 Determination of coordination ratio
The coordination reaction of Ni2+ with PSt/MA resin can be illustrated as follows[20]:
pR + Ni2+ RpNi (5)
RpNi (5)
When the coordination reaction reaches equilibrium,
 (6)
(6)
where Ke is the equilibrium constant, D is the distribution coefficient (D=Qe/ce), p is the coordination ratio, R is the carboxylic content in the PSt/MA resin (mmol). The above formula can be changed as the following equation:
lg D=lg Ke+plg R (7)
The equilibrium data are analyzed with the above linearized equation, and Fig.6 shows that the slope is the coordination ratio.
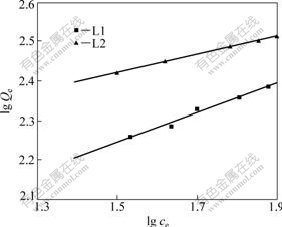
Fig.6 Relationship between lg D and lg R of L1
The slope 2.08 is consistent with the Ni-PSt/MA structure units we discussed before.
4 Conclusions
1) The PSt/MA resin has rather strong coordination ability to Ni2+ to form Ni-PSt/MA, which possess three-dimensional network structure and can’t dissolve in water and normal organic solvents.
2) Adsorption experiments reveal that the maximum adsorbing capability of PSt/MA resin is 338.12 mg/g. The isothermal adsorbing study illuminates that the adsorption is consistent with the Freundlich equation. The PSt/MA resin has good adsorption selectivity for Ni2+ and has promising use in recovering of nickel.
References
[1] WANG Xue-song, HUANG Juan, HU Huai-qiong, WANG Jing, QIN Yong. Determination of kinetic and equilibrium parameters of the batch adsorption of Ni(Ⅱ) from aqueous solutions by Na-mordenite [J]. Hazard Mater, 2007,142(1/2): 468-476.
[2] MERETUKOV M A, ORLOV P M. Metallurgy of noble metals [M]. Moscow: Metallurgiya, 1991: 415.
[3] FLEMING C A, MCMULLEN J, THOMAS K C,WELLS J A. Recent advances in the development of an alternative to the cyanidation process-based on thiosulfate leaching and resin in pulp [J]. Miner Metall Process, 2003, 20 (1): 1-9.
[4] SRIZHKO L S. Metallurgy of gold and silver [M]. Moscow: Misis, 2001: 336.
[5] XU Di, SHAO Da-dong, CHEN Chang-lun, REN An-ping, WANG Xiang-ke. Effect of pH and fulvic acid on sorption and complexation of cobalt onto bare and FA bound MX-80 bentonite [J]. Radiochim Acta, 2006, 94(2): 97-102.
[6] MERCIER L, PINNAVAIA T J. Access in mesoporous materials: Advantages of a uniform pore structure in the design of a heavy metal ion adsorbent for environmental remediation [J]. Adv Mater, 1997, 9(6): 500-503.
[7] FENG X, FRYXELL G E, WANG L Q, KIM A Y, LIU J, KEMMER K M. Functionalized monolayers on ordered mesoporous supports [J]. Science, 1997, 276(9): 923-926.
[8] UNUABONAH E I, ADEBOWAL K O, OLU-OWOLABI B I, YANG L Z, KONG L X. Adsorption of Pb (Ⅱ) and Cd (Ⅱ) from aqueous solutions onto sodium tetraborate-modified Kaolinite clay: Equilibrium and thermodynamic studies [J]. Hydrometallurgy, 2008, 93(1/2): 1-9.
[9] NAGH W S, ENDUD C S, MAYANAR. Removal of copper(Ⅱ) ions from aqueous solution onto chitosan and cross-linked chitosan beads [J]. Reactive & Functional Polymers, 2002, 50(2): 181-190.
[10] SALIBA R, GAUTHIER H, GAUTHIER R, PETIT-RAMEL M. Adsorption of copper(Ⅱ) and chromium(Ⅲ) ions onto amidoximated cellulose [J]. Journal of Applied Polymer Science, 2000, 75(13): 1624-1631.
[11] WANG C C, CHANG C Y, CHEN C Y. Study on metal ion adsorption of bifunctional chelating/ion-exchange resins [J]. Macromolecular Chemistry and Physics, 2001, 202(6): 882-890.
[12] MYASOEDOVA G V, SAVVIN S B, BLASIUS E. Chelating sorbents in analytical chemistry [J]. Critical Reviews in Analytical Chemistry, 1986, 17(1): 1-63.
[13] BISWAS M, MUKHERJEE A. Synthesis and evaluation of metal-containing polymers[J]. Advanced Polymer Science, 1994, 115: 89-123.
[14] HUANG Feng-qun, Zheng Yi-an, YANG Ying. Study on macromolecular metal complexes: synthesis, characterization, and fluorescence properties of stoichiometric complexes for rare earth coordinated with poly(acrylic acid) [J]. Journal of Applied Polymer Science, 2007, 103: 351-357.
[15] DUAN Guo-jian, YANG Ying, CUI Yu-ming. Study on macromolecular rare earth complexes (Ⅳ)—Synthesis, characterization and fluorescent properties of rare earth complexes with polymethyl acrylic acid [J]. Synthesis and Reactivity in Inorganic, Metal-organic, and Nano-metal Chemistry, 2006, 36(6): 459-463.
[16] BRUNO V. Polymer Chemistry [M]. New York: Springer-Verlag, 1973: 131.
[17] NAKAMOTO K. Infrared and Raman Spectra of Inorganic and Coordination Compounds [M]. New York: John Wily, 1986: 1-407.
[18] CHEN C Y, CHEN S Y. Adsorption properties of a chelating resin containing hydroxy group and iminodiacetic acid for copper ions[J]. Journal of Applied Polymer Science, 2004, 94: 2123-2130.
[19] REN Hui-min, TIAN Hua, LANG Hong-qi. Handbook of analytical chemistry [M]. Beijing: Chemical Industry Press, 1997: 26-28. (in Chinese)
[20] HUANG Wen-qiang, SI Zhi-huai, LI Chen-xi, HE Bing-lin. Coordination behavior of polymer-supported glycine [J]. Ion Exchange and Adsorption, 1997, 13(3): 307-312. (in Chinese)
___________________________
Corresponding author: YANG Ying; Tel: +86-931-8913610; E-mail: yangying@lzu.edu.cn
(Edited by LIU Hua-sen)