Determination of contents of eight alkaloids in fruits of Macleaya cordata (Willd) R. Br. from different habitats and antioxidant activities of extracts
来源期刊:中南大学学报(英文版)2010年第3期
论文作者:钟明 黄可龙 曾建国 黎霜 张丽
文章页码:472 - 479
Key words:Macleaya cordata (Willd) R. Br.; alkaloid; ultrasound-assisted extraction; antioxidant activity; HPLC-UV; response surface methodology
Abstract: A reliable ultrasound-assisted extraction (UAE) method combined with HPLC-UV for quantification of eight active alkaloids in fruits of Macleaya cordata (Willd) R. Br. was developed. The optimization conditions of UAE were obtained by using Box-Behnken design of response surface methodology. Chromatography was carried out using a Kromasil C18 column by gradient elution with 0.1% phosphoric acid aqueous solution for HPLC-UV. All calibration curves showed good linear correlation coefficients (R2>0.999 6) and recoveries (from 97.3% to 104.9%) were acceptable. 1,1-diphenyl-2-picrylhydrazyl (DPPH) method was employed to test the antioxidant activity of the extract from the samples. The proposed method was successfully applied to quantifying eight components in nine samples of M.cordata, and significant variations of alkaloid contents and antioxidant activity of the samples from different habitats were demonstrated. It presents a powerful proof for the selection of the best sources to extract eight kinds of alkaloids.
基金信息:the National Natural Science Foundation of China
the International Cooperation Project of Ministry of Science and Technology of China
J. Cent. South Univ. Technol. (2010) 17: 472-479
DOI: 10.1007/s11771-010-0509-1 ![]()
ZHONG Ming(钟明)1, 2, 3, HUANG Ke-long(黄可龙)1, ZENG Jian-guo(曾建国)2,
LI Shuang(黎霜)1, ZHANG Li(张丽)3
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Hunan Engineering Research Center of Botanical Extract, Changsha 410301, China;
3. Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology,
Yueyang 414006, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract: A reliable ultrasound-assisted extraction (UAE) method combined with HPLC-UV for quantification of eight active alkaloids in fruits of Macleaya cordata (Willd) R. Br. was developed. The optimization conditions of UAE were obtained by using Box-Behnken design of response surface methodology. Chromatography was carried out using a Kromasil C18 column by gradient elution with 0.1% phosphoric acid aqueous solution for HPLC-UV. All calibration curves showed good linear correlation coefficients (R2>0.999 6) and recoveries (from 97.3% to 104.9%) were acceptable. 1,1-diphenyl-2-picrylhydrazyl (DPPH) method was employed to test the antioxidant activity of the extract from the samples. The proposed method was successfully applied to quantifying eight components in nine samples of M.cordata, and significant variations of alkaloid contents and antioxidant activity of the samples from different habitats were demonstrated. It presents a powerful proof for the selection of the best sources to extract eight kinds of alkaloids.
Key words: Macleaya cordata (Willd) R. Br.; alkaloid; ultrasound-assisted extraction; antioxidant activity; HPLC-UV; response surface methodology
1 Introduction
Macleaya cordata (Willd) R. Br., locally called ‘boluhui’, is a perennial plant of the Papaveraceae family. It has been used as one of traditional Chinese medicines (TCMs) for a long time. The herb mainly distributes throughout the south, southeastern and northwest of China, and can also be found in middle part of Japan as well. As reported previously, the alkaloids were considered as main bioactive constituents of M.cordata [1-2]. The main alkaloids in this plant include sanguinarine (SA), chelerythrine (CHE), protopine (PRO), allocryptopine (ALL), dihydrosanguinarine (DHSA), dihydrochelerythrine (DHCHE), oxysanguinarine (OSA), berberine (BER) [1, 3-4]. Contemporary pharmacological studies elucidated that the alkaloids exhibited a great variety of biological and pharmacological activities such as antitumour [3, 5], antibacterial [6-7], anthelmintic [8], and anti- inflammatory properties [9-10]. Recent investigations in our laboratory have shown that the alkaloids in the extracts from the plant exhibit certain antioxidant activity. Consequently, it is important to develop effective methods for the extraction of these alkaloids from the plant.
The classical techniques for extracting alkaloid from the herb are mainly maceration, refluxing and percolation [2], but they are generally time-consuming and laborious. In recent years, ultrasound-assisted extraction (UAE) technique has been applied to increasing the yield of various biologically active compounds [11]. As a modern technique, UAE is a fast, inexpensive, and efficient alternative for conventional extraction processes in many situations. Although some of new techniques such as microwave-assisted extraction [3, 12] and supercritical CO2 extraction [13] have been applied to the extraction of alkaloids from medicinal plant, UAE is more convenient and inexpensive compared to those techniques. So it is significant to improve the extraction effect of alkaloids from the herb by using UAE method.
According to the theory of TCMs, multiple constituents are responsible for the therapeutic effects of TCMs, and the contents of ingredients may also influence therapeutic effect of Chinese herbal preparations [14]. In our previous studies, it was found that the contents of the alkaloids in samples of M.cordata from various regions were different from each other. Moreover, the antioxidant activity of the extract from the samples with different districts was distinct as well. Considering that little information is available in this aspect, it should be very important to develop a rapid, stable routine method for the determination of the contents of these alkaloids and the biological activity of the extract from the plant.
The aim of this work was to introduce a reliable ultrasound-assisted extraction combined with HPLC-UV method for quantification of eight alkaloids in nine samples of M.cordata from different habitats. The UAE operation parameters were optimized by using response surface methodology (RSM) with Box-Behnken design (BBD). The contents of eight active alkaloids were investigated by HPLC-UV under optimization conditions. Lastly, the antioxidant activities of the extracts from the samples were tested and compared. The relationship between the contents of total alkaloids and antioxidant activity of the nine extracts was demonstrated.
2 Experimental
2.1 Chemicals and standards
HPLC-grade acetonitrile and methanol were purchased from Tedia (USA). Other reagents were of analytical grade. Double deionized water was purified by Milli-Q water system (Millipore, Bedford, MA, USA) and used to prepare all buffer and sample solutions. The standards for sanguinarine and chelerythrine were purchased from Sigma (Shanghai, China), while berberine and protopine were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Owing to the non-availability of other commercial standards, a method for the purification of allocryptopine, dihydrosanguina- rine, dihydrochelerythrine and oxysanguinarine by semi-preparative HPLC was developed in our laboratory. Their identity was verified by EI-MS, 1HNMR and 13CNMR, their purity was not less than 99% according to HPLC analysis based on peak area normalization method. The chemical structures of the alkaloids are shown in Fig.1.
2.2 Plant material
Nine samples of dried fruits of M.cordata were collected from various districts in China, including Luoyang of Henan Province (lot No. HEC081101), Changsha of Hunan Province (lot No. HEC081102 and HEC081103), Hefei of Anhui Province (lot No. HEC081104 and HEC081105), Guiyang of Guizhou Province (lot No. HEC081106), Guilin of Guangxi Province (lot No. HEC081107 and HEC071008), Nanchang of Jiangxi Province (lot No. HEC081109).
2.3 Ultrasound-assisted extraction
A KQ-250B ultrasonic generator (40 kHz, 250 W) from the Kunsha Ultrasonics Co. Ltd. (Kunshan, China) was used to extract alkaloids from the plant. Approximately 1.0 g of dried fruits was accurately weighed and placed in a round-bottom flask, and suitable volume acidified methanol (pH=2) was added. The flask was immersed in the ultrasonic bath. The extraction time was selected according to the experiment design. The supernatant solution was separated by centrifuged at 4 000 r/min for 10 min. The obtained solution was filtered through a syringe filter (0.45 μm) and was diluted as required for chromatographic analysis.

Fig.1 Chemical structures of eight alkaloids identified in M.cordata: (1)—Sanguinarine; (2)—Chelerythrine; (3)Protopine; (4)—Allocryptopine; (5)—Oxysanguinarine; (6)—Berberine; (7)—Dihydrosanguinarine; (8)—Dihydrochelerythrine
2.4 HPLC-UV analysis
A Shimadzu HPLC system (Shimadzu Corporation, Kyoto, Japan) coupled with UV detector was used for quantitative determination of eight alkaloids. The UV detector was employed at the wavelength of 280 nm. Peak area was used for quantification. Chromatographic separation was carried out on a Kromasil C18 analytical column (5 μm, 250 mm×4.6 mm) at 30 ℃. A linear gradient elution of A (100% acetonitrile) and B (0.1% phosphoric acid aqueous solution) was used. The time program for the multi-step gradient was shown as follows: initial 27% (A), 0-5 min keeping 27% (A), 5-17 min linear gradient to 54% (A), 17-20 min from 54% to 75% (A), 20-25 min from 75% to 80% (A), 25-35 min keeping 80% (A), 35-40 min linear gradient to 27% (A), keeping 27% (A) at 40-45 min. The flow rate was 0.8 mL/min, and the injection volume was 5 ?L.
2.5 Antioxidant activity measurement
Antioxidant activities of M.cordata extracts were tested by measuring the ability of the extracts to scavenge the free radical DPPH (1,1-diphenyl-2- picrylhydrazyl) in vitro. The assay method was modified from that described by OLLANKETO et al [15]. For the purpose of comparing the antioxidant activity in extracts of various samples, the IC50 value, defined as the concentration of antioxidant in the reactive system necessary to decrease the initial DPPH concentration by 50%, was used as an index. To find this value, 0.1 mmol/L of DPPH in ethanol was prepared and 1.0 mL of this solution was added to a test sample (4.0 mL). The reaction mixture was shaken well and incubated at room temperature for 60 min. The absorbance of the resulting solution was read at 517 nm against a blank. All the tests and analyses were performed in triplicate and average.
3 Results and discussion
3.1 Optimization of extraction procedure
In order to optimize the extraction procedure, response surface methodology (RSM) was adopted in this work. RSM, which includes factorial design and regression analysis, helps in evaluating the effective factors, building models to study the interaction between the variables, selecting the optimum conditions of variables, and eliminating the limitations of a single-factor optimization process [16]. Before using RSM, the influence of solvent type and pH value of solution were investigated. And then, the influence of extraction methods on the content of total alkaloid (TA) was studied. The yields of eight alkaloids were summed as the yield of TA, which was used to evaluate the extraction yield. Several variables were screened for optimization of the extraction step, including extraction solvent, pH value, solvent-herb ratio, extraction time, and extraction temperature. The content of TA was selected as response variable of RSM. Experimental ranges of relevant variables were given according to the available data gathered in the preliminary experiments. The tested and the optimum values obtained for each variable at the power ultrasound of 250 W and particle size of 0.5 mm are shown in Table 1.
Table 1 Optimization of ultrasound-assisted extraction of TA from fruits of M.cordata (sample: HEC081103)

3.1.1 Selection of extraction solvent and pH value
The effect of the type of solvents on extraction efficiency was firstly determined. Four solvents, including water, acetone, ethanol and methanol were selected and evaluated. The results showed that methanol yielded more alkaloids than other extraction solvents, followed by acetone, ethanol and water. Therefore, methanol as the preferred solvent was selected. On the other hand, pH value of solution could affect the extraction efficiency as well. To investigate the influence of the parameter on extraction process, a given amount of sample (HEC081103) in acidic methanol (pH range from 2 to 5) was subjected to UAE using the same protocol mentioned above. The results indicated that the acidic medium (pH=2) enhanced the extraction efficiency of all of eight alkaloids. It was therefore decided to modify the initial pH value of the solution to about 2.
3.1.2 Optimization of extraction experiment
In this work, BBD of RSM was adopted. The BBD which consisted of 17 experiments was modified slightly from that described by ZHANG et al [16]. The design variables were the ratio of solvent to material (20:1-40:1), the extraction temperature (20-60 ℃), and the extraction time (20-30 min). Other operation conditions such as the power of ultrasonic instrument (250 W) and particle size of sample powders (about 0.5 mm) were kept at constant values.
3.1.3 Analysis of response surface methodology
Fig.2(a) shows the effect of the extraction time and temperature on the yield of TA at a fixed solvent-herb ratio of 30:1. At a definite extraction temperature, the yield of TA increased slightly with the increase of the extraction time, and nearly reached a maximum value at the point of the longest extraction time. On the other hand, extraction temperature had a remarkable effect on the extraction percent of TA at all extraction times. It can be seen that higher temperature is satisfactory to our aim. It is because higher temperature combined with the mechanical agitation by the cavitation effect of ultrasonic wave causes the increase of intermolecular interactions, giving rise to higher molecular motion and increasing the solubility. The increasing temperature may also cause opening cell matrix, and as a result, alkaloids availability for extraction increased. However, when the temperature exceeded 51 ℃, the acquired ratio of TA increased slowly, even decreased slightly. It means that higher temperature (>51 ℃) was insignificant to the extraction of alkaloids from the fruit of M.cordata using acidic methanol as extraction solvent.
Fig.2(b) depicted the effect of solvent-herb ratio and extraction temperature, showing that both also exerted a quadratic effect on the response variable. Interaction between two variables can be observed when extraction time was kept constant (25 min). In this figure, the extraction percent of TA increased with the increase of solvent-herb ratio until the value arrived at 33:1 and decreased as the ratio was over 36:1. This illustrated that higher solvent-herb ratio was not suitable for alkaloids extraction.
The effects of solvent-herb ratio and extraction time on the yield of TA are shown in Fig.2(c). The extraction time demonstrated a linear increase on the response in experimental scope from 20 to 30 min when the extraction temperature was kept constant. The effect of solvent-herb ratio on the response surface displayed a fast and curvilinear increase when solvent-herb ratio ranged from 20:1 to 33:1, but yield of TA showed a slight increase when the solvent-herb ratio was higher than 36:1. So the optimized solvent-herb ratio was 33:1. In this experiment, the optimal UAE conditions were obtained from response surface analysis as follows: solvent-herb ratio 33:1, temperature 51 ℃, time 30 min, ultrasonic power 250 W and particle size 0.5 mm.
3.2 Optimization of chromatographic condition
The chromatographic conditions were optimized to achieve an adequate resolution of adjacent peaks within a shorter time, especially when numerous similar components were to be analyzed [17]. In our previous experiment, the chromatographic condition, including gradient elution, elution systems, stationary phase, detection wavelength, and flow rate was investigated. At first, gradient elution was adopted because some of the studied compounds could not be separated by isocratic HPLC elution. The effect of mobile phase composition was also examined. It was found that acetonitrile was a better mobile phase than methanol for the separation of the alkaloids. Therefore, acetonitrile was employed as organic phase. Three different types of chromatographic columns, Kromasil C18 column, Hypersil C18 column and BDS C18 column, were tested in order to achieve an adequate separation of the alkaloid components. Kromasil C18 column was chosen for performing all chromatographic analysis due to its excellent peak shapes and high sensitivity. In order to optimize the UV detection conditions, four different wavelengths at 230, 254, 280 and 320 nm were monitored and compared. The lowest noise for these alkaloids was observed in the run at 280 nm, and it was therefore used for all the subsequent experiments. Under the proposed conditions, the samples showed good separation and high sensitivity. The representative chromatograms are shown in Fig.3. To significantly improve the peak shape and separation efficiency during HPLC-UV analysis, different buffers were used as aqueous phase: formic acid aqueous solution, trifluoroacetic acid aqueous solution, ammonium acetate-formic acid aqueous solution, phosphoric acid aqueous solution, K2HPO4 aqueous solution in various proportions. After several trials with those elution systems, it was found that 0.1% phosphoric acid aqueous solution as mobile phase led to a significant improvement on the retention behavior of the target compounds in this plant. Therefore, it was subsequently employed as mobile phase, which led to good resolution and satisfactory peak shape.
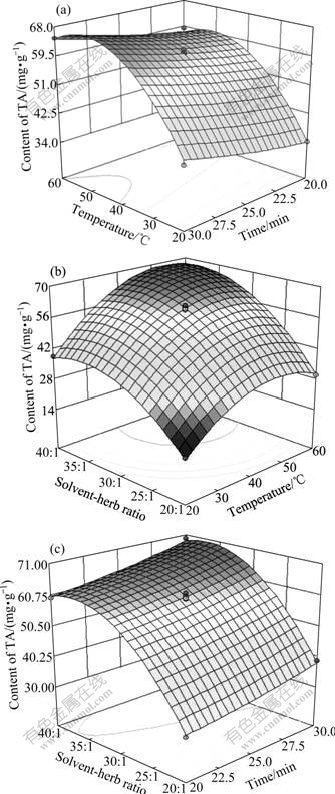
Fig.2 Response surface plots of extraction TA affected by extraction time, extraction temperature and solvent-herb ratio: (a) Solvent-herb ratio, 30:1; (b) Extraction time, 25 min;(c) Extraction temperature, 40 ℃

Fig.3 HPLC chromatograms of standard mixture (a) and sample of lot No. HEC081103 (b): 1—Protopine; 2— Allocryptopine; 3—Sanguinarine; 4—Berberine; 5— Chelerythrine; 6—Oxysanguinarine; 7—Dihydrochelerythrine; 8—Dihydrosanguinarine
3.3 Method validation
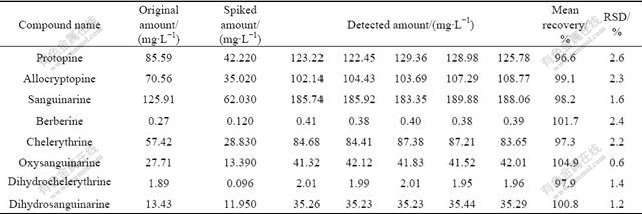
A standard stock solution containing eight reference components was prepared and diluted to a series of appropriate concentrations for the construction of calibration curves. The calibration curve for the measurement of each compound was performed with six different concentrations in triplicate using the same HPLC-UV method as described above. All calibration curves were constructed from peak areas of the reference standards versus their concentrations. Results of the regression analyses and the correlation coefficients (R2) were listed in Table 2. Good linearity (R2>0.999 6) was achieved in relatively wide concentration ranges for each of the alkaloids. The lower limit of detection (LOD) and the lower limit of quantification (LOQ) were determined as the concentrations at signal-to-noise ratios of 3 and 10, respectively. The results showed excellent correlation between the peak area and concentration of the investigated alkaloids in these chromatographic conditions. As listed in Table 3, both intra-day and inter-day reproducibilities of eight contents determined for the investigated alkaloids were less than 1.3%. The relative standard derivation (RSD) was calculated as a measurement of method stability. The accuracy of the developed method was first validated with spiking- recovery tests. Approximately 1.0 g of M.cordata (No. HEC081103) was weighed and spiked with certain amount of each reference compounds, and then treated and analyzed as described above. Each sample was analyzed in triplicate. For comparison, an unspiked sample was concurrently prepared and analyzed simultaneously. As shown in Table 4, the mean recoveries of the alkaloids were 97.3%-104.9%, with RSD values ranging from 0.6% to 2.6% (n=5). The recovery results indicated that the established method was accurate for the determination of eight components in this plant. Therefore, the method is precise, accurate and sensitive enough for simultaneously quantitative determination of those compounds from M.cordata.
3.4 Determination of eight alkaloids in samples
The established method has been successfully
Table 2 Statistical results of linear regression equation analysis in determination of eight alkaloids
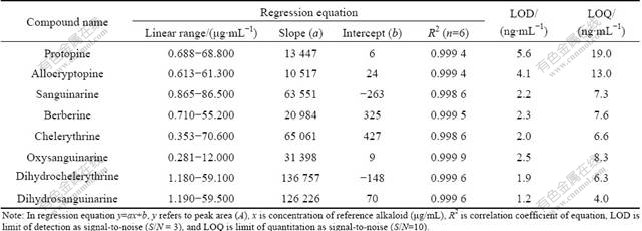
Table 3 Reproducibility of quantification of eight alkaloids for intra-day and inter-day replicates (n=5)

Table 4 Recovery of alkaloids determined by standard addition method (n=5)
applied to simultaneous determination of eight alkaloids in nine samples of M.cordata by means of the external standard methods under the optimization conditions. The typical chromatograms of the extract from the herb were shown in Fig.3. The contents of the alkaloids detected in these samples were summarized in Table 5. The total contents of eight alkaloids were ranged from 34.32 to 70.70 mg/g (average content, 53.75 mg/g). For example, the total contents of eight alkaloids in the samples from Changsha of Hunan Province (lot No. HEC081102 and HEC081103) were higher than those of others, while contents of the total alkaloid in some samples (lot No. HEC081101 and lot No. HEC081104) were very lower. It could also be observed from Table 5 that eight compounds were found in all samples and the contents of them varied significantly in different samples. Among them, sanguinarine was the most abundant component which ranged from 9.92 to 21.87 mg/g (average value of 15.20 mg/g) in nine samples. Then, protopine, allocryptopine and chelerythrine were respectively the second, the third and the fourth most dominant alkaloid and the total amount of the three compounds ranged from 46.4% to 63.1% of the total alkaloids obtained from different samples. In contrast, berberine and dihydrochelerythrine were low content alkaloids with average values of 0.029 mg/g and 0.38 mg/g, respectively, and even berberine was hardly detected in a few samples. It could also be found that no matter how many the content of total alkaloids in various sample was, the percentage of four main alkaloids (sanguinarine, chelerythrine, protopine and allocryptopine) in total alkaloids could maintain relatively stable (no more than 20% variation). In addition, it was also found that the content of an unknown compound (retention time 3.57 min) in Fig.3(b) was relatively high in some samples. The purification and structure elucidation of this compound were under investigation in our laboratory.
Table 5 Contents of alkaloids in nine samples of M.cordata (n=3)

3.5 Antioxidant activity
DPPH is widely used to determine the antioxidant activity of a given compound or extract. The effect of the antioxidants (AH) on DPPH is based on their ability to donate a hydrogen atom to DPPH, thus converting the radical into a stable molecule according to the following equation:
DPPH+AH→DPPH—H+A·
In general, it is considered that the antioxidant capacity of a molecule is equivalent to its capacity to react with free radicals. Our previous research showed that the alkaloids in the extracts exhibited certain antioxidant activity. For the purpose of comparing the antioxidant activity in various samples from different regions, IC50 value was used as an index to evaluate antioxidant activity. Based on the definition of IC50 value, the stronger the antioxidant activity (radical- scavenging activity) of a compound or an extract, the lower the IC50 value. Therefore, antioxidant compound or extract with lower IC50 value possess the better antiradical activity. IC50 value and TA contents of extracts for various samples are shown in Fig.4. The IC50 values of the DPPH antioxidant activity ranged from 578 to 1 192 mg/L for nine batches of samples. HEC081102 and HEC081103 exhibited considerably higher DPPH antioxidant activity (lower IC50 value) compared with other samples, and the lowest DPPH antioxidant activity was found in HEC081101 (higher IC50 value). By comparison of nine samples, the antioxidant activities decreased in the order of HEC081102 and HEC081103 (Hunan Province)>HEC081109 (Jiangxi Province)>HEC081107 and HEC071008 (Guangxi Province)>HEC081104 and HEC081105 (Anhui Province)>HEC081106 (Guizhou Province)>HEC081101(Henan Province). To sum up, antioxidant activity of different samples was significant. It should be attributed to different climate and soil conditions that influence the growth of the herb. The results indicated that samples of M.cordata from different habitats had different bioactivities

Fig.4 Relationship between contents of TA and antioxidant activity (IC50 value) of nine samples
4 Conclusions(1) The optimal UAE conditions for TA from the fruits of M.cordata are obtained by using response surface analysis with BBD as follows: solvent-herb ratio 33:1, extraction temperature 51 ℃, extraction time 30 min, with the power of ultrasonic instrument 250 W and particle size of sample powders 0.5 mm, using acidic methanol (pH=2) as extraction solvent.
(2) A highly sensitive and reliable method is used to simultaneously determine eight alkaloids by HPLC-UV method. Chromatographic procedure is carried out using a Kromasil C18 column by gradient elution with 0.1% phosphoric acid aqueous solution.
(3) Antioxidant activity of the extracts from nine samples of M.cordata is determined by using DPPH method. The results show that the contents of the main and minor alkaloids in the fruits of M.cordata from different regions are different from each other, so do these total alkaloids with various antioxidant activities. Thus, a powerful proof for selection of the best sources and extraction of eight alkaloids from the plant is presented.
References
[1] HU Zhi-bi, XU Ying, FENG Sheng-chu, FAN Guang-jin. Studies on the active components from fruits of Macleaya cordata (Willd) R. Br [J]. Acta Pharmaceutica Sinica, 1979, 14(9): 535-539. (in Chinese)
[2] ZHANG Fei, CHEN Bo, XIAO Song, YAO Shou-zuo. Optimization and comparison of different extraction techniques for sanguinarine and chelerythrine in fruits of Macleaya cordata (Willd) R. Br [J]. Separation and Purification Technology, 2005, 42(3): 283-290.
[3] LUO Xu-biao, CHEN Bo, YAO Shou-Zuo. Rapid determination of protopine, allocryptopine, sanguinarine and chelerythrine in fruits of Macleaya cordata by microwave-assisted solvent extraction and HPLC-ESI/MS [J]. Phytochem Analysis, 2005, 17(6): 431-438.
[4] JANA S, HANA B, HANA P, PAVEL M, EVA T. HPLC quantification of seven quaternary benzo[c]phenanthridine alkaloids in six species of the family Papaveraceae [J]. Journal of Pharmaceutical and Biomedical Analysis, 2007, 44(1): 283-287.
[5] BAI Li-ping, ZHAO Zhong-zhen, CAI Zong-wei, JING Zhi-hong. DNA-binding affinities and sequence selectivity of quaternary benzophenanthridine alkaloids sanguinarine, chelerythrine, and nitidine [J]. Bioorganic & Medicinal Chemistry, 2006, 14(16): 5439-5445.
[6] BEURIA T K, SANTRA M K, PANDA D. Sanguinarine blocks cytokinesis in bacteria by inhibiting FtsZ assembly and bundling [J]. Biochemistry, 2005, 44(50): 16584-16593.
[7] LOVASOA R R, HAVET J L, CATHERINE P, HENRI F. Solid-liquid extraction of protopine from Fumaria officinalis L.—Analysis determination, kinetic reaction and model building [J]. Separation and Purification Technology, 2007, 54(2): 253-261.
[8] SATOU T, AKAO N, MATSUHASHI R, KOIKE K, FUJITA K, NIKAIDO T. Inhibitory effect of isoquinoline alkaloids on movement of secondstage larvae of Toxocara canis [J]. Biol Pharm Bull, 2002, 25(12): 1651-1654.
[9] SAEED S A, GILANI A H, MAJOO R U, SHAH B H. Anti-thrombotic and anti-inflammatory activities of protopine [J]. Pharmacol Res, 1997, 36(1): 1-7.
[10] ZHOU H Y, MINESHITA S. The effect of berberine chloride on experimental colitis in rats in vivo and in vitro [J]. Journal of Pharmacology and Experimental Therapeutics, 2000, 294(3): 822- 829.
[11] HROM?DKOV? Z, KO?T’?LOV? Z, EBRINGEROV? A. Comparison of conventional and ultrasound-assisted extraction of phenolics-rich heteroxylans from wheat bran [J]. Ultrasonics Sonochemistry, 2008, 15(6): 1062-1068.
[12] HUANG Yi, ZHANG Tai-ming, CAO Juan, LIANG Yi-zeng. Microwave digestion polarography for determining seven trace elements in Salvia Miltiorrhiza root and compound Salvia Miltiorrhiza root injection simultaneously [J]. Journal of Central South University of Technology, 2007, 14(4): 514-519.
[13] LIU Ben, LI Wen-jing, CHANG Yi-ling, DONG Wen-hong, NI Li. Extraction of berberine from rhizome of Coptis chinensis Franch using supercritical fluid extraction [J]. Journal of Pharmaceutical and Biomedical Analysis, 2006, 41(3): 1056-1060.
[14] ZHANG Hong-ming, CHEN Shi-wei, QIN Feng, HUANG Xi, REN Ping, GU Xing-qi. Simultaneous determination of 12 chemical constituents in the traditional Chinese Medicinal Prescription Xiao-Yao-San-Jia-Wei by HPLC coupled with photodiode array detection [J]. Journal of Pharmaceutical and Biomedical Analysis, 2008, 48(5): 462-1466.
[15] OLLANKETO M, PELTOKETO A, HARTONEN K, HILTUNEN R, RIEKKOLA M L. Extraction of sage (Salvia officialis L.) by pressurized hot water and conventional methods: Antioxidant activity of the extracts [J]. European Food Research and Technology, 2002, 215(2): 158-163.
[16] ZHANG Qing-an, ZHANG Zhi-qi, YUE Xuan-feng, FAN Xue-Hui, LI Tao, CHEN Shou-fen. Response surface optimization of ultrasound-assisted oil extraction from autoclaved almond powder [J]. Food Chemistry, 2009, 116(2): 513-518.
[17] LIANG Ming-jin, ZHANG Wei-dong, HU Jiang. Simultaneous analysis of alkaloids from Zanthoxylum nitidum by high performance liquid chromatography–diode array detector–electrospray tandem mass spectrometry [J]. Journal of Pharmaceutical and Biomedical Analysis, 2006, 42(2): 178-183.
Foundation item: Project(20576142) supposed by the National Natural Science Foundation of China; Project(2009DFA31270) supported by the International Cooperation Project of Ministry of Science and Technology of China
Received date: 2009-10-19; Accepted date: 2009-12-25
Corresponding author: HUANG Ke-long, PhD, Professor; Tel: +86-731-88870827; E-mail: kelonghuang@yahoo.com.cn
(Edited by YANG You-ping)

