Photocatalytic activity of bauxite-tailings supported nano-TiO2
来源期刊:中南大学学报(英文版)2010年第4期
论文作者:卢清华 胡岳华 王梦
文章页码:755 - 759
Key words:TiO2; bauxite-tailings; phenol; photocatalytic activity
Abstract: TiO2/bauxite-tailings (TiO2/BTs) composites were prepared via a chemical liquid deposition method and characterized by X-ray diffractometry (XRD), scanning electronic microscopy (SEM) and N2 adsorption analysis. The photocatalytic performance of TiO2/BTs composites was evaluated with UV–Vis spectrophotometer following the changes of phenol concentration under different illumination time. Effects of the calcination temperature, the pH and the cycles on the photocatalytic activity of TiO2/BTs composites were investigated. The composites calcined at 500 and 600 ℃ exhibit the best photocatalytic performance, and the phenol degradation ratios reacting for 40 and 160 min reach 35% and 78% respectively under the same conditions, higher than those of 29% and 76% of the Degussa P25(TiO2). The ability of TiO2/BTs500 (BTs500 represents bauxite-tailings calcined at 500 ℃) composites to degrade phenol increases with decreasing pH.
J. Cent. South Univ. Technol. (2010) 17: 755-759
DOI: 10.1007/s11771-010-0552-y![]()
LU Qing-hua(卢清华) , HU Yue-hua(胡岳华), WANG Meng(王梦)
School of Resources Processing and Bioengineering, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2010
Abstract: TiO2/bauxite-tailings (TiO2/BTs) composites were prepared via a chemical liquid deposition method and characterized by X-ray diffractometry (XRD), scanning electronic microscopy (SEM) and N2 adsorption analysis. The photocatalytic performance of TiO2/BTs composites was evaluated with UV–Vis spectrophotometer following the changes of phenol concentration under different illumination time. Effects of the calcination temperature, the pH and the cycles on the photocatalytic activity of TiO2/BTs composites were investigated. The composites calcined at 500 and 600 ℃ exhibit the best photocatalytic performance, and the phenol degradation ratios reacting for 40 and 160 min reach 35% and 78% respectively under the same conditions, higher than those of 29% and 76% of the Degussa P25(TiO2). The ability of TiO2/BTs500 (BTs500 represents bauxite-tailings calcined at 500 ℃) composites to degrade phenol increases with decreasing pH.
Key words: TiO2; bauxite-tailings; phenol; photocatalytic activity
1 Introduction
Since suspended TiO2 powders present some drawbacks in their separation and recovery from water, supported TiO2 catalysts have increasingly attracted much attention in recent years because of their potential application to photocatalytic degradation of organic pollutants in water and air [1]. Numerous methods, such as liquid phase deposition [2], sputtering [3], chemical vapor depositions [4], spray pyrolysis [5], microwave irradiation [6], ion beam enhanced deposition [7], and sol-gel method [8] were employed for the deposition of photocatalytic TiO2 coatings on porous supporting matrices, for example, silica, alumina, molecular sieve, and activated carbon [9-12]. Recently, some natural minerals, especially clay minerals, have been used to make TiO2/clay composite powders through chemical and physical treatment because of low cost and good adsorption performance. In aqueous dispersions, clays have been used in combination with TiO2 photocatalyst degradation to enhance the removal of organic pollutants [13]. Intercalation of TiO2 nanoparticles into the clay mineral structure is expected to be beneficial to the photocatalytic fields, providing resistance to aggregation. By hosting organic solutes to ensure their effective interaction with TiO2 particles and active oxidants generated upon light absorbent, clay-TiO2 composites can lead to increased photocatalytic activity [14-17]. Photocatalytic activity of clay-supported TiO2 has been reported not only in aqueous solution but also in degradation of gaseous substances [18-19].
Bauxite-tailings (BTs) are the aluminosilicate-waste generated during improving ratio of aluminum to silicon in bauxite by beneficiation methods and have the characteristics of large output, fine particle and high activity. The main minerals are diaspore, kaolinite, illite and pyrophyllite. Some investigations and trials for the utilization of BTs in cement, building materials and ceramics have been carried out in recent years. Furthermore, BTs are also desirable to develop absorbent materials for the treatment of waste streams containing dissolved heavy metals because of their mineral compositions. It is discovered that BTs modified by FeCl3?6H2O have a higher capacity to remove heavy metal ions and dyes from the industrial wastewater and that the calcined BTs have larger surface area than pure clay minerals [20]. Based on these facts, investigations about BTs used as a support for TiO2 photocatalyst were carried out in this work.
2 Experimental
2.1 Materials
The raw materials used were BTs (supplied by Zhengzhou Research Institute of Chalco, China), which were composed of Al2O3 (39.5%), SiO2 (28.9%), TiO2 (3.1%), MgO (0.5%), CaO (0.6%), total Fe (7.3%), S (0.1%), K2O (4.7%) and Na2O (0.8%) (in mass fraction). The loss on ignition (LOI) of the as-received BTs was about 12.74%. The main minerals in BTs were determined to be diaspore, kaolinite, anatase, hematite and quartz by X-ray diffractometry (XRD). BTs were milled for 5 h with planet-type ball mill in order to fine the particle size. Tetrabutyl titanate Ti(OC4H9)4 (CR grade), triethanol-amine C6H15O3N (AR grade), absolute ethanol (AR grade), nitric acid(AR grade) and phenol (AR grade) were used as additives. Degussa P25 TiO2, mainly composed of anatase phase, with a BET surface area of 50 m2/g and an average particle size of 30 nm, was used as reference sample.
2.2 Sample preparation
2.2.1 TiO2 sol stock dispersion
TiO2 sol stock dispersion was prepared by mixing tetrabutyl titanate, Ti(OC4H9)4, with nitric acid, triethanolamine, nanopure water and absolute ethanol, in which the Ti(OC4H9)4 concentration was 0.4 mol/L, the molar ratio of H2O to Ti(OC4H9)4 to C6H15O3N was 10:6:0.1, and pH was 1.87. Then, TiO2 stock dispersion was diluted to 0.05 mol/L with absolute ethanol and agitated for 2 h for the preparation of the composites.
2.2.2 Preparation of TiO2/BTs composites
TiO2/BTs composites were prepared as follows: 1.6 g of BTs was weighed and dispersed in 200 mL water. The dispersion was stirred while 100 mL TiO2 sol was slowly added, and continued to stir for 6 h after completing the addition. After being settled for 2 h, the solid deposits were separated and dried at 80 ℃ for 6 h, and then calcined in air for 1 h at 400, 500, 600 and 700 ℃, respectively. Finally, TiO2/BTs400, TiO2/BTs500, TiO2/BTs600 and TiO2/BTs700 composites were obtained.
2.3 Characterization
The morphologies of the composites were examined by scanning electron microscope (SEM, JSM-6700F, Japan). XRD patterns were recorded on a RIGAKU D/max-2550VB+18 kW powder diffractometer with Cu Kα radiation (λ=0.154 180 6 nm), and a graphite monochrometer was used for the diffracted beam. N2 adsorption and desorption isotherms were measured using a Quantachrome Autosorb-1MP surface area and porosity analyzer after the samples were degassed in vacuum at 300 ℃ for 3 h. BET surface areas were calculated from the BET plot. Pore size distributions were calculated using the BJH model.
2.4 Evaluation of photocatalytic activity
The photocatalytic degradation was carried out with AM sunlight under clear sky in summer (July). The photocatalytic activity of samples was evaluated with Shimadzu UV-Vis-2450 spectrometer. The mass of photocatalyst used was 1 g and the initial concentration of phenol solution was 0.6 g/L in each trial. At the beginning, 100 mL fresh phenol solution and 1 g catalyst powder were put into the cylindrical glass vessels of uniform diameter and stirred for 30 min in dark. Then, the vessels were placed under the sunlight. 10 mL sample was taken out at time intervals to analyze the concentration of phenol. The dependence of phenol- concentration with illumination time revealed the degradation ratio. A series of solar experiments of required reaction conditions were carried out simultaneously in order to ensure the same number of light quanta.
3 Results and discussion
3.1 XRD analysis
XRD patterns of the TiO2/BTs samples are shown in Fig.1. The spectra exhibit the same characteristics of anatase, aluminum and metakaolin at various heat temperatures. With increasing temperature, the diffraction peaks of anatase gradually become sharper and stronger, showing that crystalline structure tends to be uniform. Oppositely, the intensity of peak of Al2O3 in TiO2/BTs composites, resulting from diaspore dehydrating, weakens gradually with increasing temperature. No diffraction peaks of rutile are detected in TiO2/BTs composites. Actually, the phase transition temperature of TiO2 is susceptible to various factors such as preparation method, dopants, particle size and shapes [21]. Generally, the transition from pure anatase phase to rutile phase appears at about 500 ℃. However, rutile phase cannot be found in composites even at the calcining temperature over 700 ℃ due to the effect of multi-compositions in BTs.
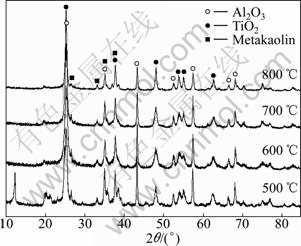
Fig.1 XRD patterns of TiO2/BTs composites calcined at different temperatures
3.2 Change of specific surface area
The specific surface areas for BTs and TiO2/BTs composites treated at different temperatures are shown in Fig.2. Obviously, TiO2/BTs composites exhibit larger area than BTs, but the dependence on the temperature for both samples is the same. The surface area begins to increase, reaches the maximum at 500 ℃, and then decreases with increasing treatment temperature. It can be explained as follows: diaspore begins to dehydrate at about 300 ℃ and then turns into porous particles, which leads to the increase of the surface area of samples. The dehydration process of diaspore is completed till 500 ℃, so the surface area reaches the highest. With further increasing the treatment temperature the holes in the particles collapse and then lead to the decrease of the surface area. The distributions of pore sizes for BTs and TiO2/BTs composites treated at 500 ℃ (BTs500 and TiO2/BTs500) shown in the illustration are almost the same, which indicates that the loaded TiO2 has no effect on the pore structure of BTs and is responsible for the larger specific surface area of TiO2/BTs composites. The largest specific surface area of TiO2/BTs composites is 2.01 times as large as that of BTs.
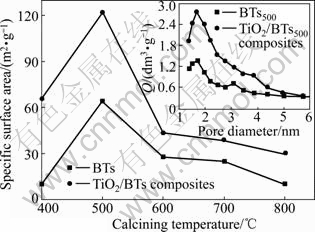
Fig.2 Specific surface areas for BTs and TiO2/BTs composites calcined at different temperatures (Q represents quantity adsorbed)
3.3 Morphologies of TiO2/BTs composites
SEM images for TiO2/BTs500 composites are shown in Fig.3, which indicates changes in surface morphology after TiO2 is loaded onto BTs. The surface of TiO2 on BTs is non-uniform and fluctuant. The distribution of TiO2 grains is quite uniform with a grain size of 5-20 nm. Some agglomerates exist at the grain boundary due to the difficulty of coating on irregular shape of BTs. It can be also seen that there are some claviform grains in TiO2/BTs500 composites besides sheet shape grains.
3.4 Photocatalytic activity
The photocatalytic activity of TiO2/BTs composites calcined at different temperatures was studied and compared with that of Degussa P25 and BTs under the same conditions. The results are shown in Fig.4. It can be seen that: (1) BTs do not affect the phenol degradation, and the phenol concentration reduction is caused by the sorption in the place of the degradation illuminating for 40 min; (2) the photocatalytic activity of BTs results from loaded-TiO2, and among TiO2/BTs composites, TiO2/BTs500 and TiO2/BTs600 composites have the highest photocatalytic activity, and the phenol degradation ratios reach 35% and 78% respectively under the same conditions illuminating for 40 and 160 min, which are 29% and 76% higher than those of the Degussa P25; and (3) although TiO2/BTs500 and TiO2/BTs600 composites have the same degradation ratio for phenol at 160 min, they always have not the same photocatalytic activity throughout the process of photocatalytic reaction. The photocatalytic activity of TiO2/BTs500 composites is higher than that of TiO2/BTs600 composites in the initial 40 min. The above phenomena show that the photocatalytic activity is related to the specific surface area and the crystalline of the composites. The sorption predominates in the initial stage of catalytic reaction, and thus TiO2/BTs500 composites with larger specific surface area show higher photocatalytic activity; while the oxidation predominates as the reaction processes, and TiO2/BTs600 composites with higher crystallization show higher photocatalytic activity.
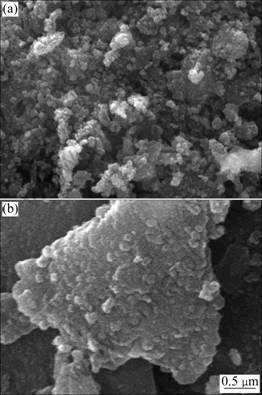
Fig.3 SEM images of TiO2/BTs500 composites: (a) In low magnification; (b) In high magnification
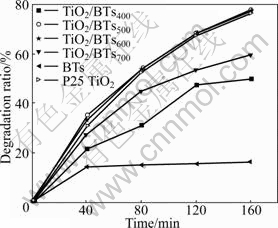
Fig.4 Photocatalytic activities of different samples
The effect of initial pH on the photocatalytic activity of TiO2/BTs500 composites is shown in Fig.5. It can be seen that lower pH is beneficial to the phenol degradation because TiO2 is an amphoteric compound that will form TiOH of binary acid after hydration in aqueous solution. Different acid-base balances under different initial pH values are shown as follows [22]:
pH<pHPZC: TiOH+H+![]() TiOH2+ (1)
TiOH2+ (1)
pH>pHPZC: TiOH+OH-![]() TiO-+H2O (2)
TiO-+H2O (2)
The isoelectric point (PZC) of TiO2 is 3.5-6.4 [23]. The surface of TiO2 is mainly TiOH2+ under lower pH, so the positive ξ potential causes to transfer photo-electron to the surface of TiO2 easily and to suppress photo- carrier complex.
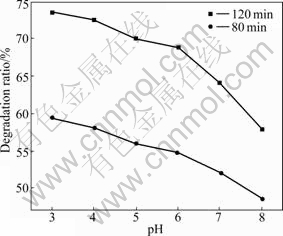
Fig.5 Effect of initial pH on degradation ratio of phenol
The photocatalytic activity of recycled TiO2/BTs500 composites was also investigated. There is 60 min illumination for recycled TiO2/BTs500 composites to eliminate phenol adsorbed before being reused. The effect of recycling times of TiO2/BTs500 composites on the degradation ratio of phenol is shown in Fig.6. It is clear that the photocatalytic activity of fresh photocatalyst is better than that of the used photocatalysts. The photocatalysts used almost have the same photocatalytic activity, probably because there is small amount of free TiO2 in TiO2/BTs500 composites, which cannot be separated and lead to the decrease of TiO2 in the next recycle. Thereby, the photocatalytic activity of the used photocatalysts decreases.
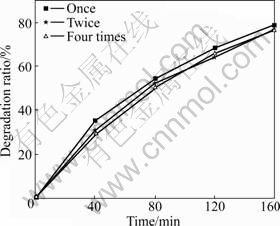
Fig.6 Effect of recycling times of TiO2/BTs500 composites on degradation ratio of phenol
4 Conclusions
(1) TiO2/BTs composites were prepared via hydrolysis precipitation method and characterized by SEM, XRD and N2 adsorption techniques.
(2) TiO2 loaded on BTs is anatase phase that tends towards integral crystalline structure with increasing treatment temperature. Its grain sizes are from 5 to 20 nm.
(3) The specific surface area for both BTs and TiO2/BTs composites as a function of the temperature is similar. However, TiO2/BTs composites show larger surface area than BTs at all treatment temperatures, and the largest specific surface area of them is 2.01 times as large as that of BTs.
(4) TiO2/BTs500 and TiO2/BTs600 composites are of the highest photocatalytic activity. Their phenol degradation ratios reach 35% and 78% under the same conditions reacting for 40 and 160 min, higher than those of 29% and 76% of the Degussa P25, respectively.
(5) The ability of TiO2/BTs500 composites to degrade phenol increases with decreasing pH. The photocatalytic activity of TiO2/BTs500 composites slightly degrades along with the first trial. Cycling times almost has no effect on the photocatalytic activity of the used TiO2/BTs500 composites.
References
[1] SONG Hai-yan, JIANG Hong-fu, LIU Xing-qing, MENG Guang-yao. Nano TiO2 deposited on crude mineral and the photoactivity to the degradation of chloroform [J]. American Journal of Environmental Sciences, 2006, 2(2): 60-65.
[2] YU H G, LEE S C, AO C H, YU J G. Low-temperature fabrication and photocatalytic activity of clustered TiO2 particles formed on glass fibers [J]. Journal of Crystal Growth, 2005, 280(3/4): 612-619.
[3] SATO Y, UEBAYASHI A, ITO N, KAMIYAMA T, SHIGESATO Y. High rate deposition of photocatalytic TiO2 films by dc magnetron sputtering using a TiO2-x target [J]. Journal of Vacuum Science and Technology A: Vacuum, Surfaces and Films, 2008, 26(4): 903-907.
[4] BESSERGENEV V G, PEREIRA R J F, MATEUS M C, KHMELINSKII I V, VASCONCELOS D A. Study of physical and photocatalytic properties of titanium dioxide thin films prepared from complex precursors by chemical vapour deposition [J]. Thin Solid Films, 2006, 503(1/2): 29-39.
[5] SUGIYAMA O, OKUYA M, KANEKO S. Photocatalytic ability of TiO2 porous film prepared by modified spray pyrolysis deposition technique [J]. Journal of the Ceramic Society of Japan, 2009, 117: 203-207.
[6] SUN Shen-mei, JIANG Yin-shan, YU Li-xin, LI Fang-fei, YANG Zheng-wen, HOU Tian-yi, HU Da-qiang, XIA Mao-sheng. Enhanced photo-catalytic activity of microwave-treated TiO2 pillared montmorillonite [J]. Materials Chemistry and Physics, 2006, 98(2/3): 377-381.
[7] WAN L, LI J F, FENG J Y, SUN W, MAO Z Q. Improved optical response and photocatalysis for N-doped titanium oxide (TiO2) films prepared by oxidation of TiN [J]. Applied Surface Science, 2007, 253(10): 4764-4767.
[8] GAMBHIRE A B, LANDE M K, MANDALE A B, PATIL K R, ARBAD B R. Photocatalytic activity and characterization of sol-gel-derived Cr(III)-doped TiO2-coated active carbon composites [J]. Philosophical Magazine, 2008, 88(5): 767-779.
[9] IDA J, YOSHIKAWA T, MATSUYAMA T, YAMAMOTO H. TiO2 coating on silica particles by deposition of sol-gel-derived nanoparticles [J]. Advanced Powder Technology, 2007, 18(3): 329-348.
[10] FUJINO T, HATTORI T. Activation of photocatalytic coatings applied on aluminum oxidation film [J]. Journal of Japan Institute of Light Metals, 2006, 56(4): 197-202.
[11] REDDY J K, DURGAKUMARI V, SUBRAHMANYAM M, SREEDHAR B. Structure and photocatalytic activity studies of TiO2-supported over Ce-modified Al-MCM-41 [J]. Materials Research Bulletin, 2009, 44(7): 1540-1546.
[12] YUAN Ru-sheng, GUAN Rong-bo, ZHENG Jing-tang. Effect of the pore size of TiO2-loaded activated carbon fiber on its photocatalytic activity [J]. Scripta Materialia, 2005, 52(12): 1329-1334.
[13] K?ROLY M, ANDR?S F, IMRE D, ISTV?N I, ANDR?S D. TiO2-based photocatalytic degradation of 2-chlorophenol adsorbed on hydrophobic clay [J]. Environmental Science and Technology, 2002, 36(16): 3618-3624.
[14] HIROSHI Y, SHIQEO H, SHOJI Y. Photocatalytic activities of microcrystalline TiO2 incorporated in sheet silicates of clay [J]. Journal of Physical Chemistry, 1989, 93(12): 4833-4837.
[15] KANEKO T, SHIMOTAUMA H, KAJIKAWA M, HATAMACHI T, KODAMA T, KITAYAMA Y. Synthesis and photocatalytic activity of titania pillared clays [J]. J Porous Mater, 2001, 8(4): 295-301.
[16] MOGYOR?SI K, D?K?NY I, FENDLER J H. Preparation and characterization of clay mineral intercalated titanium dioxide nanoparticles [J]. Langmuir, 2003, 19(7): 2938-2946.
[17] KUN R, MOGYOR?SI K, D?K?NY I. Synthesis and structural and photocatalytic properties of TiO2/montmorillonite nanocomposites [J]. Applied Clay Science, 2006, 32(1/2): 99-110.
[18] FASSIER M, CHOUARD N, PEYRATOUT C S, SMITH D S, RIEGLER H, KURTH D G, DUCROQUETZ C, BRUNEAUX M A. Photocatalytic activity of oxide coatings on fired clay substrates [J]. Journal of the European Ceramic Society, 2009, 29(4): 565-570.
[19] KIBANOVA D, TREJO M, DESTAILLATS H, CERVINI S J. Synthesis of hectorite-TiO2 and kaolinite-TiO2 nanocomposites with photocatalytic activity for the degradation of model air pollutants [J]. Applied Clay Science, 2009, 42(3/4): 563-568.
[20] WANG Yu-hua, LAN Ye, HU Yue-hua. Adsorption mechanisms of Cr(VI) on the modified bauxite tailings [J]. Minerals Engineering, 2008, 21(12/14): 913-917.
[21] YANG Bing-chu, GAO Fei, LIU Xiao-yan, ZHANG Li. Effect of oxygen partial pressure on microstructure and absorption characteristics of TiO2 thin films [J]. J Cent South Univ Technol: Science and Technology, 2008, 39(1): 64-68. (in Chinese)
[22] ZHANG Xi-wang, WANG Yi-zhong, LI Guo-ting. Effect of operating parameters on microwave assisted photocatalytic degradation of azo dye X-3B with grain TiO2 catalyst [J]. Journal of Molecular Calalysis A: Chemical, 2005, 237(1/2): 199-205.
[23] TANALA K, PADERMPOLE K, HISANAGA T. Photocatalytic degradation of commercial azo dyes [J]. Water Res, 2000, 34(1): 327-333.
Foundation item: Project(2005CB623701) supported by the National Key Basic Research Program of China
Received date: 2009-12-10; Accepted date: 2010-03-01
Corresponding author: HU Yue-hua, Professor; Tel: +86-731-88876483; E-mail: huyuehua@mail.csu.edu.cn
(Edited by CHEN Wei-ping)

