 Electrochemical behavior of YAG laser-welded NiTi shape memory alloy
Electrochemical behavior of YAG laser-welded NiTi shape memory alloy
YAN Xiao-jun(阎小军), YANG Da-zhi(杨大智), LIU Xiao-peng(刘晓鹏)
School of Materials Science and Engineering, Dalian University of Technology, Dalian 116024, China
Received 11 July 2005; accepted 20 February 2006
Abstract: Electrochemical behaviors of laser-welded Ti-50.6%Ni(mole fraction) shape memory alloy and the base metal in 0.9% NaCl solution were investigated by electrochemical techniques as corrosion potential measurement, linear and potentiodynamic polarization. The results indicate that the laser-welded NiTi alloy is less susceptible to pitting and crevice corrosion than the base metal, which is demonstrated by the increase in polarization resistance(Rp) and pitting potential(φpit) and decrease in corrosion current density(Jcorr) and mean difference between φpit and φprot values. It is confirmed by scanning electron microscope micrographs that pits could be observed on the surface of base metal but not on the surface of laser-welded alloy after potentiodynamic tests. An improvement of corrosion resistance of laser-welded NiTi alloy could be attributed to almost complete dissolution of inclusions upon laser welding.
Key words: NiTi alloy; shape memory alloy; laser welding; electrochemical behavior; polarization
1 Introduction
NiTi shape memory alloy has been widely used for medical devices, such as orthodontic wire, guide wire and stent. Recently, due to the fabrication of the micro medical devices and economic reasons, laser welding NiTi alloy to itself or other metals has drawn a lot of attention. Although many research projects have been conducted on this subject[14], the data are far from sufficient. Particularly, corrosion resistance of the laser-welded NiTi alloy is little known. HSU et al[5] used CO2 laser to join binary Ti50Ni50 alloy and investigated the corrosion characteristics of weld structures. The results revealed that after welding Ti50Ni50 had the satisfactory performance in 1.5 mol/L H2SO4 and HNO3 solutions. In contrast, the weld had worse corrosion resistance than base metal in artificial saliva due to the presence of intermetallic particles in the welded structure. Villermaux et al[6] and MAN et al[7] used laser to melt the surface of NiTi alloys. The authors reported that laser surface melting could cause some new phases in the melted layer but significantly improve the corrosion resistance of NiTi alloys. The reason for this was attributed to the increased amount of TiO2 and the higher ratio of Ti to Ni on the surface. From the aforementioned literature survey, it can be seen that the results about corrosion resistance of laser-welded NiTi alloy are contradictory.
Corrosion resistance of NiTi alloy is an important property for the long-term stability and biocompatibility of implants and medical devices. In addition to the release of ions in the physiological environment, the corrosion process will also result in the deterioration of dimensional parameters of the corroding body. Small and geometrically-complex devices can be susceptible to failure due to mechanically-assisted corrosion[8]. Thus, when choosing a method for fabricating medical devices, evaluation and optimization of the corrosion properties should be performed carefully. However, systemic study of corrosion resistance of laser welded NiTi alloy was scarcely carried out. It was the motivation of this study to investigate the corrosion characteristics of laser-welded NiTi(WM), while base metal(BM) was used as a reference.
2 Experimental
Ti-50.6%Ni plate, hot-rolled at 900℃ with thickness of 1.0 mm was used in this study. A Nd-YAG laser was used to perform the laser welding. Full-penetration bead-on-plate welds were made by proper selection of processing parameters. The bead width on the top surface was 2 mm. After welding, no postweld heat treatments were made on these welds.
The electrochemical experiments of specimens with or without welding were conducted in 0.9% NaCl solution(37℃). The electrochemical cell was a conventional three-electrode cell consisting of a working electrode, a saturated calomel reference electrode(SCE), and a platinum counter electrode. The specimens were mechanically polished using SiC paper in successive grades from 200 to 1500-grit prior to electrochemical tests. The exposed surface area of specimens to the solution was 10 mm×10 mm for the base metal(BM) and 10 mm×2 mm for the welds(WM). Three different electrochemical tests were executed: monitoring of the corrosion potential(φcorr) during 32 h, measurement of the polarization resistance(Rp) by applying a polarization from –10 to +10 mV versus φcorr with a scan rate of 0.1 mV/s, a cyclic polarization starting from 100 mV more active (more cathodic) than the open circuit potential (OCP) and increasing the anodic values at a constant rate of 1 mV/s to higher potentials. The scan direction was reversed until the protection potential(φprot) was achieved or the potential was 0 mV with respect to the OCP. Because of the well-known problem of reproducibility of corrosion results, four specimens were tested for each experiment. Surface morphologies of the specimens before and after the cyclic potentiodynamic tests were observed by using a scanning electron microscope (JSM-5600LV). The difference of inclusions distribution between WM and BM was observed using an electron probe microanalyzer (EPMA-1600).
3 Results and discussion
3.1 Corrosion potential measurements of specimens tested
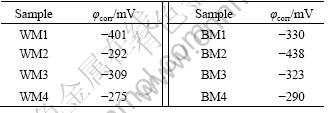
The aim of corrosion potential measurements is to understand the corrosion behavior of the specimens under equilibrated conditions in the simulated body environment. Corrosion potential measured at the end of 32 h period following immersion is considered pseudo- steady state potential and referred as φcorr. The time dependence of corrosion potential of laser welded NiTi(WM) in 0.9% NaCl solution is given in Fig.1. It can be seen that the φcorr values vary from sample to another, suggesting that the φcorr value is not reproducible. An overview of the measured φcorr values is given in Table 1.
Generally, φcorr value is indicative for the ionization tendency of materials in specific media. The ionization tendency decreases with increasing φcorr values[9]. As shown in Table 1, the φcorr values of BM are a little more negative than those of WM.

Fig.1 Time dependence of φcorr of laser-welded NiTi monitored during 32 h
Table 1 Overview of open circuit potentials recorded after 32 h
Although polished, a residual oxide layer remains on the surface of specimen. The dissolution of that oxide layer is indicated by a decrease in φcorr as the sample is dipped in the test solution. The dissolution reaction may be represented by
TiO2+2H2O= Ti4++4OH- (1)
The build-up of a passive layer of corrosion products is indicated by an asymptotical increase in φcorr. The formation reaction may be represented by
Ti+2H2O=TiO2+4H++4e (2)
In many cases, meta-stable pitting is observed on sudden changes in φcorr.
3.2 Linear polarization experiments
Generally, the corrosion rate of medical alloys is very low in the human body as the resting potentials of these alloys in isolated state are significantly lower than their breakdown potentials. Therefore, it is difficult to measure actual value of the corrosion rate. Linear polarization technique is a method of measuring low corrosion rate. The polarization resistance(Rp) is determined by
 (3)
(3)
The value of Jcorr can be calculated by
 (4)
(4)
where ba is the slope of anodic Tafel curve and the bc is the slope of cathodic Tafel curve.
Fig.2 shows Rp values calculated during the linear polarization experiments. It can be seen that the Rp value of WM is a little higher than that of BM. The higher the Rp value, the lower the corrosion current density (Jcorr). Fig.3 shows the calculated the corrosion current densities (Jcorr) for WM and BM. It can be seen that the Jcorr value WM is lower than that of BM. A lower Jcorr value is thought to indicate a better resistance to general corrosion.
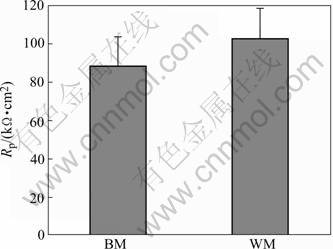
Fig.2 Polarization resistance calculated during linear polarization experiments

Fig.3 Corrosion current density calculated during linear polarization experiments
3.3 Cyclic potentiodynamic polarization experiments
The cyclic potentiodynamic polarization curves of WM and BM in 0.9% NaCl solution are shown in Fig.4. It can be seen that the breakdown potential (φpit) of the BM is about 638 mV. For the WM, it appears that no obvious passive film breakdown occurred. The corrosion resistance parameters obtained from the cyclic potentiodynamic polarization curves are shown in Table 2. SEM observations of BM and WM after cyclic potentiodynamic tests are shown in Fig.5. After the corrosion tests, pits could be observed on the surface of the BM. For the WM, pits could not be observed clearly at the same magnification.

Fig.4 Cyclic polarization curves of base metal and laser-welded NiTi
Table 2 Corrosion resistance parameters obtained from cyclic polarization curves


Fig.5 Surface morphologies of base metal(a) and laser-welded NiTi(b) after corrosion test
Most reports concerning corrosion resistance of NiTi alloy are mainly based on the results of potentiodynamic polarization curves[10-14]. In this study, corrosion resistance of the samples was characterized by the pitting corrosion potential(φpit) and the corrosion current density (Jcorr). It is well known that the pitting corrosion potential (φpit) represents the potential above which a preexisting passive film breaks down and pits originate on the free surface of the specimen. In other words, the φpit is a measure of the region where a surface layer is stable and corrosion resistant. Thus a higher or more positive value of φpit would indicate a large region of corrosion resistance[15]. The Jcorr value illustrates how much a material will be lost during the corrosion process. Hence the higher the Jcorr value, the more the material lost[8]. It can be seen from Fig.3 that the corrosion current density of WM is a little lower than that of BM. The mean φpit value and passive range for WM (φpit: >1 220 mV; passive range: >1 539 mV) were much higher than those for BM (φpit: 638 mV; passive range: 951 mV), as shown in Table 2. Furthermore, the difference between φpit and φprot, Δφ, is related to the pitting and crevice corrosion resistance of the material[16-18]. The lower the difference is, the more corrosion resistant the material is. In this study (Table 2), the BM had a much higher mean Δφ (about 655 mV) than the WM (<20 mV). These indicated that the WM was less susceptible to pitting and crevice corrosion than the BM.
Corrosion resistance of NiTi alloy relies on the presence of a passive film on the surface. VILLERMAUX points out that mechanically polished NiTi is autopassivated. VANDENKERCKHOVE points out that Nitinol is readily passivated even in de-aerated solutions, forming surface oxide layers with semi- conducting nature [10]. Several studies have demon- strated that this passive film predominantly consists of a titanium oxide layer (TiO2) similar to these formed on Ti alloys. This oxide layer serves two purposes as follows: 1) It increases the stability of the surface layer by protecting the bulk material from corrosion; 2) It creates a physical and chemical barrier against Ni oxidation by modifying the oxidation pathways of the Ni[13]. Various factors, such as surface finish quality, the amount of residues, the inhomogeneity of microstructures and defects, affect corrosion resistance of NiTi alloy[19, 20]. The surface quality is very important for NiTi alloy because it will strongly affect the nature of the passive film. One of the possible reasons for the better corrosion resistance of WM than that of BM in the above studies could be related to the surface topography, as many
authors have shown that the smoother surface possesses a better corrosion resistance[12]. From SEM, as shown in Fig.6(a), many black particles appear on the surface of base metal. Fig.6(b) shows that the content of carbon reduces obviously after laser welding. Therefore we can judge those black particles to be carbide inclusions. This is in agreement with that in Ref.[21]. However, the WM shows a clean SEM appearance with only a few carbide inclusions in it. The inclusion is a kind of defect inside the metallic material. Once the inclusions on the surface of metal are pulled-out due to the weak bonding, a lot of pores will be formed on the metallic surface. It is very difficult for a metal to form a uniform and continuous passive film on a porous surface. On the other hand, it is possible to form a uniform and continuous passive film on a clean metallic surface[7]. Shabalovskaya et al[21] reported that the surface concentration of foreign particles (inclusions and mechanically imbedded particles) might be an important factor in the control of NiTi corrosion resistance. The less the surface particles are, the higher the corrosion resistance is. Particles, such as NiTiOxCy and Ti3NiC, are easily soluble and anodic relative to NiTi base. Therefore, an improvement of corrosion resistance of laser-welded NiTi could be attributed to almost complete dissolution of carbide inclusions upon laser welding.

Fig.6 Difference of inclusions(a) and distribution of carbon (b) in base metal and laser-welded NiTi alloy
4 Conclusions
The electrochemical behaviors of laser welded Ti-50.6%Ni shape memory alloy have been investigated through a systematic comparison between the weld and the base metal. The electrochemical experiments reveal that laser welded NiTi alloy has lower corrosion current density (Jcorr), much higher pitting corrosion potentials and wider passive range than base metal. At the same time, the laser welded NiTi alloy has lower Δφ values than the base metal. In general, NiTi alloy is less susceptible to pitting and crevice corrosion after laser welding. The improvement of corrosion resistance of the laser-welded NiTi alloy is ascribed to the sharp decrease of carbides on the surface layer.
References
[1] Ogata Y, Takatugu M, Kunimasa T, Uenishi K, Kobayashi K F. Tensile strength and pseudo-elasticity of YAG laser spot melted Ti-Ni shape memory alloy wires [J]. Materials Transactions, 2004, 45(4): 1070-1076.
[2] HALL P C. Laser welding nitinol to stainless steel [A]. Proceedings of the International Conference on Shape Memory and Superelastic Technologies [C]. California SMST Society, Inc, 2003. 219-228.
[3] YAN Xiao-jun, YANG Da-zhi, LIU Li-ming. Microstructures and properties of laser spot-welded joint of superelastic NiTi alloy wire [J]. The Chinese Journal of Nonferrous Metals, 2005, 15(1): 19-23. (in Chinese)
[4] Tuissi A, Besseghini S, Ranucci T, Squatrito F, Pozzi M. Effect of Nd-YAG laser welding on the functional properties of the Ni-49.6at.%Ti [J]. Mater Sci Eng A, 1999, 273-275: 813-817.
[5] HSU Y T, WANG Y R, WU S K, Chen C. Effect of CO2 laser welding on the shape-memory and corrosion characteristics of TiNi alloys [J]. Metall Mater Trans A, 2001, 32A: 569-576.
[6] Villermaux F, Tabrizian M, Yahia L H, Meunier M, Prion D L. Excimer laser treatment of NiTi shape memory alloy biomaterials [J]. Applied Surface Science, 1997, 109/110: 62-66.
[7] MAN H C, CUI Z D, YUE T M. Corrosion properties of laser surface melted NiTi shape memory alloy [J]. Scripta Materialia, 2001, 45: 1447-1453.
[8] Venugopalan R, Trépanier C. Assessing the corrosion behaviour of Nitinol for minimally invasive device design [J]. Min Invas Ther & Allied Technol, 2000, 9(2): 67-74.
[9] Darabara M, Bourithis L, Zinelis S, Papadimitriou G D. Susceptibility to localized corrosion of stainless steel and NiTi endodontic instruments in irrigating solutions [J]. International Endodontic Journal, 2004, 37: 705-710.
[10] Shabalovskaya S A. Surface, corrosion and biocompatibility aspects of nitinol as an implant material [J]. Bio-Medical Materials and Engineering, 2002, 12: 69-109.
[11] Veldhuizen A G, Wever D J, De Vries J, Busscher H J, Uges D R A, Van Horn J R. Electrochemical and surface characterization of a nickel-titanium alloy [J]. Biomaterials, 1998, 19: 761-769.
[12] Rondelli G. Corrosion resistance tests on NiTi shape memory alloy [J]. Biomaterials, 1996, 17: 2003-2008.
[13] Rondelli G, Vicentini B. Localized corrosion behavior in simulated human body fluids of commercial Ni-Ti orthodontic wires [J]. Biomaterials, 1999, 20: 785-792.
[14] Rondelli G, Vicentini B. Evaluation by electrochemical tests of the passive film stability of equiatomic NiTi alloy also in presence of stress-induced martensite [J]. J Biomed Mater Res, 2000, 51: 47-54.
[15] Trépanier C, Tabrizian M, Yahia ? H, Bilodeau L, Piron D L. Effect of the modification of the oxide layer on NiTi stent corrosion resistance [J]. J Biomed Mater Res, 1998, 43: 433-440.
[16] HUANG H H. Corrosion resistance of stressed NiTi and stainless steel orthodontic wires in acid artificial saliva [J]. J Biomed Mater Res, 2003, 66(4): 829-839.
[17] Cissé O, Savadoga O, Wu M, Yahia ? H. Effect of surface treatment of NiTi alloy on its corrosion behavior in Hanks’ solution [J]. J Biomed Mater Res, 2002, 61(3): 339-345.
[18] Es-Souni M, Es-Souni M, Fischer-Brandies H. On the properties of two binary NiTi shape memory alloys. Effect of surface finish on the corrosion behaviour and in vitro biocompatibility [J]. Biomaterials, 2002, 23: 2887-2894.
[19] Es-Souni m, Es-Souni m, Fischer-Brandies h. On the properties of two binary NiTi shape memory alloys. Effects of surface finish on the corrosion behaviour and in vitro biocompatibility [J]. Biomaterials, 2002, 23: 2887-2894.
[20] Nakayama Y. In vivo measurement of anodic polarization of orthopaedic implant alloys: Comparative study of in vivo and in vitro experiments [J]. Biomaterials, 1989, 10: 420-424.
[21] Shabalovskaya S, Anderegg J, Rondelli G, Xiong J P. The effect of surface particulates on the corrosion resistance on nitinol wire [A]. Proceedings of the International Conference on shape memory and superelastic technologies [C]. California SMST Society, Inc, 2003. 399-408.
Foundation item: Project(2002AA326010) supported by the Hi-tech Research and Development Program of China; Project(50471066) supported by the National Natural Science Foundation of China
Corresponding author: YAN Xiao-jun; Tel: +86-411-84708441; E-mail: dreamto2008@sohu.com
(Edited by LONG Huai-zhong)