
Microwave permittivity of SiC-Al2O3 composite powder prepared by sol-gel and carbothermal reduction
LIU Xiao-kui(刘晓魁), LUO Fa(罗 发), ZHU Dong-mei(朱冬梅), ZHOU Wan-cheng(周万城)
State Key Laboratory of Solidification Processing, Northwestern Polytechnical University, Xi’an 710072, China
Received 10 April 2006; accepted 25 April 2006
Abstract: SiC-Al2O3 composite powder was prepared by sol-gel and carbothermal reduction method. The powder synthesized was characterized by X-ray diffraction(XRD) and scanning electron microscopy(SEM) to confirm the phase formation, and the thermodynamic analysis was performed systematically. Moreover, the variation of its microwave permittivity with different atomic ratio of Al/Si was investigated in the frequency range of 8.2-12.4 GHz. The results show that, the powder obtained consists of spherical particles of 300-400 nm in diameter, which are composed of SiC and Al2O3 microcrystal with the grain size of approximately 45 nm. The results of XRD accord with those of the thermodynamic analysis. It is impossible for Al atoms to dissolve in the lattice of SiC during the carbothermal reduction process. Along with the increase of atomic ratio of Al/Si in the xerogel, the amount of Al2O3 in the powder synthesized increases, which reduces both e′, the real part of complex permittivity, and tgd(e″/e′), the dissipation factor, where e″ is the imaginary part of complex permittivity.
Key words: sol-gel method; carbothermal reduction; SiC-Al2O3; composite powder; microwave permittivity
1 Introduction
Silicon carbide (SiC) is a promising wear and high-temperature material, owing to its excellent properties for high-temperature structural applications, including high stiffness and fairly good oxidation resistance at high-temperature[1, 2]. In addition, SiC can be utilized as lossy additive for microwave processing, which can couple with microwave efficiently[3-5]. Due to the strong covalent bonding in SiC, sintering additives (AlN, Al2O3 and B4C etc) are commonly adopted to obtain well-sintered SiC ceramic materials. The characteristics, such as shape, microstructure and grain size, of the starting SiC powder and the additive powder, have great influences on the properties of SiC ceramics sintered[1, 5-7].
In the past decades, sol-gel techniques have been utilized to synthesize ceramic composite powders successfully. It was found that, the extensive composite homogeneity and dispersion achieved through sol–gel approaches can improve both physical and mechanical properties of products[8-12].
In the present work, SiC-Al2O3 composite powder was synthesized by sol-gel and carbothermal reduction method. The reaction process was analyzed by XRD and thermodynamic calculations, and the microwave permittivity of the composite powder with different Al2O3 contents was measured to investigate the possibility of solution of aluminum atoms in SiC lattice.
2 Experimental
SiO2-Al2O3 sol with different Al/Si ratios was prepared with TEOS (tetraethylorthosilicate), Al(NO3)3, saccharose (C12H22O11), ethylalcohol and deionized water as starting materials by the following procedures. Al(NO3)3 and saccharose were firstly dissolved in deionized water. TEOS was mixed with the solution and agitated using magnetic stirrer at room temperature for 10 min. Then HCl was added to adjust the mixture pH to 3-4. Finally, the mixture was further stirred for hydrolyzing for 1 h to get a transparent sol.
The sol was left at 60 ℃ in the air until complete gelation. The xerogel of SiO2-Al2O3 was carbonized at 600 ℃, and then treated at 1 300 ℃, 1 450 ℃, 1 600℃ and 1 700 ℃ for 1 h in an argon atmosphere respectively.
The phase composition of the reaction products obtained was determined by XRD (Cu target, Ka), and the morphology was observed by SEM. The measurement of microwave permittivity is in the frequency range of 8.2-12.4 GHz, based on measurements of the reflection and transmission modudi in the fundamental wave-guide mode TE10. The powder of reaction products was dispersed in melting wax, then the mixtures were cast into molds (10.16 mm×22.86 mm×2 mm). After calibrated with an intermediate of a short circuit and blank holder, reflection and transmission coefficients were obtained with the help of an automated measuring system (HP8510B network analyzer). The powder to be measured was oxidized at 700 ℃ in air to remove excess carbon and washed with dilute hydrofluoric acid to remove remaining silica. The sample consisted of 20%(mass fraction) powder and 80% wax.
3 Results and discussion
3.1 Characterization of reaction products
The XRD patterns of reaction products of SiO2-Al2O3 xerogel with Al/Si atomic ratio of 15/85 are shown in Fig.1. It can be learnt that, the treating temperature has great effect on the phase composition of the products. There is no obvious peak in the pattern of the product treated at 1 300 ℃, which indicates that the powder is still composed of amorphous phases. When the treating temperature rises to 1 450 ℃, there appear weak peaks indexed as mullite (3Al2O3?2SiO2) and silicon carbide. Accompnied by the rise of treating temperature, the peaks become intense. There exist peaks indexed as mullite, SiC and Al2O3 in the XRD pattern of the product obtained at 1 600 ℃. When treated at 1 700 ℃, the product obtained is composed of SiC and Al2O3, and no amorphous phase can be found in the XRD pattern.
Fig.2 shows the XRD patterns of reaction products
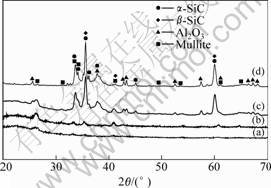
Fig.1 XRD patterns of reaction products with Al/Si atomic ratio of 15/85 at different treating temperatures: (a) 1 300 ℃; (b) 1 450 ℃; (c) 1 600 ℃; (d) 1 700 ℃
with different Al/Si ratios treated at 1 700 ℃. The peaks indexed as Al2O3 become weaker with the decrease of Al/Si ratios, which indicates that there is less Al2O3 in the products.
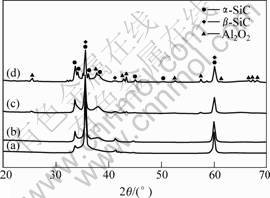
Fig.2 XRD patterns of reaction products treated at 1 700 ℃, in which Al/Si atomic ratios are 1/99 (a), 5/95 (b), 10/90 (c) and 15/85 (d), respectively
Fig. 3 shows the SEM micrograph of the reaction product treated at 1 700 ℃. It can be seen that the product obtained consists of spherical particles of 300-400 nm in diameter. The mean crystalline size of SiC and Al2O3, calculated from the full width at half maximum of peak using SCHERRER’s method, is about 45 nm. Then it can be inferred that, the particles in the products are polycrystalline.
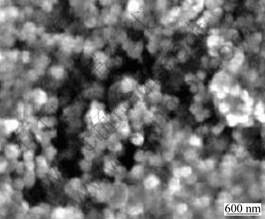
Fig.3 SEM micrograph of SiC- Al2O3 composite powder
According to the results of XRD and the SEM micrograph, it can be known that, Al atoms can exist in mullite, Al2O3, and amorphous phases at the treating temperatures under 1 700 ℃, and only in Al2O3 when treated at 1 700 ℃. SiC can be synthesized at the temperature higher than 1 300 ℃ by carbothermal reduction, and the amount of SiC increases with increasing treating temperature. The reaction products of xerogel at 1 700 ℃ are the composite powder of SiC- Al2O3.
3.2 Thermodynamic analysis
The possible reactions of SiO2-Al2O3 xerogel during the heat-treatment are listed in Table 1, and the thermodynamic analysis of the possible reactions is performed according to Gibbs-Helmhotz equation:
ΔGr=ΔHr-TΔ Sr (1)
Table 1 Possible reactions during heat-treatment of SiO2-Al2O3 xerogel
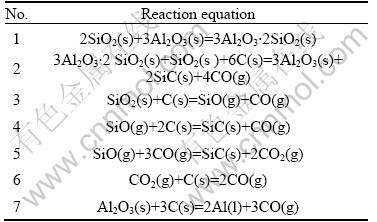
All the pressure values of gas phases are took as 0.1 MPa for the simplification of calculation. The relationship between ΔGr, the change of GIBBS free energy, and the temperature(T) was calculated systemically, and is shown in Fig.4.
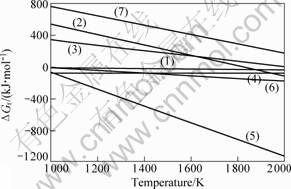
Fig.4 Relationship between ΔGr and temperature for products
According to the thermodynamic analysis, ΔGr for reaction (1) keeps negative at the temperatures higher than 700 ℃. The reason, that the actual tempera-
ture under which mullite phase can be formed is fairly higher than 700 ℃, lies in the small absolute value of ΔGr for reaction (1).
ΔGr for reaction (2) decreases with increasing temperature, and turns to negative when temperature rises to 1 560 ℃, which indicates that, mullite cannot coexist with SiO2 and C steadily above 1 560 ℃.
The synthesis of SiC is by means of reactions (3)-(5). Due to the negative value of ΔGr for reactions (4) and (5) over 700 ℃, the decisive factor for the forming of SiC by carbothermal reduction is whether reaction (3) can be carried out. The thermodynamic analysis indicates that, ΔGr for reaction (3) turns to negative at 1 750 ℃, which is higher than the actual temperature that SiC can be synthesized. The main reason lies in the thermodynamic analysis on the assumption that all the pressure of gas phase is 0.1 MPa, while the actual pressure of SiO(g) and CO(g) is very low in the treating atmosphere. Moreover, the homogeneous dispersion achieved through sol–gel approaches may also reduce the reaction temperature.
In addition, ΔGr for reaction (7) keeps positive at 2 027 ℃. This indicates that, Al2O3 cannot react with carbon, and there is no possibility of the existence of Al in gaseous or liquid phase at 1 700 ℃.
3.3 Microwave permittivity analysis
Fig.5 shows the microwave permittivity of the mixture of SiC-Al2O3 composite powder obtained in the frequency range of 8.2-12.4 GHz. It can be found that, e?, the real part of complex permittivity and tg d(e″/e′), the dissipation factor, where e″ is the imaginary part of complex permittivity, decrease with the increase of the Al/Si ratio in the powder.
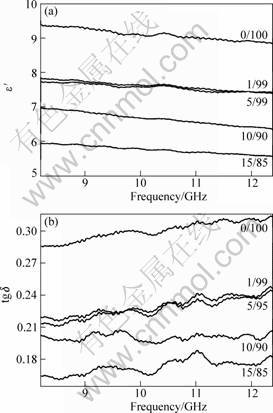
Fig.5 Permittivity of SiC-Al2O3 composite powder with different atomic ratios of Al/Si: (a) e′-frequency; (b) tg d- frequency
According to the dielectric mixing law of polyphase materials[3, 13, 14]:
lne=f1lne1+f2lne2 (2)
where e, e1 and e2 are the permittivities of composite, component 1 and component 2, respectively; f1 and f2 are the volume fractions of the component phases, the complex permittivity of composite material is decided by the components.
For the powder synthesized, the main component phases are SiC, a lossy material, and Al2O3, a low-loss material. On account that mullite and Al2O3 cannot be deoxidize during the course of carbothermal reduction according to the result of XRD and thermodynamic analysis, there is no possibility of solution of Al atoms in SiC in the way of in-situ doping.
In addition, doping of SiC cannot be performed via diffusion at 1 800 ℃ due to the strong covalent bonding in SiC. So it can be inferred that, Al atoms in the composite powder synthesized can exist only in the form of Al2O3, and it is impossible for Al atoms to dissolve in SiC prepared with carbothermal reduction method. Accompanied by the increase of Al/Si ratios in the SiO2-Al2O3 xerogel, there will be more Al2O3 formed in the SiC-Al2O3 composite powder obtained.
It was found that, doping of SiC induces such defects as dangling bonds and unpaired electrons, which move in response to the electric field, and lead to higher ability of absorbing microwave energy [15-17]. The variation of microwave permittivity of SiC-Al2O3 composite powder obtained also indicates that, there is no Al atoms doped in SiC lattice. Increasing of atomic ratio of Al/Si in the xerogel results in more Al2O3 in SiC-Al2O3 composite powder synthesized, which induces lower values of e′ and tgd(e″/e′).
4 Conclusions
1) SiC-Al2O3 composite powder can be prepared by sol-gel and carbothermal reduction method. The powder synthesized consists of spherical particles of 300-400 nm in diameter, which are composed of SiC and Al2O3 microcrystals with the mean grain size of approximately 45 nm.
2) There is no possibility of solution of Al atom in the lattice of SiC via sol-gel and carbothermal reduction method. Along with the increase of atomic ratio of Al/Si in the xerogel, the amount of Al2O3 in the composite powder increases, which reduces both e′ and tgd(e″/e′) of SiC-Al2O3 composite.
References
[1] KIRIANOV A, YAMAGUCHI A. Sintering and physicochemical properties of composition in the SiC-Al2O3-La2O3-Cr2O3 system II. Oxidation resistance of pressureless sintered compacts in the SiC-Al2O3-La2O3-Cr2O3 system[J]. Ceram Int, 2000, 26: 447-453.
[2] KAVECKY S, JANEKOVA B, MADEJOVA J, SAJGALIK P. Silicon carbide powder synthesis by chemical vapour deposition from silane/acetylene reaction system[J]. J Eur Ceram Soc, 2000, 20: 1939-1946
[3] SUELEISER K. Microwave behavior of silicon carbide/high alumina cement composites[D]. Gainesville: University of Florida, 2001.
[4] ELIAS F N, RUTH H G A K. Al2O3/mullite/SiC powders synthesized by microwave-assisted carbothermal reduction of kaolin[J]. Ceram Int, 2001, 27: 815-819.
[5] MULLA M A, KRSTIC V D. Pressureless sintering of β-SiC with Al2O3 additions[J]. J Mater Sci, 1994, 29: 934-938.
[6] PADTURE N P. In suit-toughered silicon carbide[J]. J Am Ceram Soc, 1994, 77: 519-523.
[7] HWANG K T, AUH K H, KIM C S. Influence of SiC particle size and drying method on mechanical properties and microstructure of Si3N4/SiC nanocomposite[J]. J Mater Lett, 1997, 32(4): 251-257.
[8] RODEGHIDERO E D, MOORE B C, WOLKENBERG B S, WUTHENOW M, TSE O K, GIANNELIS E P. Sol-gel synthesis of ceramic matrix composites[J]. Mater Sci Eng, 1998, A244: 11-21.
[9] RICE R W. Ceramic composites-processing challenges[J]. Ceram Eng Sci Proc, 1981, 2(7-8): 493-508.
[10] BECHER P F. Transient thermal stress behavior in ZrO-toughened Al2O3[J]. J Am Ceram Soc, 1981, 64(1): 37-39.
[11] RODEGHIERO E D, TSE O K, CHISAKI J, GIANNELIS E P. Synthesis and properties of Ni/α-Al2O3 composites via sol-gel[J]. Mater Sci Eng, 1995, A195: 151-161.
[12] BOULLE A, OUDJEDI Z, GUINEBRETIERE R, SOULESTIN B, DAUGER A. Ceramic nano composites obtained by Sol-gel coating of submicron powders[J]. Acta Mater, 2001, 49: 811-816.
[13] SIHVOLA A. A review of dielectric mixing models[R]. Springfield: National Technical Information Service, 1997.
[14] GERSHON D L. Complex permittivity measurements and mixing laws of ceramic materials and application to microwave processing[D]. College Park: University of Maryland, 1999.
[15] BERNHOLE J, KAJIHARA S A, WANG C, ANTONELLI A, DAVIS R F. Theory of native defects, doping and diffusion in diamond and silicon carbide[J]. Mater Sci Eng, 1992, B11(1-4): 265-272.
[16] SUZUKI M, HASEGAWA Y, AIZAWA M, NAKARA Y, OKUTANI T, UOSAKI K. Characterization of silicon carbide-silicon nitrogen composite ultrafine particles synthesized using a CO2 laser by silicon-29 magic angle spinning NMR and ESR[J]. J Am Ceram Soc, 1995, 78(1): 83-89.
[17] ZHAO D L, ZHOU W C. Preparation and microwave permittivity of nano Si/C/N composite powders suspended in different matrixes[J]. J Inorganic Mater, 2001, 16(5): 909-914.
(Edited by YANG You-ping)
Foundation item: Project (50572090) supported by the National Natural Science Foundation of China
Corresponding author: LIU Xiao-kui; Tel: +86-29-88494574; Fax: +86-29-88494574; E-mail: xiaokui69@126.com