Trans. Nonferrous Met. Soc. China 26(2016) 1112-1117
Flotation of low-grade bauxite using organosilicon cationic collector and starch depressant
Xin-yang YU1, Hao-lin WANG1, Qiang-qiang WANG1, Bo FENG1, Hong ZHONG2
1. School of Resource and Environment Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 15 June 2015; accepted 25 February 2016
Abstract: The flotation of diaspore and three kinds of silicate minerals, including kaolinite, illite and pyrophyllite, using an organosilicon cationic surfactant (TAS101) as collector and starch as depressant was investigated. The results show that both diaspore and aluminosilicate minerals float readily with organosilicon cationic collector TAS101 at pH values of 4 to 10. Starch has a strong depression effect for diaspore in the alkaline pH region but has little influence on the flotation of aluminosilicate minerals. It is possible to separate diaspore from aluminosilicate minerals using the organosilicon cationic collector and starch depressant. Further studies of bauxite ore flotation were also conducted, and the reverse flotation separation process was adopted. The concentrates with the mass ratio of Al2O3 to SiO2 of 9.58 and Al2O3 recovery of 83.34% are obtained from natural bauxite ore with the mass ratio of Al2O3 to SiO2 of 6.1 at pH value of 11 using the organosilicon cationic collector and starch depressant.
Key words: bauxite; reverse flotation; organosilicon cationic collector; starch depressant
1 Introduction
China has abundant bauxite ore resources, however, more than 98% of the bauxite ores are the diasporic type with the characteristics of complex mineral composition, low A/S (mass ratio of Al2O3 to SiO2) and difficulty to process. For diasporic bauxite with A/S less than 8, a sintering process or a combination of sintering and Bayer process can be used [1]. However, the sintering process caused environmental damage and wasted energy. Therefore, it needs to increase the A/S of the diasporic ores by flotation method and get a concentrate that can be processed directly by the Bayer process [2,3].
The gangue minerals in bauxites are mainly kaolinite, pyrophyllite and illite [4]. Direct flotation has been shown to be an efficient method for the desilicating of diasporic bauxite [5,6]. However, its disadvantages, such as the difficulty in dewatering concentrate and its high reagent consumption, have restricted its wide application in industry. Therefore, reverse flotation for diasporic-bauxite ore desilicating has been studied [7-9].
In recent years, many flotation collectors for aluminosilicate minerals in the reverse flotation process, notably, quaternary ammonium salts, N-(2-aminoethyl)-dodecanamide, N-(2-aminoethyl)-1-naphthalene-acet- amide, N-dodecyl-1,3-diaminopropanes, γ-alkyl- propylamines, dedecylguannidine sulfate, Gemini quaternary ammonium, dodecyl tertiary amines and alkylguanidine, have been reported effective in collecting aluminosilicate minerals [10-17]. However, most of these collectors are not used in industry due to their deficiencies, such as the excessive amount of foams, high cost, high reagent consumption and low selectivity.
Cationic organosilicon primary ammonium, a new surfactant with bioactivity, is now widely used in the fields of fabric finishing, chemical engineering, pharmaceuticals and agricultural chemicals. However, there is little information regarding the use of this surfactant for mineral flotation. Therefore, in this work, the systematical reverse flotation separation experiments of bauxite ores were conducted in the presence of an organosilicon cationic collector and starch depressant.
2 Experimental
2.1 Materials and reagents
The diaspore, kaolinite, pyrophyllite and illite materials used in the present study were obtained from Xiaoguan of Henan Province, Jiaxian of Henan Province, Qingtian of Zhejiang Province and Ouhai of Zhejiang Province of China, respectively. They were handpicked and then crushed and ground to less than 0.074 mm in a porcelain mill. The purities of the materials are higher than 95% based on mineralogical analysis, X-ray diffraction (XRD) and chemical analysis.
Diasporic-bauxite ore was obtained from Henan Province, China. This sample with Al2O3 head assay of 64.32% and SiO2 head assay of 10.52% was mainly composed of 65.22% diaspore, 9.8% kaolinite, 6.2% illite, 4.7% pyrophyllite and 0.6% chlorite detected by the XRD analysis and mineralogical analysis, and the A/S of the bauxite was 6.11. The elements analysis results of the bauxite are shown in Table 1.
Table 1 Elements analysis of bauxite (mass fraction, %)
NaCO3, NaOH and HCl were used as pH modifiers and distilled water was used in all experiments. The new collector of organosilicon primary amine (TAS101) has a molecular structural formula as follows:

where R represents (CH2)3NH(CH2)2.
2.2 Methods
2.2.1 Micro-flotation
Flotation tests were carried out with an XFG5-35 flotation machine with effective cell volume of 40 mL, at the impeller speed fixed at 1800 r/min. 3.0 g mineral samples and suitable amount of distilled water were added. The pH values were adjusted with 0.01 mol/L hydrochloric acid solution and 0.01 mol/L sodium hydroxide solution. After adding the desired amount of collectors, the suspension was agitated for 3 min, and then the pH value was measured. The flotation was sustained for 5 min. The concentrates and tailings were weighed separately after filtration and drying, and the recovery was calculated. The A/S was determined by silicon-molybdenum blue colorimetry.
2.2.2 Bench scale flotation
The ore samples were crushed to -2 mm, riffled into representative samples of 500 g. For each flotation experiment, the samples were ground in a mild steel rod mill. The flotation tests were performed in an XFD-63 flotation cell (self aeration) whose volume for flotation was 1.5 L using an agitation speed of 1800 r/min. The solid density in the flotation cell was 50% by mass. During the conditioning, depressant, collector and frother were respectively added and conditioned for 5 min to allow reagent adsorption. After the conditioning, the flotation started with the injection of air in the flotation cell, and the air flow rate was kept at 0.1 Nm3/h monitored with a flow meter. Flotation was performed for 12 min and the concentrates was collected.
2.2.3 Zeta potential measurements
Isoelectric points (IEP) of mineral samples were determined by measuring the electrophoretic mobility of aqueous dispersions as a function of pH value in a zeta potential meter. For theses measurements, 30 mg samples were added into 1 mmol/L KNO3 solution and ultrasonicated for 30 min, magnetically stirred for 10 min and the pH value was adjusted using HCl or KOH. The zeta potential of samples was then measured using a zeta plus potential meter.
3 Results and discussion
3.1 Role of organosilicon cationic collector and starch in separation of diaspore from aluminosilicates
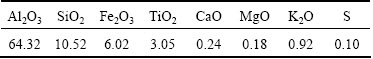
The effect of starch dosage on the flotation of diaspore and aluminosilicates with 2×10-4 mol/L collectors is shown in Fig. 1. It can be seen from Fig.1 that the flotation of four kinds of minerals is all affected by the presence of starch, but the starch is more effective for the depression of diaspore than that of kaolinite, illite and pyrophyllite. With the increase of the starch dosage, the floatability of diaspore shows an obvious decrease. When the starch dosage reaches 450 mg/L, the flotation selectivity is basically formed at pH value of 10 with TAS101 as collector.
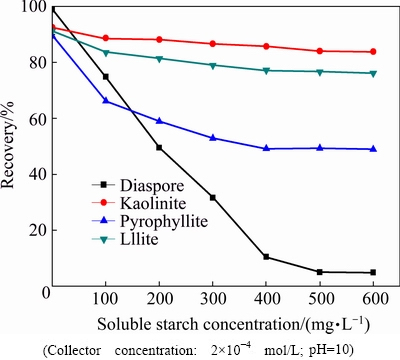
Fig. 1 Effect of starch dosage on flotation of diaspore and aluminosilicates
Figure 2 presents the floatability of minerals as a function of pH value in the absence and presence of depressant. In the case of diaspore, the recovery is high when depressant is not added and it keeps at about 95% from pH values of 4 to 8. With the addition of starch, the recovery of diaspore decreases sharply which is less than 10% at all the pH values. However, for other three aluminosilicate minerals, the recovery only slightly decreases in the presence of starch, to a much less extent than the decrease of diaspore.

Fig. 2 Effect of pH value on flotation of diaspore and aluminosilicates in absence (a) and presence (b) of starch
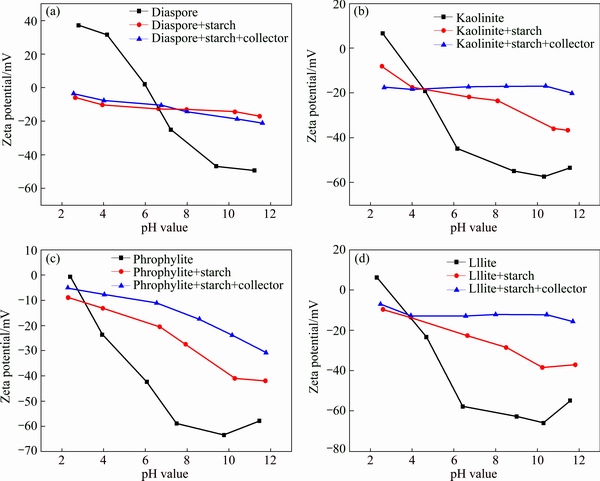
Fig. 3 Effect of collector and starch on zeta potential of diaspore and aluminosilicates at different pH values
The effect of collector and starch on the zeta potentials of diaspore and aluminosilicates at different pH values is shown in Fig. 3. The result shows that the IEPs of disapore, illite, pyrophyllite and kaolinite are 6.2, 2.4, 3.0 and 3.4, respectively. With the addition of starch, the zeta potentials of four minerals show pronounced shift towards positive direction when the pH value is above the IEP, indicating that the starch molecules have been adsorbed onto those minerals surfaces. The effect of collector on the zeta potentials of diaspore and aluminosilicates with the pre-adsorption of starch is also shown in Fig. 3. For aluminosilicates, the zeta potentials still show shift towards positive direction, illustrating that collector can also adsorb on the aluminosilicates particles in the presence of strarch. However, it is not the same for the diaspore. It can be seen that with the addition of collector, the zeta potential of diaspore never changed.

Fig. 4 Flotation flowsheet of roughing of bauxite
Fig. 5 Effect of grinding time on flotation of bauxite

Fig. 6 Effect of collector dosage on flotation of bauxite
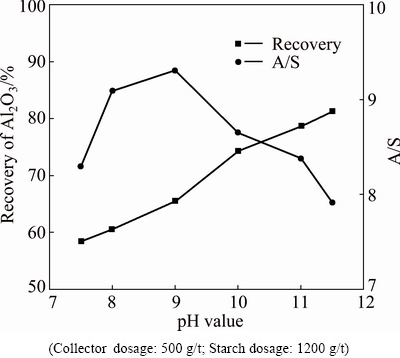
Fig. 7 Effect of pH value on flotation of bauxite

Fig. 8 Effect of starch dosage on flotation of bauxite
3.2 Flotation behavior of bauxite ore sample
The flotation behavior of a low-grade bauxite ore was studied. The flotation separation flowsheet is shown in Fig. 4, and the results are shown in Figs. 5 to 8.
As most minerals are finely disseminated and intimately associated with the gangue minerals, they must be initially liberated before separation. The effective liberation of bauxite minerals is the foundation of improving flotation performance. Thus, the effect of grinding time on bauxite mineral flotation was investigated and the results are shown in Fig. 5. The grinding pulp density is about 66%. The grinding time increases from 10 to 15 min. According to the result shown in Fig. 5, increasing the grinding time from 10 to 13 min increases the A/S from 6.7 to 8.4 and decreases the recovery from 89% to 79%. However, the grinding time longer than 13 min produces little increase of A/S but still decreases the recovery. Thus, 13 min is considered as the optimum grinding time. When the grinding time is 13 min, the fraction of particles below 0.074 mm approximately accounts for 81% of the feed.
The effect of collector dosage on the flotation of bauxite was studied and the results are shown in Fig. 6. An increase of the collector dosage from 350 to 500 g/t increases the A/S of concentrates from 7.1 to 8.4 and which then never changes with the further increase of collector dosage from 500 to 600 g/t. On the other hand, the recovery of concentrates decreases from 85% to 79% with the increase of collector dosage from 350 to 500 g/t. Hence, the collector dosage of 500 g/t was maintained as it gives the best flotation performance.
The effect of pH value on bauxite flotation was studied and the results are shown in Fig. 7. It can be seen from Fig. 7 that increasing the pH value from 7.5 to 11 improves the recovery of the concentrate greatly and the A/S first increases and then decreases. According to Fig. 7, the optimum flotation pH value is 11.
The effect of starch dosage on the flotation of bauxite was studied and the results are shown in Fig. 8. An increase of the starch dosage from 400 to 1200 g/t increases the A/S of concentrate from 5.6 to 8.4, which then never changes with the further increase of starch dosage. On the other hand, the recovery of concentrate decreases from 88% to 74% with the increase of starch dosage from 400 to 1400 g/t. Hence, the starch dosage of 1200 g/t was used as it gives the best flotation performance.
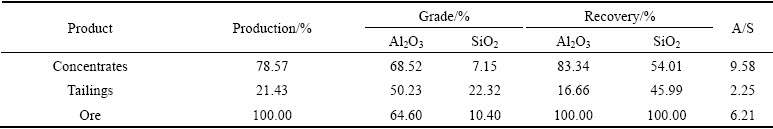
The flotation separation flowsheet of closed-circuit is shown in Fig. 9 and the results are listed in Table 2. The bauxite concentrates with A/S of 9.58 and Al2O3 recovery of 83.34% are obtained from the low grade bauxite ore (mass ratio of Al to Si of 6.21) at pH value of about 11, when the dosages of Na2CO3, collector and starch are 600, 500 and 1200 g/t, respectively.

Fig. 9 Flowsheet of closed-circuit of bauxite reverse flotation
Table 2 Results of closed-circuit of bauxite reverse flotation
TAS101 is an organosilicon cationic collector. Compared with other bauxite flotation collectors, TAS101 produced small amount of flotation concentrates which is in favour of the subsequent filtration and dehydration of flotation foam product. Also, this reagent is affected little by the slime and a deslime operation does not need to be included in the flotation flowsheet, thus the flowsheet is simple.
4 Conclusions
1) Both diaspore and aluminosilicate minerals float readily with organosilicon cationic collector TAS101 at pH values of 4 to 11.
2) Starch has a strong depression effect for diaspore in the alkaline pH region but has little effect on the flotation of aluminosilicate minerals.
3) It is possible to separate silicate mineral from diaspore by reverse flotation using TAS101 as collector and starch as depressant at pH value of 11. The concentrates with A/S of 9.85 and Al2O3 recovery of 83.34% are obtained from low grade bauxite ore (A/S is 6.21).
References
[1] SMITH P. The processing of high silica bauxites—Review of existing and potential processes [J]. Hydrometallurgy, 2009, 98(1): 162-176.
[2] PAPANASTASSIOU D, CSOKE B, SOLYMAR K. Improved preparation of the greek diasporic bauxite for Bayer-process [C]//Light Metals: Proceedings of TMS Annual Meeting. Washington: TMS, 2002: 67-74.
[3] RAO D S, DAS B. Characterization and beneficiation studies of a low grade bauxite ore [J]. Journal of the Institution of Engineers, 2014, 95(2): 81-93.
[4] ZHONG Hong, LIU Guang-yi, XIA Liu-yin, LU Yi-ping, HU Yue-hua, ZHAO Sheng-gui, YU Xin-yang. Flotation separation of diaspore from kaolinite, pyrophylite and illite using three cationic collector [J]. Minerals Engineering, 2008, 21(12-14): 1055-1061.
[5] HUANG Gen, ZHOU Chang-chun, LIU Jiong-tian. Effects of different factors during the de-silication of diaspore by direct flotation [J].International Journal of Mining Science and Technology,2012, 22(3): 341-344.
[6] LU Yi-ping, ZHANG Guo-fan, FENG Qi-ming, OU Le-ming. A novel collector RL for flotation of bauxite [J].Journal of Central South University of Technology, 2002, 9(1): 21-24.
[7] LIU Guang-yi, ZHONG Hong, HU Yue-hua, ZHAO Shin-min, XIA Jin-lan. The role of cationic polyacrylamide in the reverse flotation of diasporic bauxite [J]. Minerals Engineering, 2007, 20(13), 1191-1199.
[8] HU Yue-hua. Progress in flotation de-silica [J]. Transactions of Nonferrous Metals Society of China, 2003, 13(3): 656-662.
[9] HU Yue-hua, JIANG Hao, WANG Dian-zuo. Electrokinetic behavior and flotation of kaolinite in CTAB solution [J]. Minerals Engineering, 2003, 16(11): 1221-1223.
[10] CAO Xue-feng, HU Yue-hua, XU Jing. Synthesis of γ-alkoxy- propylamines and their collecting properties on aluminosilicate minerals [J]. Journal of Central South University of Technology, 2004, 11(3): 280-285.
[11] GUAN Feng, ZHONG Hong, LIU Guang-yi. Flotation of aluminosilicate minerals using alkylguanidine collectors [J]. Transactions of Nonferrous Metals Society of China, 2009, 19(1): 228-234.
[12] LIU Chang-miao, HU Yue-hua, CAO Xue-feng. Substituent effects in kaolinite flotation using dodecyl tertiary amines [J]. Mineral Engineering, 2009, 22(9-10): 849-852.
[13] XIA Liu-yin, ZHONG Hong, LIU Guang-yi. Flotation separation of the aluminosilicates from diaspore by a Gemini cationic collector [J]. International Journal of Mineral Processing, 2009, 92(1): 74-83.
[14] XIA Liu-yin, ZHONG Hong, LIU Guang-yi. Flotation techniques for separation of diaspore from bauxite using Gemini collector and starch depressant [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(3): 495-501.
[15] ZHAO Shi-min, ZHONG Hong, LIU Guang-yi. Effect of quaternary ammonium salts on flotation behavior of aluminosilicate minerals [J]. Journal of Central South University of Technology, 2007, 14(4): 500-503.
[16] ZHAO Shi-min, WANG Dian-zuo, HU Yue-hua, XU Jing. Flotation of aluminosilicates using N-(2-aminoethyl)-1-naphthaleneacet- amide [J]. Mineral Engineering, 2003, 16(10): 1031-1033.
[17] ZHAO Shi-min, WANG Dian-zuo, HU Yue-hua, XU Jing. The flotation behaviour of N-(3-aminopropyl)-dodecanamide on three aluminosilicates [J]. Mineral Engineering, 2003, 16(12): 1391-1395.
有机硅阳离子捕收剂和淀粉抑制剂分选铝土矿
余新阳1,王浩林1,王强强1,冯 博1,钟 宏2
1. 江西理工大学 资源与环境工程学院,赣州 341000;2. 中南大学 化学与化工学院,长沙 410083
摘 要:以有机硅阳离子表面活性剂TAS101为捕收剂,淀粉为抑制剂,研究一水硬铝石和3种硅酸盐矿物高岭石、叶腊石和伊利石的浮选行为。结果表明,在pH值范围为4-10时,TAS101对一水硬铝石和3种硅酸盐矿物均有较强的捕收能力。在碱性pH条件下,淀粉对一水硬铝石有较强的抑制效果但不会影响硅酸盐矿物的浮选。使用淀粉作为抑制剂,TAS101作为捕收剂能够实现一水硬铝石和3种硅酸盐脉石的浮选分离。对铝土矿浮选闭路试验进行进一步研究,采用反浮选流程,使用淀粉作为抑制剂,TAS101作为捕收剂,在pH=11条件下进行浮选,当原矿Al2O3与SiO2的质量比为6.1时,可以获得Al2O3与SiO2的质量比为 9.58,Al2O3回收率为83.34%的精矿。
关键词:铝土矿;反浮选;有机硅阳离子捕收剂;淀粉抑制剂
(Edited by Mu-lan QIN)
Foundation item: Project (51304085) supported by the National Natural Science Foundation of China; Project (GJJ12363) supported by the Education Department of Jiangxi Province, China; Project (20142BAB216021) supported by the Natural Science Foundation of Jiangxi Province, China
Corresponding author: Hong ZHONG; Tel/Fax: +86-731-88830654; E-mail: zhongh@csu.edu.cn
DOI: 10.1016/S1003-6326(16)64209-7