Phase change materials as quenching media for heat treatment of 42CrMo4 steels
来源期刊:中南大学学报(英文版)2020年第3期
论文作者:Milad SAKKAKI Farhad SADEGH MOGHANLOU Soroush PARVIZI Haniyeh BAGHBANIJAVID Aziz BABAPOOR Mehdi SHAHEDI ASL
文章页码:752 - 761
Key words:phase change materials; heat treatment; quenchant; 42CrMo4 steel; microstructure; mechanical property
Abstract: In the present work, paraffin phase change material is used as quenchant for the heat treatment of 42CrMo4 alloy and compared with water, air, and CuO doped paraffin. The samples were prepared based on ASTM E 8M-98 standard for tensile test and then heated up to 830 °C, kept for 4 h in an electric resistance furnace and then quenched in the mentioned media. Elastic modulus, yield strength, ultimate tensile strength, elongation, and modulus of toughness were determined according to the obtained stress-strain curves. Moreover, the hardness and microstructural evolution were investigated after the heat treatment at different media. The samples quenched in paraffin and CuO-doped paraffin are higher in ultimate tensile strength (1439 and 1306 MPa, respectively) than those quenched in water (1190 MPa) and air (1010 MPa). The highest hardness, with a value of HV 552, belonged to the sample quenched in CuO-doped paraffin. The microstructural studies revealed that the non-tempered steel had a ferrite/pearlite microstructure, while by quenching in water, paraffin and CuO-doped paraffin, ferrite/martensite microstructures were achieved. It is also observed that using the air as quenchant resulted in a three-phase bainite/martensite/ferrite microstructure.
Cite this article as: Milad SAKKAKI, Farhad SADEGH MOGHANLOU, Soroush PARVIZI, Haniyeh BAGHBANIJAVID, Aziz BABAPOOR, Mehdi SHAHEDI ASL. Phase change materials as quenching media for heat treatment of 42CrMo4 steels [J]. Journal of Central South University, 2020, 27(3): 752-761. DOI: https://doi.org/ 10.1007/s11771-020-4328-8.

J. Cent. South Univ. (2020) 27: 752-761
DOI: https://doi.org/10.1007/s11771-020-4328-8

Milad SAKKAKI1, Farhad SADEGH MOGHANLOU1, Soroush PARVIZI2,Haniyeh BAGHBANIJAVID2, Aziz BABAPOOR3, Mehdi SHAHEDI ASL1
1. Department of Mechanical Engineering, University of Mohaghegh Ardabili, Ardabil, Iran;
2. Department of Materials Engineering, Shahid Rajaee Teacher Training University, Lavizan, Tehran, Iran;
3. Department of Chemical Engineering, University of Mohaghegh Ardabili, Ardabil, Iran
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract: In the present work, paraffin phase change material is used as quenchant for the heat treatment of 42CrMo4 alloy and compared with water, air, and CuO doped paraffin. The samples were prepared based on ASTM E 8M-98 standard for tensile test and then heated up to 830 °C, kept for 4 h in an electric resistance furnace and then quenched in the mentioned media. Elastic modulus, yield strength, ultimate tensile strength, elongation, and modulus of toughness were determined according to the obtained stress-strain curves. Moreover, the hardness and microstructural evolution were investigated after the heat treatment at different media. The samples quenched in paraffin and CuO-doped paraffin are higher in ultimate tensile strength (1439 and 1306 MPa, respectively) than those quenched in water (1190 MPa) and air (1010 MPa). The highest hardness, with a value of HV 552, belonged to the sample quenched in CuO-doped paraffin. The microstructural studies revealed that the non-tempered steel had a ferrite/pearlite microstructure, while by quenching in water, paraffin and CuO-doped paraffin, ferrite/martensite microstructures were achieved. It is also observed that using the air as quenchant resulted in a three-phase bainite/martensite/ferrite microstructure.
Key words: phase change materials; heat treatment; quenchant; 42CrMo4 steel; microstructure; mechanical property
Cite this article as: Milad SAKKAKI, Farhad SADEGH MOGHANLOU, Soroush PARVIZI, Haniyeh BAGHBANIJAVID, Aziz BABAPOOR, Mehdi SHAHEDI ASL. Phase change materials as quenching media for heat treatment of 42CrMo4 steels [J]. Journal of Central South University, 2020, 27(3): 752-761. DOI: https://doi.org/ 10.1007/s11771-020-4328-8.
1 Introduction
Heat treatment is the process of timed heating and cooling of materials in order to change their microstructure and related mechanical properties. Annealing, normalizing, tempering, and quenching are the most important heat treatment methods often used to modify the microstructure and mechanical properties of engineering materials. Heat treatment is a basic process in steel manufacturing, aluminum processing, and glass manufacturing. Water, brine, polymers, oils, salts, and gases are commonly used as quenching media [1-4]. Improper quenching may cause geometrical distortion, undesirable residual stresses, and unpleasant mechanical properties [5]. The cooling rate of quench medium has a strong effect on properties of the specimen [6, 7]. A good quenching result can be obtained if the cooling rate is equal to or higher than the critical value for the martensite formation [5].
When hot metal is submerged into a liquid medium, the liquid layer in contact with the metal wall immediately vaporizes and a vapor film (vapor blanket) separates the metal surface and liquid. This vapor film acts as an insulator, and the heat transfer occurs predominately as a result of radiation. By decreasing the surface temperature, the vapor film starts to collapse, and liquid comes into contact with the hot surface. At this stage, the bubble boiling initiates and rapid absorption of heat from hot surface occurs. This stage, which corresponds to violent bubble boiling, has the maximum cooling rate during the quenching. By decreasing the temperature below the boiling point, the nucleate boiling stops and heat transfer continues as a result of convection mechanism between liquid medium and metal surface which is slow compared to two-phase heat transfer [8-10].
The important factors which influence the metallurgical transformation during quench hardening are workpiece specifications, type of quenchant, and facilities related to the quenching process [11]. The properties of quenchant like density, thermal conductivity, specific heat, and wetting properties also have a significant effect on the quenching process [5]. The conventional quenchants have low thermal conductivities, which decrease the heat transfer rate from hot specimens. Quenching liquids with different thermal conductivities lead to obtaining different mechanical properties [12]. The heat transfer by the fluids can be enhanced by active and passive methods. Active methods employ external energies such as electric field, magnetic field or blender to improve the heat transfer, while the passive methods need no external forces and augment the heat transfer by change in geometry or improve the thermal properties of the fluids [13, 14]. One of the recently used methods to enhance the thermal conductivity of weakly conductive materials is the suspension of fine particles with higher thermal conductivities than their base fluids such as water, ethylene glycol, and oils. These types of mixtures, which are called nanofluids, can result in thermal conductivity values significantly higher than those predicted by classical solid–liquid mixture models [15, 16]. The thermal conductivity of nanofluids depends on some factors like particle size and particle concentration. The reported results showed that by decreasing the particle size, thermal conductivity increases, and by increasing the particle fraction in the fluid, thermal conductivity raises [17-19].
PARK et al [20] first experimented application of nanofluids as quenching media. High-temperature copper spheres were quenched in alumina nanofluids in order to investigate the effect of suspended nanoparticles on film boiling heat transfer. Even though high particle concentrations were used, from 5 vol. % to 20 vol. %, nanofluids showed lower boiling heat transfer rate than pure water. Quenching of nickel-plated copper sphere was performed due to the fact that copper spheres can be assumed thermally lumped. Superior thermal properties of carbon nanotube (CNT) caused higher critical heat flux, boiling heat transfer rate, and Leidenfrost point compared to water. KIM et al [21] investigated the quenching of steel and zircaloy spheres in nanofluids with 0.1% alumina, silica, and diamond nanoparticles. The results showed that film boiling heat transfer in nanofluids and water is almost identical. Deposited nanoparticles had a remarkable impact on critical heat flux, while diamond, unlike alumina and silica particles, had no significant effect. In the next step quench front speed during rodlet quenching was compared after each test, which proved that deposited nanoparticles increase the cooling rate and quench front speed. As mentioned before, it is necessary to use new types of quenchants to obtain the desired microstructure and mechanical properties.
The phase change materials (PCM) are the group of materials which absorb (or dissipate) a large amount of heat when experiencing phase change. The high heat transfer as the form of latent heat, makes PCMs a good candidate in wide applications in thermal energy storage units [22-25], heat transfer devices [26-28] and air conditioning and thermal comfort inside buildings [29]. PCMs are categorized into three basic types of organic, inorganic, and eutectic materials [23, 30, 31]. These types of materials have advantages like high latent heat value, appropriate solid-liquid phase change temperature, thermal reliability, and low cost [32, 33]. Paraffin is one of the organic PCMs which is widely used. Unfortunately, the low thermal conductivity of PCMs [34, 35] has a negative effect on heat transfer rate [36, 37] and limits their practical applications. The thermal properties of PCMs can be enhanced by dispersion of nano size particle [38]. The thermal conductivity of dispersed particles affects the PCM overall thermal conductivity. Solid materials like metals and ceramics [16, 39] have high thermal conductivities and can be used to improve the thermal properties of pure paraffin [40].
The literature review showed that utilizing PCMs as heat transfer media is investigated widely, but there is lack of researches on PCMs application in the field of heat treatment. Therefore, the present work aims to investigate the using paraffin (a phase change material) and the case of dispersing CuO nano-particles in paraffin as quenchant. Effects of such quenching media on the microstructure and mechanical properties of heat-treated samples were studied experimentally. The investigated material was 42CrMo4 steel, which is common chromium-molybdenum steel with high strength and hardenability. This material also has high fatigue strength and good low-temperature impact toughness. It is used in the automotive and machine manufacturing industries such as axle, gear, connecting rod, gear, engine cylinder, spring and fishing tools [41].
2 Experimental
2.1 Materials and process
A sample of 42CrMo4 steel bar was purchased from a local supplier located in Tabriz, Iran. The chemical composition of the as-received steel bar was determined by the quantometry (PMI-Master Smart) analysis according to ASTM E415–17 [42] standard. The results are given in Table 1. The specimens were prepared by machining based on ASTM E 8M–98 [43] standard for tensile test, with the dimensions illustrated in Figure 1. All specimens were subjected to a similar heating cycle in an electric resistance furnace to reach the austenitization temperature of 830 °C with a heating rate of 10 °C/min and kept for 4 h dwell time. Finally, the austenitized samples were quenched in different media: air, water, solid paraffin, and CuO-doped (1 wt. %) solid paraffin.
2.2 Characterizations
The mechanical and microstructural properties of heat-treated and non-tempered specimens were investigated. For hardness determination and microstructural study of polished surfaces, the probable oxide films formed during the heat treatment were eliminated by grinding and polishing. Metallographic studies were performed to observe the microstructural changes after the heat treatment process by a scanning electron microscope (Tescan Vega II, Czech Republic). In this regard, both the polished and fracture (broken after tensile test) surfaces of specimens were investigated. The crystalline structure of polished surfaces of the specimens was made visible by Nital etchant (4%) for 5 s. The ferrite volume fraction in the microstructures was estimated using ImageJ software. Hardness and tensile properties of the specimens were measured by standard techniques (ASTM E92-82 [44] and ASTM E 8M–98 [43], respectively). Average hardness values were determined by taking four indentations at different positions of the polished surfaces using a Vickers hardness tester. Tensile tests were performed at room temperature (20 °C) by a universal testing machine (Santam STM-150, Iran). The specimens were kept under a cross-head speed of 2 mm/min and hooked up to a data-logger. Load-elongation records were recorded and then converted to stress-strain curves. The yield strength, ultimate tensile strength, elastic modulus, elongation, and modulus of toughness were determined according to the obtained stress-strain graphs. The highest stress in the elastic region was assumed as yield strength. The maximum stress in the stress-strain curve was considered ultimate tensile strength. The elastic modulus was obtained by calculating the slope of curve in the elastic region. The elongation was achieved by multiplying the fracture strain by 100. The modulus of toughness was estimated as the area under the stress-strain curve by integrating up to the fracture point.
Table 1 Chemical composition of as-received 42CrMo4 steel (mass fraction, %)
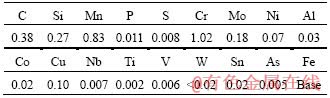
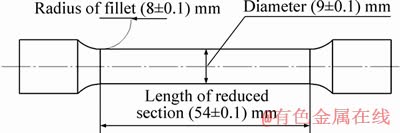
Figure 1 Dimensions of as-prepared samples for heat treatment investigations
3 Results and discussion
3.1 Microstructure
The SEM micrographs of different heat-treated samples are shown in Figure 2. As shown in the figure, the non-tempered sample (Figure 2(a)) has a ferrite/pearlite structure. The results show that the samples quenched in water, paraffin and CuO-doped paraffin (Figures 2(c)-(e)) have a ferritic-martensitic structure [45, 46] but the air-quenched sample has a three-phase bainite- martensite-ferrite microstructure (Figure 2(b)) [47-50]. The formation of bainite reduces the size of martensitic envelopes. Figure 2(b) shows that the bainite breaks out of the prior austenitic grain boundaries. This causes the austenitic grains to be partitioned into smaller portions and, consequently, reduces the size of the martensite packets, which results in incomplete austenite decomposition and formation of bainite. The dispersion and fragmentation of martensite strips with a short distance between the martensite blocks, separated by relatively flexible bainite, leads to the improved ductility (as reported in Figure 6) [51].
In all martensitic states, the carbide precipitation that occurs during the tempering stage can be observed. According to Figure 2(e), nano carbide precipitations are dispersed in the structure of tempered specimens at the boundaries of martensitic lathes and inside them. These particles are more in the sample which was quenched in CuO-doped paraffin than the other ones. After the tempering, the carbides precipitate in the form of M3C [41]. Carbide has a greater hardness than the martensitic phase with lower toughness; therefore, the carbide distribution in the matrix increases the hardness.
The optical microstructures of the quench- tempered and non-tempered specimens are shown in Figure 3. The microstructures related to the water, paraffin, and CuO-doped paraffin quenchants consist of ferrite (bright area) and martensite (brown area). The ferrite volume fractions in Figures 3(c)-(e) are 10%, 33% and 24%, respectively. It was reported that the thermal conductivity of paraffin as a phase change material was enhanced with the addition of CuO particles [52]. Martensite, as a hard phase, has increased the strength, as shown in Figure 6. Formation of martensite and upper bainite in the ferrite matrix, according to Figures 2(b) and 3(b), increases the strength and modulus of toughness compared to the non-tempered specimen. However, due to the diffusion transformation, the formation of upper bainite caused lower strength in comparison with the samples quenched in water, paraffin, and CuO-doped paraffin.

Figure 2 SEM microstructures of 42CrMo4 steels:
Figure 4 shows the fracture surfaces of samples after the tensile tests. The fractographs presented in Figures 5(c)-(e) are a mixture of ductile and brittle fracture modes, because of the existence of brittle phases at the grain boundaries, precipitation, or empty spaces causing a quasi- cleavage fracture [53]. In these figures, clusters of the fine dimples, that indicate the ductile fracture, are identified, and a combination of both types of fractures has been shown. For example, the arrows in Figure 4(d) show the ductile and brittle fracture modes [41, 45, 54, 55]. One of the most important microstructural features in controlling the formation and release of microcracks is the local dislocation densities and the mean ferrite path [56]. Thus, by increasing the amount of carbide, as shown in Figure 4(e), the fracture mode is dominantly brittle. In the macro-fractograph of the non-tempered sample, presented in Figure 5, a ductile fracture surface can be seen. A large number of micro- cavities and micro-cracks appear on the fracture surface macroscopically. The fracture surface in the center of the block prevents vertical cracks in the shear fracture area [57]. In Figure 4(b), the voids, and microvoids in the ductile fracture surface can be seen due to the evolution of a three-phase ferritic-bainitic-martensitic microstructure [51].

Figure 3 Optical micrographs of 42CrMo4 steels:

Figure 4 Fracture micrographs of 42CrMo4 steels:

Figure 5 Fracture macrograph of non-tempered 42CrMo4 steel
Martensitic and bainitic transformations cause the distortion and high-density dislocations that can assist the cracks to be nucleated. The small dimples with more heterogeneity in size at the fracture surface are due to the existence of large interphase boundaries of ferrite-martensite and ferrite-bainite [58].
3.2 Mechanical properties
Different cooling rates have a significant effect on the microstructure of steels. Increasing the cooling rate causes the progressed martensite formation [46, 53, 59, 60]. As reported in Table 2, the hardness values of all heat-treated samples are more than that of non-tempered one. The hardness value of sample quenched in CuO-doped paraffin is the highest in comparison with the other ones. Such a result can be attributed to the nano-particle doping of PCM cooling media with higher thermal conductivity [52]. The relatively lower hardness of the sample quenched in air, compared to the other heat-treated ones, is related to the formation of above-discussed three-phase microstructure.
Figure 6 shows the stress-strain curves of the different investigated specimens. The results show that by quenching in all media, the fracture stress increases compared to the non-tempered specimen. The non-tempered 42CrMo4 steel is a ductile alloy which shows an elastic behavior up to the yield stress of 514 MPa. The ultimate tensile strength of this specimen is 704 MPa, and after the necking, the specimen fails at fracture stress of 466 MPa. Based on Figure 6, the quenching in air has raised the yield stress to 876 MPa and the ultimate tensile strength to 1010 MPa. Despite increasing the strength of specimen, the fracture occurs at lower elongation which means that the specimen has been a few brittle compared to the non-tempered steel. Quenching in water makes the specimen show different behavior from the non-tempered and air-quenched materials. While the strength has increased considerably, the water-quenched specimen showed a brittle behavior. The interesting result, which can be observed in Figure 6, is that the employing paraffin and CuO-doped paraffin as quenchants resulted in a similar tensile behavior obtained by water as a cooling medium. However, it should be noted that both PCM quenchants (pure and CuO-doped paraffin) have significant strengthening influences, as listed in Table 2, compared to the water. The maximum tensile strength obtained by CuO-doped paraffin as quenchant is between the values achieved by the water and pure paraffin media. Such an observation comes back to the heat transfer mechanism during the quenching process.
Table 2 Mechanical properties of non-tempered and heat-treated samples at different cooling media


Figure 6 Stress-strain curves for samples quenched at different media
As mentioned before, there are three mechanisms of heat transfer through the quenching process, i.e., film boiling, nucleate boiling, and convective heat transfer. As the specimen submerged in the quenchant, a film of vapor covers the specimen which acts as an insulator. By decreasing the temperature, nucleate boiling initiates, which has the highest heat transfer rate during the quenching and by more temperature decreasing, convective heat transfer begins, which is again weak heat transfer mechanism. The properties of applied quenchants like thermal conductivity, heat capacity, viscosity, and density directly affect these heat transfer mechanisms [61-64]. Paraffin does not boil as quickly as water. This allows the specimen to absorb more initial heat and cool for longer periods of time, and the risk of entering pearlite or bainite noses decreases. Lower thermal conductivity and higher viscosity of paraffin compared to water result in lower heat transfer coefficient and related heat transfer rate at the convection phase. As a result of slow heat rate at convection phase of paraffin, the related stresses release and the risk for cracking decreases in the material. Therefore, quenching in pure paraffin shows higher ultimate strength than water. Adding CuO nano-particles to paraffin, decreased the ultimate strength compared to pure paraffin. As it was mentioned before, dispersing nano-particles in pure liquid, enhances the thermal properties. It seems that the increased thermal conductivity of CuO-doped paraffin resulted in increased heat transfer rate. It is proven before that increased heat rate increases the risk of cracking in the specimen. Table 2 also shows that the yield stress values of all quenched materials are the same, and the type of quenchant has no significant effect on this value. It is also observable that the yield stress of quenched specimens is higher than that of the non-tempered material.
4 Conclusions
Paraffin as a phase change material and CuO-doped paraffin were used for the heat treatment of 42CrMo4 alloy steels. The microstructure and mechanical properties of heat- treated samples were investigated, and the results were compared with the non-tempered steel and samples quenched in water and air. The highest ultimate tensile strength (1439 MPa) belonged to the sample quenched in paraffin; however, the hardest alloy, with a hardness value of HV 552, was obtained by heat-treating in the paraffin doped with nano-CuO particles. A two-phase ferrite/martensite microstructure was observed by employing the paraffin as quenching medium, regardless of the addition of CuO as the dopant. A similar microstructure was also observed in the water- quenched sample. Using the air as quenchant led to the formation of a three-phase bainite/martensite/ ferrite microstructure.
References
[1] HUSSEIN A K, ABBAS L K, HASAN W N. Effect of quenching media variations on the mechanical behavior of martensitic stainless steel [J]. Al-Khwarizmi Eng J, 2019, 15: 1-12. DOI: 10.22153/kej.2019.11.002.
[2] CANBAY C A, KARADUMAN O, UNLU N, BAIZ S A, OZKUL I. Heat treatment and quenching media effects on the thermodynamical, thermoelastical and structural characteristics of a new Cu-based quaternary shape memory alloy [J]. Compos Part B, 2019, 174: 106940. DOI: 10.1016/j.compositesb.2019.106940.
[3] RAYGAN S, RASSIZADEHGHANI J, ASKARI M. Comparison of microstructure and surface properties of AISI 1045 steel after quenching in hot alkaline salt bath and oil [J]. J Mater Eng Perform, 2009, 18: 168-173. DOI: 10.1007/ s11665-008-9273-x.
[4] de ANDRES GARCIA C, CARUANA G, ALVAREZ L. Control of M23C6 carbides in 0.45C–13Cr martensitic stainless steel by means of three representative heat treatment parameters [J]. Mater Sci Eng A, 1998, 241: 211-215. DOI: 10.1016/S0921-5093(97)00491-7.
[5] GRUM J, BOZIC S, ZUPANCIC M. Influence of quenching process parameters on residual stresses in steel [J]. J Mater Process Technol, 2001, 114: 57–70. DOI: 10.1016/S0924- 0136(01)00560-X.
[6] BIRCH R, WANG S, TONG V S, BRITTON T B. The effect of cooling rate and grain size on hydride microstructure in zircaloy-4 [J]. J Nucl Mater, 2019, 513: 221-225. DOI: 10.1016/j.jnucmat.2018.11.011.
[7] PEDERSEN L, ARNBERG L. The effect of solution heat treatment and quenching rates on mechanical properties and microstructures in AlSiMg foundry alloys [J]. Metall Mater Trans A, 2001, 32: 525-532. DOI: 10.1007/s11661-001- 0069-y.
[8] HAKAN GUR C, PAN J. Handbook of thermal process modeling steels [M]. CRC Press, 2008. DOI: 10.1201/ 9781420003581.
[9] LISCIC B, TENSI H M, LUTY W. Theory and technology of quenching [M]. Berlin, Heidelberg: Springer Berlin Heidelberg, 1992. DOI: 10.1007/978-3-662-01596-4.
[10] HALL D D, MUDAWAR I. Experimental and numerical study of quenching complex-shaped metallic alloys with multiple, overlapping sprays [J]. Int J Heat Mass Transf, 1995, 38: 1201-1216. DOI: 10.1016/0017-9310(94)00244-P.
[11] LISCIC B. State of the art in quenching[M]//Quenching and Carburizing. London: The Institute of Materials, 1993: 1–32.
[12] FAKIR R, BARKA N, BROUSSEAU J. Mechanical properties analysis of 4340 steel specimen heat treated in oven and quenching in three different fluids [J]. Met Mater Int, 2018, 24: 981-991. DOI: 10.1007/s12540-018-0120-9.
[13] MOGHANLOU F S, KHORRAMI A S, ESMAEILZADEH E, AMINFAR H. Experimental study on electrohydrodynamically induced heat transfer enhancement in a minichannel [J]. Exp Therm Fluid Sci, 2014, 59: 24-31. DOI: 10.1016/j.expthermflusci.2014.07.019.
[14] WEBB R L, KIM N H. Principles enhanced heat transfer [M]. CRC Press, 2005.
[15] EASTMAN J A, PHILLPOT S R, CHOI S U S, KEBLINSKI P. Thermal transport in nanofluids [J]. Annu Rev Mater Res, 2004, 34: 219-246. DOI: 10.1146/annurev.matsci.34.052803. 090621.
[16] PIL J S, CHOI S U S. Effects of various parameters on nanofluid thermal conductivity [J]. J Heat Transfer, 2007, 129: 617. DOI: 10.1115/1.2712475.
[17] TIWARI A K, GHOSH P, SARKAR J. Performance comparison of the plate heat exchanger using different nanofluids [J]. Exp Therm Fluid Sci, 2013, 49: 141-151. DOI: 10.1016/j.expthermflusci.2013.04.012.
[18] CHOUGULE S S, SAHU S K. Comparative study of cooling performance of automobile radiator using Al2O3-water and carbon nanotube-water nanofluid [J]. J Nanotechnol Eng Med, 2014, 5: 010901. DOI: 10.1115/1.4026971.
[19] MADHESH D, PARAMESHWARAN R, KALAISELVAM S. Experimental investigation on convective heat transfer and rheological characteristics of Cu–TiO2 hybrid nanofluids [J]. Exp Therm Fluid Sci, 2014, 52: 104-115. DOI: 10.1016/j.expthermflusci.2013.08.026.
[20] PARK H S, SHIFERAW D, SEHGAL B R, KIM D K, MUHAMMED M. Film boiling heat transfer on a high temperature sphere in nanofluid [C]// 2004 ASME Heat Transf Eng Summer Conf. Charlotte, NC, 2004.
[21] KIM H, DEWITT G, MCKRELL T, BUONGIORNO J, HU L. On the quenching of steel and zircaloy spheres in water- based nanofluids with alumina, silica and diamond nanoparticles [J]. Int J Multiph Flow, 2009, 35: 427-438. DOI: 10.1016/j.ijmultiphaseflow.2009.02.004.
[22] FARID M M. Solar energy storage with phase change [J]. J Sol Energy Res, 1986, 4: 11-29.
[23] LANE G A. Solar heat storage phase-chang materials [M]// CRC Press, 2018. DOI: 10.1201/9781351076746.
[24] MORCOS V H. Investigation of a latent heat thermal energy storage system [J]. Sol Wind Technol, 1990, 7: 197-202. DOI: 10.1016/0741-983X(90)90087-I.
[25] LI J H, ZHANG G, WANG J Y. Investigation of a eutectic mixture of sodium acetate trihydrate and urea as latent heat storage [J]. Sol Energ, 1991, 47: 443-445. DOI: 10.1016/0038- 092X(91)90112-A.
[26] HIMPEL M, HIEBLER S, SCHWEIGLER C, HELM M. Long-term test results from a latent heat storage developed for a solar heating and cooling system [C]// Proc Euro Sun 2010 Conf. Freiburg, Germany: International Solar Energy Society, 2010: 1-8. DOI: 10.18086/eurosun.2010.16.08.
[27] MORRISON D J, ABDEL-KHALIK S I. Effects of phase- change energy storage on the performance of air-based and liquid-based solar heating systems [J]. Sol Energy, 1978, 20: 57-67. DOI: 10.1016/0038-092X(78)90141-X.
[28] DAS S, DUTTA T K. Mathematical modeling and experimental studies on solar energy storage in a phase change material [J]. Sol Energy, 1993, 51: 305-312. DOI: 10.1016/0038-092X(93)90142-B.
[29] TYAGI V V, BUDDHI D. PCM thermal storage in buildings: A state of art [J]. Renew Sustain Energy Rev, 2007, 11: 1146-1166. DOI: 10.1016/j.rser.2005.10.002.
[30] SHARMA A, TYAGI V V, CHEN C R, BUDDHI D. Review on thermal energy storage with phase change materials and applications [J]. Renew Sustain Energy Rev, 2009, 13: 318-345. DOI: 10.1016/j.rser.2007.10.005.
[31] ZALBA B, MARIN J M, CABEZA L F, MEHLING H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications [J]. Appl Therm Eng, 2003, 23: 251-283. DOI: 10.1016/S1359- 4311(02)00192-8.
[32] MA G, LIU S, XIE S, JING Y, ZHANG Q, SUN J, JIA Y. Binary eutectic mixtures of stearic acid-n-butyramide/ n-octanamide as phase change materials for low temperature solar heat storage [J]. Appl Therm Eng, 2017, 111: 1052-1059. DOI: 10.1016/j.applthermaleng.2016.10.004.
[33] MENG Z N, ZHANG P. Experimental and numerical investigation of a tube-in-tank latent thermal energy storage unit using composite PCM [J]. Appl Energy, 2017, 190: 524-539. DOI: 10.1016/j.apenergy.2016.12.163.
[34] WATANABE T, KIKUCHI H, KANZAWA A. Enhancement of charging and discharging rates in a latent heat storage system by use of PCM with different melting temperatures [J]. Heat Recover Syst CHP, 1993, 13: 57-66. DOI: 10.1016/0890-4332(93)90025-Q.
[35] MEHLING H, HIEBLER S, ZIEGLER F. Latent heat storage using a PCM-graphite composite material [M]. Terrastock, 2000.
[36] JEGADHEESWARAN S, POHEKAR S D, KOUSKSOU T. Conductivity particles dispersed organic and inorganic phase change materials for solar energy storage—An exergy based comparative evaluation [J]. Energy Procedia, 2012, 14: 643-648. DOI: 10.1016/j.egypro.2011.12.989.
[37] SHUKLA A, BUDDHI D, SAWHNEY R L. Thermal cycling test of few selected inorganic and organic phase change materials [J]. Renew Energy, 2008, 33: 2606-2614. DOI: 10.1016/j.renene.2008.02.026.
[38] SCIACOVELLI A, COLELLA F, VERDA V. Melting of PCM in a thermal energy storage unit: Numerical investigation and effect of nanoparticle enhancement [J]. Int J Energy Res, 2013, 37: 1610-1623. DOI: 10.1002/er.2974.
[39] NEKAHI S, VAJDI M, MOGHANLOU F S, VAFERI K, MOTALLEBZADEH A, OZEN M, AYDEMIR U, SHA J, SHAHEDI ASL M. TiB2–SiC-based ceramics as alternative efficient micro heat exchangers [J]. Ceram Int, 2019, 45(15): 19060-19067. DOI: 10.1016/j.ceramint.2019.06.150.
[40] CUI W, YUAN Y, SUN L, CAO X, YANG X. Experimental studies on the supercooling and melting/freezing characteristics of nano-copper/sodium acetate trihydrate composite phase change materials [J]. Renew Energy, 2016, 99: 1029-1037. DOI: 10.1016/j.renene.2016.08.001.
[41] SUN C, FU P X, LIU H W, LIU H H, DU N Y. Effect of tempering temperature on the low temperature impact toughness of 42CrMo4-V steel [J]. Metals (Basel), 2018, 8(4): 232. DOI: 10.3390/met8040232.
[42] ASTM E415-17. Standard test method for analysis of carbon and low-alloy steel by sparkatomic emission spectrometry [S]. 2017.
[43] ASTM E 8M-98. Standard test methods for tension testing of metallic materials [S]. 1998.
[44] ASTM E92-82. Standard test methods for vickers hardness of metallic materials [S]. 2003.
[45] AHMAD E, MANZOOR T, ALI K L, AKHTER J I. Effect of microvoid formation on the tensile properties of dual-phase steel [J]. J Mater Eng Perform, 2000, 9: 306-310. DOI: 10.1361/105994900770345962.
[46] SHAIKH K, INAMDAR S, QURESHI H, BIDRI U, ZAID S. To study the behaviour of materials AISI 1050, 1090, 4140 using conventional quenching and nano quenching [J]. Int J Eng Trends Technol, 2016, 38: 81-84. DOI: 10.14445/ 22315381/IJETT-V38P216.
[47] CLAESSON E. Development of a heat treatment method to form a duplex microstructure of lower bainite and martensite in AISI 4140 steel [D]. Stockholm, Sweden: Department of Material Science and Engineering, Royal Institute of Technology, 2014.
[48] CHUAIPHAN W, SRIJAROENPRAMONG L, PINPRADUB D. The effects of heat treatment on microstructure and mechanical properties of AISI 4140 for base cutter cane harvester [J]. Adv Mater Res, 20013, 774-776: 1059-1067. DOI: 10.4028/www.scientific.net/ AMR.774-776.1059.
[49] PORUKS P, YAKUBTSOV I, BOYD J D. Martensite–ferrite interface strength in a low-carbon bainitic steel [J]. Scr Mater, 2006, 54: 41-45. DOI: 10.1016/j.scriptamat.2005.09.012.
[50] GURUMURTHY B M, SHARMA S S, GOWRI SHANKAR M C, WALIA R, AGARWAL T. Study on dual-phase structure of AISI4140 steel [J]. Indian J Sci Technol, 2016, 9: 1-6. DOI: 10.17485/ijst/2016/v9i33/93526.
[51] SHAKERIFARD B, GALAN LOPEZ J, HISKER F, KESTENS L A I. Effect of banding on micro-mechanisms of damage initiation in bainitic/martensitic steels [J]. Mater Sci Eng A, 2018, 735: 324-335. DOI: 10.1016/j.msea.2018. 08.049.
[52] LEONG K Y, ABDUL RAHMAN M R, GURUNATHAN B A. Nano-enhanced phase change materials: A review of thermo-physical properties, applications and challenges [J]. J Energy Storage, 2019, 21: 18-31. DOI: 10.1016/j.est. 2018.11.008.
[53] KANG S S, BOLOURI A, KANG C G. The effect of heat treatment on the mechanical properties of a low carbon steel (0.1%) for offshore structural application [J]. Proc Inst Mech Eng Part L: J Mater Des Appl, 2012, 226: 242-251. DOI: 10.1177/1464420712438502.
[54] KIM H, LIU Z, CONG W, ZHANG H C. Tensile fracture behavior and failure mechanism of additively-manufactured AISI 4140 low alloy steel by laser engineered net shaping [J]. Materials (Basel), 2017, 10: 1283. DOI: 10.3390/ma10111283.
[55] SAEIDI N, EKRAMI A. Comparison of mechanical properties of martensite/ferrite and bainite/ferrite dual phase 4340 steels [J]. Mater Sci Eng A, 2009, 523: 125-129. DOI: 10.1016/j.msea.2009.06.057.
[56] CAI X L, FENG J, OWEN W S. The dependence of some tensile and fatigue properties of a dual-phase steel on its microstructure [J]. Metall Trans A, 1985, 16: 1405-1415. DOI: 10.1007/BF02658673.
[57] FENG J, FRANKENBACH T, WETTLAUFER M. Strengthening 42CrMo4 steel by isothermal transformation below martensite start temperature [J]. Mater Sci Eng A, 2017, 683: 110-115. DOI: 10.1016/j.msea.2016.12.013.
[58] SALEMI A, ABDOLLAH-ZADEH A, MIRZAEI M, ASSADI H. A study on fracture properties of multiphase microstructures of a CrMo steel [J]. Mater Sci Eng A, 2008, 492: 45-48. DOI: 10.1016/j.msea.2008.02.043.
[59] CALIK A. Effect of cooling rate on hardness and microstructure of AISI 1020, AISI 1040 and AISI 1060 Steels [J]. International Journal of the Physical Sciences, 2009, 4(9): 514-518.
[60] de SILVA R A, de SOUZA L F G, MORALES E V, RIOS P R, de BOTT I S. Formation of microphases and constituents from remaining austenite decomposition in API X80 steel under different processing conditions [J]. Mater Res, 2015, 18: 908-917. DOI: 10.1590/1516-1439.315214.
[61] SAKKAKI M, SADEGH MOGHANLOU F, VAJDI M, PISHGAR F, SHOKOUHIMEHR M, SHAHEDI ASL M. The effect of thermal contact resistance on the temperature distribution in a WC made cutting tool [J]. Ceram Int, 2019, 45(17): 22196-22202. DOI: 10.1016/j.ceramint.2019.07.241.
[62] SADEGH MOGHANLOU F, VAJDI M, SHA J, MOTALLEBZADEH A, SHOKOUHIMEHR M, SHAHEDI ASL M. A numerical approach to the heat transfer in monolithic and SiC reinforced HfB2, ZrB2 and TiB2 ceramic cutting tools [J]. Ceram Int, 2019, 43(13): 15892-15897. DOI: 10.1016/ j.ceramint.2019.05.095.
[63] SADEGH MOGHANLOU F, VAJDI M, MOTALLEBZADEH A, SHA J, SHOKOUHIMEHR M, SHAHEDI ASL M. Numerical analyses of heat transfer and thermal stress in a ZrB2 gas turbine stator blade [J]. Ceram Int, 2019, 45(14): 17742-17750. DOI: 10.1016/j.ceramint. 2019.05.344.
[64] VAFERI K, NEKAHI S, VAJDI M, SADEGH MOGHANLOU F, SHOKOUHIMEHR M, MOTALLEBZADEH A, SHA J, SHAHEDI ASL M. Heat transfer, thermal stress and failure analyses in a TiB2 gas turbine stator blade [J]. Ceram Int, 2019, 45(15): 19331- 19339. DOI: 10.1016/j.ceramint.2019.06.184.
(Edited by YANG Hua)
中文导读
石蜡相变材料作42CrMo4钢的热处理淬火介质
摘要:采用石蜡相变材料作为42CrMo4合金热处理的淬火介质,并与用水、空气和CuO掺杂石蜡作淬火介质进行比较。根据ASTME8M-98标准制备拉伸试样,将试样在电阻炉中加热至830 °C,然后保温4 h,再在上述介质中淬火。根据得到的应力-应变曲线确定弹性模量、屈服强度、极限抗拉强度、伸长率和韧性模量。此外,还研究了不同介质热处理后的硬度和显微组织演化。石蜡与CuO掺杂石蜡淬火样品的极限抗拉强度(分别为1439和1306 MPa)高于在水(1190 MPa)和空气(1010 MPa)中淬火样品的。CuO掺杂石蜡淬火样品的硬度最高,为HV552。显微组织研究表明,非淬火钢具有铁素体/珠光体组织,而在水、石蜡和CuO掺杂石蜡中淬火,可获得铁素体/马氏体组织;还观察到,以空气为淬火介质,形成贝氏体/马氏体/铁素体三相组织。
关键词:相变材料;热处理;淬火;42CrMo4钢;显微组织;力学性能
Received date: 2019-06-25; Accepted date: 2019-09-26
Corresponding author: Mehdi SHAHEDI ASL, PhD, Associate Professor; Tel: +98-9123277186; E-mail: shahedi@uma.ac.ir; ORCID: 0000-0002-9915-2367

