CeCl3和苯并三唑对自然时效和人工时效AA2024铝合金耐蚀性的协同效应
来源期刊:中国有色金属学报(英文版)2020年第6期
论文作者:Bore JEGDIC Biljana BOBIC Bojana RADOJKOVIC Jovanka KOVACINA Dunja MARUNKIC
文章页码:1478 - 1490
关键词:铝合金;点蚀;缓蚀剂;铈;苯并三唑
Key words:aluminium alloy; pitting corrosion; corrosion inhibitor; cerium; benzotriazole
摘 要:分析自然时效和人工时效AA2024铝合金在0.5 mol/dm3 NaCl溶液中的腐蚀行为,溶液中分别加入环境友好的缓蚀剂:10 mmol/dm3 CeCl3,10 mmol/dm3 BTA和5 mmol/dm3 CeCl3 + 5 mmol/dm3 BTA混合缓蚀剂。本研究的目的是确定混合缓蚀剂的协同效应水平,并解释这种效应的本质。采用电化学阻抗谱(EIS)研究缓蚀剂层的腐蚀性能,通过动电位极化试验研究其抑制点蚀形成和点蚀生长的能力。扫描电子显微镜(SEM/EDS)的结果表明,自然时效铝合金中形成的点蚀尺寸小于人工时效合金中形成的点蚀尺寸。在自然时效合金测试的96 h内以及在人工时效合金测试的后期,均观察到混合缓蚀剂对腐蚀性能的协同效应,但没有发现混合缓蚀剂对点蚀形成和点蚀生长的协同效应。
Abstract: The corrosion behaviours of naturally aged and artificially aged AA2024 aluminium alloys in 0.5 mol/dm3 NaCl solution in the presence of environmentally friendly corrosion inhibitors of 10 mmol/dm3 CeCl3, 10 mmol/dm3 BTA and the inhibitor mixture (5 mmol/dm3 CeCl3 + 5 mmol/dm3 BTA) were analyzed. The goal of this work was to determine the level of the synergistic effect of the inhibitor mixture and to explain the nature of this effect. Corrosion properties of the inhibitor layer were studied using the electrochemical impedance spectroscopy (EIS), while the resistance to pit formation and pit growth was studied by applying potentiodynamic polarisation tests. The results of scanning electron microscopy (SEM/EDS) showed that the size of pits formed in naturally aged aluminium alloy was smaller than that formed in artificially aged alloy. The synergistic effect of the inhibitor mixture on corrosion properties of naturally aged alloy was observed throughout 96 h, and in later phases of testing of artificially aged alloy. The synergistic effect of the inhibitor mixture was not noticed on pit formation and pit growth.
Trans. Nonferrous Met. Soc. China 30(2020) 1478-1490
Bore JEGDIC, Biljana BOBIC, Bojana RADOJKOVIC, Jovanka KOVACINA, Dunja MARUNKIC
University of Belgrade, Institute for Chemistry, Technology and Metallurgy, Belgrade, Serbia
Received 17 September 2019; accepted 25 March 2020
Abstract: The corrosion behaviours of naturally aged and artificially aged AA2024 aluminium alloys in 0.5 mol/dm3 NaCl solution in the presence of environmentally friendly corrosion inhibitors of 10 mmol/dm3 CeCl3, 10 mmol/dm3 BTA and the inhibitor mixture (5 mmol/dm3 CeCl3 + 5 mmol/dm3 BTA) were analyzed. The goal of this work was to determine the level of the synergistic effect of the inhibitor mixture and to explain the nature of this effect. Corrosion properties of the inhibitor layer were studied using the electrochemical impedance spectroscopy (EIS), while the resistance to pit formation and pit growth was studied by applying potentiodynamic polarisation tests. The results of scanning electron microscopy (SEM/EDS) showed that the size of pits formed in naturally aged aluminium alloy was smaller than that formed in artificially aged alloy. The synergistic effect of the inhibitor mixture on corrosion properties of naturally aged alloy was observed throughout 96 h, and in later phases of testing of artificially aged alloy. The synergistic effect of the inhibitor mixture was not noticed on pit formation and pit growth.
Key words: aluminium alloy; pitting corrosion; corrosion inhibitor; cerium; benzotriazole
1 Introduction
The aluminium alloys of 2xxx series (Al-Cu- Mg) are high strength alloys with a wide application in the airline industry and various industries. The reason for this is a favourable ratio of the strength to the density of these alloys [1]. These alloys in chloride solutions often experience localized forms of corrosion, such as pitting corrosion, intergranular corrosion, exfoliation corrosion, and stress corrosion cracking [2,3]. The microstructure and corrosion characteristics of AA2024 alloy and other aluminium alloys series 2xxx were analyzed in detail [1-11]. AA2024 (Al-Cu-Mg) aluminium alloy is commonly used after precipitation hardening at 190 °C (artificially aged alloy). This alloy is also commercially used after natural aging at room temperature (naturally aged alloy).
In recent decades, a great deal of attention has been dedicated to finding ecologically acceptable corrosion inhibitors for aluminium alloys. A large number of papers are devoted to the study of inhibitory effect of cerium and cerium salts in the case of AA2024 aluminium alloy [12-23]. The inhibitory effect of cerium in aluminium alloys is explained by the formation of insoluble oxides and/or hydroxides on the surface of cathodic inclusions and in a lower extent on the aluminium alloy matrix. On the cerium oxide and hydroxide, surface cathodic reaction of oxygen reduction becomes much slower, and consequently overall corrosion rate is significantly reduced.
Benzotriazole (BTA) is known as an effective corrosion inhibitor of copper and its alloys [24-26]. BTA belongs to a group of environmentally acceptable corrosion inhibitors. It has been found that BTA in chloride solutions has an inhibitory effect on AA2024 aluminium alloy [27-29]. BTA molecule is deprotonated (in the presence of formed OH- anions), and a complex with Cu+ ion is formed (CuBTA). Benzotriazole- based film is formed over the entire surface of AA2024 alloy, on intermetallic particles (IMPs) which contain copper, and on the alloy matrix which also contains copper. The film formation is favoured in the presence of chloride ions in the inhibitor-containing solution [29]. COELHO et al [30] considered that the inhibitory effect of BTA is due to the formation of a BTA layer over the passive layer of AA2024 alloy. The BTA layer slows down both the anodic and the cathodic reaction of the corrosion process.
It has been shown [31] that the inhibitor mixture (CeCl3+BTA) has a synergistic effect on the coupled aluminium/copper metals in NaCl solution. The results of the EIS tests showed a much lower synergistic effect of the inhibitors (CeCl3+BTA) in the case of AA2024 aluminium alloy than in the coupled Al/Cu metals [30]. According to Ref. [30], the CuBTA layer is formed over the layer of Ce-oxide/hydroxide.
AA2024 aluminium alloy has a complex microstructure. In addition to the strengthening precipitates of Al2CuMg (S-phase) and Al2Cu (θ-phase), the alloy contains intermetallic particles. Intermetallic particles, such as Al2CuMg, Al2Cu, Al(FeMnSi), Al3Fe, and Al7Cu2Fe, which are present in AA2024 alloy, have been studied in Ref. [32]. Due to the presence of elements of various electronegativities, the corrosion and electrochemical behaviours of these particles are very complex. The electrochemical and corrosion properties of intermetallic particles in AA2024 aluminium alloy were analyzed [32-40].
The greatest attention of researchers was devoted to the examination of the electrochemical properties of intermetallic particles Al2CuMg (S-phase). Al2CuMg particles are anodic at the start of the examination in NaCl solution, but due to a selective dissolution of Mg and Al, the particles become enriched in copper and thus highly cathodic. Clusters of Al2Cu and Al2CuMg particles are the most common sites of localized corrosion in AA2024 aluminium alloy [33]. The electrochemical behaviour of intermetallic particles in AA2024 aluminium alloy was analyzed in chloride solutions in the presence of CeCl3 [41-48].
Few papers have been published discussing the synergistic effect of CeCl3 and BTA on the corrosion behaviour of AA2024 alloy [30,31]. However, none of the studies considered the influence of the microstructure formed by natural and artificial aging of the AA2024 alloy on the synergetic effect of these inhibitors. Also, there have been no papers dealing with the synergistic effect of the aforementioned inhibitors to the resistance on pit formation and pit growth of the AA2024 alloy in different thermal states.
The aim of this work was to analyze the corrosion behaviour of AA2024 alloy in the chloride solution in the presence of environmentally acceptable corrosion inhibitors (CeCl3 and BTA). The influence of AA2024 alloy tempers (naturally aged and artificially aged) to resistance to general corrosion, and the resistance to pit formation and pit growth was also considered. The goal was also to determine the level of the synergistic effect of the inhibitor mixture (CeCl3+BTA) and to explain the nature of the synergistic effect.
2 Experimental
2.1 Material
The AA2024 aluminium alloy samples were made of an extruded 25 mm-diameter rod. The thickness of the samples was 6 mm. Table 1 gives the chemical composition of the AA2024 aluminium alloy.
Table 1 Chemical composition of AA2024 alloy (wt.%)

After annealing at 495 °C for 1 h, the samples were quenched in water and naturally aged for minimum 7 days (naturally aged alloy, NA). After natural aging, some of the samples were annealed at 190 °C for 16 h (artificially aged alloy, AA). Prior to testing, the samples were ground with abrasive paper of increasing fineness, to a fineness of 1000 grit, degreased with ethanol, washed with distilled water, and dried. Until the beginning of the tests, the samples were kept in a desiccator.
2.2 Methods
2.2.1 SEM/EDS analysis
The morphology of pits observed on the naturally and artificially aged samples after cyclic polarization tests in NaCl solution in the presence of the inhibitor mixture (CeCl3+BTA) was analyzed by a scanning electron microscope (SEM, JEOL JSM-6610LV), equipped with energy dispersive spectroscopy (EDS). The composition of intermetallic particles on the AA2024 alloy surface was determined using the SEM/EDS method. Before the analysis of the composition of intermetallic compounds, the samples were ground, polished, and then ultrasonically degreased in ethanol.
2.2.2 Electrochemical impedance spectroscopy (EIS) measurement
The electrochemical impedance spectroscopy measurements were performed in a 0.5 mol/dm3 NaCl solution under ambient conditions, with addition of the corrosion inhibitors (5 mmol/dm3 CeCl3 + 5 mmol/dm3 BTA). The measurements were performed by a GAMRY Reference 1010E Potentiostat/Galvanostat/ZRA. A classic three- electrode cell arrangement was used. The working electrode was AA2024 alloy (1 cm2 test surface). The counter electrode was a Pt mesh. The reference electrode was a saturated calomel electrode (SCE). The measurements were performed at the corrosion potential (φcorr) over a frequency range from 100 kHz to 0.1 Hz using a 10 mV amplitude of sinusoidal voltage. Experiments were repeated three times and characteristic results were obtained.
2.2.3 Potentiodynamic polarization test
Pitting corrosion tests were performed with the same electrochemical instrument as in the EIS measurements. Scanning with a sweep rate of 1 mV/s was applied after establishing the constant φcorr (up to 60 min). The potentiodynamic polarization started from the cathodic potential -0.250 V versus the corrosion potential (φcorr) and scanning was reversed when a current density of 500 μA/cm2 was reached. The scanning was terminated after reaching the initial corrosion potential (φcorr). The pitting potential (φpit) was determined with the potential at which the current increases by an order of magnitude upon a 10 mV potential change, in accordance with Ref. [49]. Charge density (q), defined as a charge passed during the reverse scan, was determined by the analysis of the polarization curves. Experiments were repeated three times and characteristic results were obtained.
3 Results and discussion
3.1 Corrosion characteristics of AA2024 alloy in the presence of inhibitor mixture (CeCl3+ BTA)
The corrosion characteristics of AA2024 alloy were tested on naturally and artificially aged samples. The microstructure of the naturally aged AA2024 alloy was formed at room temperature. Formed GP zones have a significant influence on the strength of the alloy. The GP zones are solute atoms collected into clusters, and they have the same crystal structure as the solid solution. The GP zones are of several nanometers in size [6].
During quenching of naturally aged AA2024 alloy, coarse intermetallic particles enriched in copper precipitate along grain boundaries and copper-depleted zones are formed close to grain boundaries. The solid solution inside the grains has a significantly higher copper concentration and a more positive potential relative to the narrow zones near the grain boundaries. Due to the difference in the potential between the grains and the zones along the grain boundaries, intergranular corrosion often occurs, i.e. the dissolution of the zones along the grain boundaries. Naturally aged AA2024 alloy is also susceptible to stress corrosion cracking. The mechanism of stress corrosion cracking of AA2024 alloy is much more complex than that of intergranular corrosion and depends on the properties of hardening precipitates, types of dislocation motion, etc.
In the case of artificially aged AA2024 alloy, precipitation hardening of AA2024 alloy at 190 °C occurs with formation of coherent GP zones. These zones are transformed to the semi-coherent S'-phase, and later to the coherent stable S-phase. Often, the θ-phase is precipitated in a smaller amount. During artificial aging, there is a decrease in the copper concentration inside grains due to the formation of coarse precipitates enriched in copper inside grains. The copper concentrations in the solid solution inside grains and in the narrow zones near the grain boundaries are equalized. As a result, galvanic coupling between grains and grain boundaries is minimized. For the artificially aged AA2024 alloy there is a lower copper concentration in the solid solution than for the naturally aged alloy. The artificially aged AA2024 alloy is more resistant to intergranular corrosion, exfoliation corrosion, and stress corrosion cracking than naturally aged alloy.
In this work, the resistance to general corrosion and pitting corrosion of naturally and artificially aged AA2024 alloys was examined in NaCl solution and in the presence of CeCl3 and BTA inhibitors. In our previous papers [50,51] it was shown that the naturally aged alloy in NaCl solution without inhibitors, is more resistant to the general corrosion and pitting corrosion than the artificially aged alloy. Also, CeCl3 and BTA (added individually to NaCl solution) have been shown to be more effective corrosion inhibitors of the naturally aged alloy than the artificially aged alloy.
Figure 1 shows the EIS diagrams for the naturally and artificially aged AA2024 alloys after testing in NaCl solution without inhibitor and in the presence of the inhibitor mixture (CeCl3+BTA). As it can be seen in Fig. 1(a), for the naturally aged AA2024 alloy the resistance of passive oxide film (Rf) in NaCl without inhibitor is very low (about 4.4 kΩ·cm2). After 1 h testing in the presence of the inhibitor mixture (CeCl3+BTA), an extremely high resistance of inhibitor film (Rf =220.1 kΩ·cm2) was obtained. After 48 and 96 h, the Rf value decreased to a certain extent in comparison to that after 1 h.
Figure 1(b) shows the EIS results for the artificially aged AA2024 alloy in NaCl solution and in the presence of the inhibitors (CeCl3+BTA). As it can be seen that, the value of Rf in NaCl solution without inhibitor is low (about 2.8 kΩ·cm2). The value of Rf is lower than that for the naturally aged alloy in NaCl solution due to a lower copper concentration in the solid solution of the artificially aged AA2024 alloy. Significantly high Rf values are obtained in the presence of the inhibitors. After 1 h, the value of Rf=50.6 kΩ·cm2 and after 96 h, Rf=101.3 kΩ·cm2. The results of the EIS measurements are given in Table 2.
Figure 2 shows the equivalent electric circuit (EEC) used to fit the results of the impedance measurements (EIS). The EEC is composed of electrolyte resistance (Re), constant phase element for the inhibitive and/or passive oxide film (CPEf), resistance of the inhibitive and/or passive oxide film (Rf), constant phase element (CPEdl) for electrochemical double layer and charge transfer resistance (Rct). A time constant specific to the inhibitive film could not be distinguished from the time constant related to the passive oxide film. During EIS testing, a significant scattering of results occurred at low frequencies, so that fitting of test results (Rct and CPEdl) at low frequencies was not possible.
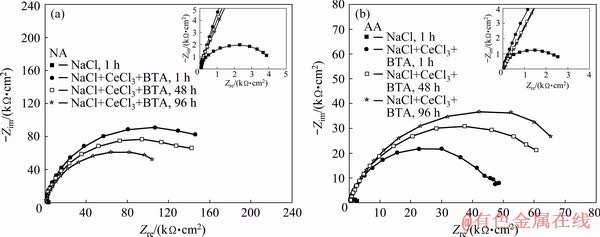
Fig. 1 Nyquist diagrams for naturally aged (NA) (a) and artificially aged (AA) (b) aluminium alloys
Table 2 Results of EIS test for naturally and artificially aged aluminium alloys


Fig. 2 Equivalent electric circuit for AA2024 alloy in NaCl solution, and in the presence of inhibitor mixture (CeCl3+BTA)
The testing results for naturally aged AA2024 alloy in the presence of the inhibitors (CeCl3+BTA) in NaCl solution are similar to those for testing AA2024 alloy in the presence of CeCl3. However, in the presence of the inhibitors (CeCl3+BTA), a higher inhibitor film resistance (Rf) was obtained than that in the presence of CeCl3. This indicates the synergistic effect of the inhibitor mixture (CeCl3+BTA). It can be assumed that cerium from the inhibitor mixture (CeCl3+BTA) has a dominant influence on the corrosion resistance of naturally aged alloy.
Cerium-oxides/hydroxides are primarily precipitated on the cathodic inclusions. Cerium is also precipitated on the naturally aged AA2024 alloy matrix due to a more positive corrosion potential (more precisely, Volta potential) of the naturally aged alloy compared to a corrosion potential of the artificially aged alloy. The results of the SEM/EDS analysis confirmed this observation (Fig. 3 and Table 3). Spectra 2 and 3 refer to the aluminium matrix, while spectrum 1 refers to the aluminium matrix and IMPs (see Fig. 3(a)). The average cerium content on the matrix and IMPs (spectrum 1) is higher than that on the matrix alone. Spectrum 1 comprises a relatively small number of IMPs (Fig. 3(a)), so that the cerium content on IMPs is higher than that listed in Table 3. The cerium content on intermetallic inclusions (Fig. 3(b)) is significantly higher in the centre of IMPs than that at some distance from the centre of IMPs (Spectrum 1).

Fig. 3 SEM/EDS locations of chemical composition measurements for naturally aged aluminium alloy after immersion in NaCl solution with inhibitor mixture (CeCl3+BTA) for 48 h (a) and 96 h (b)
Table 3 Chemical composition of naturally aged aluminium alloy (wt.%)

BTA from the inhibitor mixture (CeCl3+BTA) shows the additional inhibitory effect. BTA forms CuBTA complexes on IMPs (which contain copper) and on the 2024 alloy matrix. This statement is confirmed by the electrochemical measurements using microelectrodes, and by XPS and Tof-SIMS methods [29]. Nevertheless, copper is present in its metallic form in the alloy and the inhibitive effect is also associated with the formation of a thin adsorbed BTA layer on the aluminium matrix, as detected by Tof-SIMS analysis [29].
According to the results obtained in this study, the inhibitor BTA from the inhibitor mixture (CeCl3+BTA) plays a dominant inhibiting role in the case of the artificially aged alloy.
Figure 4 shows the time dependency of the film resistance (Rf) and the constant phase element (CPEf) for the naturally and the artificially aged alloys in NaCl solution and in the presence of the inhibitor mixture (CeCl3+BTA).

Fig. 4 Time dependency of film resistance Rf (a) and constant phase element CPEf (b)
For the naturally aged alloy, in the presence of the inhibitor mixture (CeCl3+BTA), the Rf value decreases over time, while the CPE value increases over the same period. A similar behaviour was observed for both tempers of AA2024 alloy, in NaCl solution in the presence of CeCl3 [50]. The decrease in the Rf value over time was explained by the appearance of cracks in the cerium-oxide/ hydroxide layer deposited on the cathodic inclusions and by partial delaminating of this layer. At the formed cracks and sites of delaminating, the cathodic reaction of the corrosion process is facilitated, and Rf value decreases [50]. As shown in Ref. [50], a rather compact cerium layer is deposited over time near cathodic inclusions, where pits are most often formed. An increase of pit formation resistance over time was observed in the presence of CeCl3 [50], as well as in the presence of the inhibitors (CeCl3+BTA) in this work.
The φcorr value for the naturally aged alloy is more positive than that for the artificially aged AA2024 alloy. In accordance with the microstructure of AA2024 alloy, described above (Section 3.1), in the case of naturally aged alloy (NA temper), the Volta potential of the grains in the matrix is much more positive than that of the regions close to the grain boundaries. In our view, the cathodic reaction of oxygen reduction takes place not only on IMPs but also on the grain surfaces to a significant extent. The anodic reaction takes place predominantly in regions close to the grain boundaries (where the copper content is lower and the Volta potential is more active).
For the artificially aged alloy, in the presence of the inhibitors (CeCl3+BTA), the value of Rf increases over time. However, there is an unusual increase in the CPEf values over the same period. There is also an increase in the resistance of the alloy to pit formation over time. Such behaviour of the artificially aged alloy is similar to the behaviour of AA2024 alloy in NaCl solution in the presence of BTA [51].
An increase in the Rf value and an increase in the resistance of the alloy to pit formation over time are explained by the formation of CuBTA complex, not only on IMPs which contain copper, but also on the rest of the alloy surface [51]. The increase in the CPEf value over time is explained by water molecules (which have a significantly higher dielectric permeability relative to BTA molecules) being incorporated into the BTA layer. The formed layer, consisting of the CuBTA and water molecules, has a higher capacitance than the CuBTA layer [51].
In the artificially aged AA2024 alloy, there is a significant depletion of the matrix in the alloying elements (Cu) during the precipitation hardening process. This results in the shift of the φcorr to the more active values. BTA is cathodic and anodic corrosion inhibitor for AA2024 alloy, so its stronger influence in the inhibitor mixture (CeCl3+BTA) on the corrosion behaviour of the artificially aged AA2024 alloy is understandable.
Figure 5 shows the polarization diagrams for the naturally aged and artificially aged AA2024 alloys obtained during the pitting corrosion testing in NaCl solution and in the presence of the corrosion inhibitor mixture (CeCl3+BTA). As it can be seen in Fig. 5, in NaCl solution, the pitting potential (φpit) is close to or coincides with the corrosion potential (φcorr). This is characteristic for many aluminium alloys in chloride solutions.
It is commonly believed that pitting corrosion of AA2024 alloy occurs on the edges of cathodic inclusions. S-phase (Al2CuMg) particles are the most common places for pit formation in AA2024 alloy. Due to a selective dissolution of Al and Mg from the S-phase, the dimensions of the S-phase are reduced, the S-phase becomes significantly cathodic, and pit formation occurs on its edges.
From the polarization diagram in Fig. 5, it can be seen that the corrosion current density (Jcorr) is considerably lower in the presence of the inhibitor mixture (CeCl3+BTA) than that in the NaCl solution without the inhibitor. The Jcorr in NaCl solution is 1.80 μA/cm2, while in the presence of the inhibitor mixture it is 0.18 μA/cm2 after 1 h, for the naturally aged alloy (Table 4). After 96 h, the Jcorr is still significantly lower than that in NaCl solution without inhibitors (Table 4). The inhibitor mixture (CeCl3+BTA) shows a stronger inhibitory effect after 1 h than that after 96 h.
For the artificially aged alloy, the Jcorr value is 4.2 μA/cm2 in NaCl solution without inhibitors, while in the presence of inhibitors, the Jcorr values are 1.05 and 0.52 μA/cm2, after 1 and 96 h, respectively (Table 4).
The polarization of the working electrode started at potential of -250 mV versus φcorr which facilitates the occurrence of the cathodic reaction (OH- ions evolution). This may slightly affect the deposition process of cerium-oxide/hydroxide and the formation of the layer of CuBTA complex. However, the possible influence of OH- ions was neglected, because all experiments were performed under the same conditions.

Fig. 5 Potentiodynamic polarization diagrams of naturally aged (a) and artificially aged (b) aluminium alloys
Table 4 Results of polarization test for naturally aged (NA) and artificially aged (AA) aluminium alloys

For the naturally aged alloy, in the presence of the inhibitors, an increase in pit formation resistance over time was observed (Table 4). The higher value (φpit-φcorr) indicates a higher resistance of the alloy to pit formation.
The results of the potentiodynamic measurements and the EIS results indicate that different factors affect the pitting corrosion (pit formation) in comparison to the general corrosion. MANSFELD [52] suggested that the determination of the pitting corrosion resistance of aluminium alloys in NaCl solution without inhibitors and in the presence of corrosion inhibitors can be performed on the basis of the results obtained during EIS measurements.
In the presence of the inhibitor mixture (CeCl3+BTA), a decrease in pit growth resistance over time is observed. These results are in accordance with a new theory of pitting corrosion, proposed by FRANKEL et al [53]. According to this theory, in simplified terms, the higher the resistance of the alloy to pit formation is, the lower the resistance of the same alloy to pit growth is. A similar dependence exists in artificially aged alloy (Table 4).
Figure 6 shows the SEM/SEI (secondary electron image) micrographs of the surface of the naturally and the artificially aged AA2024 alloys after the potentiodynamic tests in the presence of the inhibitor mixture (CeCl3+BTA). It can be noticed that the dimensions of pits formed in the naturally aged alloy (Fig. 6(a)) are of significantly smaller dimensions than those formed in the artificially aged alloy (Fig. 6(b)). This is in agreement with the amount of the charge q having passed during the pit growth (Table 4). Larger dimensions of the formed pits on the artificially aged alloy (Fig. 6(b)) are probably due to the lower corrosion resistance of this temper, compared to the naturally aged alloy.
3.2 Synergistic effect of inhibitor mixture (CeCl3+BTA)
Figure 7 shows the characteristic results of the EIS tests of the naturally aged AA2024 alloy (Figs. 7(a) and (b)) and artificially aged AA2024 alloy (Figs. 7(c) and (d)) in NaCl solution without any inhibitor, in the presence of individually added inhibitors (10 mmol/dm3 CeCl3, 10 mmol/dm3 BTA), and in the presence of the inhibitor mixture (5 mmol/dm3 CeCl3 + 5 mmol/dm3 BTA). The Nyquist semicircles for NaCl solution without inhibitors are barely visible in Fig. 7 due to a very low value of Rf in NaCl solution (Table 2).

Fig. 6 SEM/SEI micrographs of alloy surface after potentiodynamic test (1 h) in the presence of inhibitor mixture (CeCl3+BTA)
In the presence of the inhibitor mixture (CeCl3+BTA), a significant synergistic effect of the inhibitors was observed after 1 and 96 h, for naturally aged AA2024 alloy, respectively (Figs. 7(a) and (b)). Cerium-oxides/hydroxides are precipitated on the cathodic inclusions. BTA forms CuBTA complex on the cathodic inclusions which contain copper. Each inhibitor forms inhibitor layer on a specific type of inclusions, i.e. on inclusions of a particular composition. Also, BTA complexation reactions on Cu-rich surfaces (IMPs and matrix) might compete with cerium precipitation. It has been also observed that cerium precipitates on the matrix (Table 3).
In this work, the SEM/EDS analysis has shown that intermetallic particles of different compositions are present in both tempers of AA2024 alloy. Also, it was observed that the content of cerium on the intermetallic inclusions was significantly higher than that on the surface of AA2024 alloy matrix (Table 3). The classification of the intermetallic particles in AA2024 alloy, based on the contents of copper, iron and magnesium, is given in Ref. [54].
For the artificially aged AA2024 alloy, a significant synergistic effect of the inhibitor mixture (5 mmol/dm3 CeCl3 + 5 mmol/dm3 BTA) was observed after 96 h of the EIS test, compared to 10 mmol/dm3 CeCl3 and 10 mmol/dm3 BTA added individually (Fig. 7(d)). After 1 h, the synergistic effect of the inhibitor mixture was not observed (Fig. 7(c)).

Fig. 7 Nyquist diagrams for naturally aged (a, b) and artificially aged (c, d) aluminium alloys after 1 h (a, c) and 96 h (b, d)

Fig. 8 Potentiodynamic polarization diagrams for naturally aged aluminium alloy after 1 h (a) and 96 h (b)
Figure 8 shows the polarization diagrams obtained during the pitting corrosion test of the naturally aged AA2024 alloy in NaCl solution without inhibitors, in the presence of individually added inhibitors (10 mmol/dm3 CeCl3, 10 mmol/dm3 BTA), and in the presence of the inhibitor mixture (5 mmol/dm3 CeCl3 + 5 mmol/dm3 BTA). Figure 8(a) shows the results obtained after 1 h and 96 h of testing. The synergistic effect of the inhibitor mixture (CeCl3+BTA) on the resistance to general corrosion is clearly visible as a decrease in the corrosion current density after 1 and 96 h of testing.
In general, the synergistic effect of the inhibitor (CeCl3+BTA) on the resistance to pit formation and pit growth was not observed clearly enough.
Figure 9 shows the polarization diagrams for the artificially aged alloy in NaCl solution, in the presence of corrosion inhibitors CeCl3 and BTA added individually (10 mmol/dm3 CeCl3 or 10 mmol/dm3 BTA), and in the presence of (5 mmol/dm3 CeCl3 + 5 mmol/dm3 BTA) to NaCl solution. Polarization diagrams were recorded after EIS measurements.
The results of the polarization measurements of the artificially aged alloy are in accordance with those of the EIS measurements. The Jcorr in the presence of the inhibitor mixture (CeCl3+BTA) is the lowest after 96 h of testing (Fig. 9).
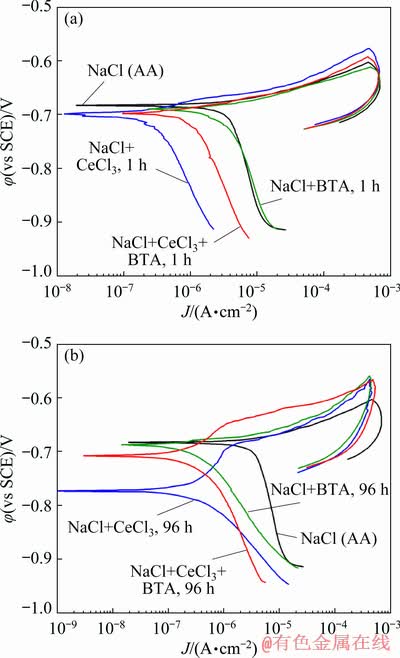
Fig. 9 Potentiodynamic polarization diagrams for artificially aged aluminium alloy after 1 h (a) and 96 h (b)
In general, in chloride solutions, cerium is a more efficient corrosion inhibitor for AA2024 alloy than BTA. The corrosion resistance of the naturally aged AA2024 alloy in the presence of the inhibitor mixture (CeCl3+BTA) is higher than that of the artificially aged alloy, over the entire test period. BTA is a less effective corrosion inhibitor of the AA2024 alloy than CeCl3 and its full effect is achieved after a certain period of time. Therefore, after 96 h of testing, the corrosion resistance of the artificially aged alloy is higher than that after 1 h.
4 Conclusions
(1) The microstructure of AA2024 alloy formed during natural and artificial aging processes has a great influence on the resistance of the alloy to general corrosion and pitting corrosion.
(2) The microstructure of alloy has a great influence on the inhibitory effect of CeCl3 and BTA, added individually, or added as the inhibitor mixture, to NaCl solution.
(3) A significant synergistic effect of the inhibitor mixture (CeCl3+BTA) on the resistance to general corrosion is observed in both tempers of AA2024 alloy.
(4) Each inhibitor forms protective film on the specific intermetallic inclusions, i.e. on inclusions with a specific chemical composition.
(5) BTA complexation reactions on Cu-rich surfaces (intermetallic inclusions and matrix) might compete with cerium precipitation.
(6) The synergistic effect of the inhibitor mixture (CeCl3+BTA) on the resistance to pit formation and pit growth is not clearly observed in either naturally aged or artificially aged AA2024 alloy.
(7) In chloride solutions, cerium is a more efficient corrosion inhibitor than BTA, for naturally aged and artificially aged AA2024 alloys.
Acknowledgments
This work was financially supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 451-03-68/2020-14/200026).
References
[1] HATCH E J. Aluminum properties and physical metallurgy [M]. Ohio: ASM International, 1984.
[2] DAVIS R J. Corrosion of aluminum and aluminum alloys [M]. Ohio: ASM International, 1999.
[3] SINJAVSKIJ S V, VALJKOV D V, KALININ D V. Corrosion and protection of aluminium alloys [M]. Moskva: Metallurgia, 1986. (in Russian)
[4] YIN Mei-jie, CHEN Jiang-hua, WANG Shuang-bao, LIU Zi-ran, CHA Li-mei, DUAN Shi-yun, WU Cui-lan. Anisotropic and temperature-dependent growth mechanism of S-phase precipitates in Al-Cu-Mg alloy in relation with GPB zones [J]. Transactions of Nonferrous Metals Society of China, 2016, 26: 1-11.
[5] FONTANA G M, STAEHLE W R. Advances in corrosion science and technology [M]. Volume 2. New York, London: Plenum Press, 1972.
[6] BROOKS C R. Principles of heat treating of nonferrous alloys [M]. Volume 4. Ohio: ASM International, 1991: 1861-1960.
[7] LI Hui-zhong, ZHU Ze-xiao, LIANG Xiao-peng, LI Peng-wei, QI Ye-long, LV Feng, HUANG Lan, Effect of T6-treatments on microstructure and mechanical properties of forged Al-4.4Cu-0.7Mg-0.6Si alloy [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 2539-2547.
[8] KRYMSKIY S, SITDIKOV O, AVTOKRATOVA E, MARKUSHEV M. 2024 aluminum alloy ultrahigh-strength sheet due to two-level nanostructuring under cryorolling and heat treatment [J]. Transactions of Nonferrous Metals Society of China, 2020, 30: 14-26.
[9] DONATUS U, THOMPSON G E, OMOTOYINBO J A, ALANEME K K, ARIBO S, AGBABIAKA O G, Corrosion pathways in aluminium alloys [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 55-62.
[10] ACOSTA G, VELEVA L, LOPEZ J L, LOPEZ-SAURI D A, Contrasting initial events of localized corrosion on surfaces of 2219-T42 and 6061-T6 aluminum alloys exposed in Caribbean seawater [J]. Transactions of Nonferrous Metals Society of China, 2019, 29: 34-42.
[11] MENG Qiang, LIU Yang, KANG Ju, FU Rui-dong, GUO Xiao-yan, LI Yi-jun, Effect of precipitate evolution on corrosion behavior of friction stir welded joints of AA2060-T8 alloy [J]. Transactions of Nonferrous Metals Society of China, 2019, 29: 701-709.
[12] HARVEY G T. Cerium-based conversion coatings on aluminium alloys: A process review [J]. Corrosion Engineering Science and Technology, 2013, 48: 248-269.
[13] HU T, SHI H, WEI T, LIU F, FAN S, HAN En-Hou. Cerium tartarate as a corrosion inhibitor for AA 2024-T3 [J]. Corrosion Science, 2015, 95: 152-161.
[14] ROSERO-NAVARRO C N, CURIONI M, BINGHAM R, DURAN A, APARICIO M, COTTIS A R, THOMPSON E G. Electrochemical techniques for practical evaluation of corrosion inhibitor effectiveness: Performance of cerium nitrate as corrosion inhibitor for AA2024T3 alloy [J]. Corrosion Science, 2010, 52: 3356-3366.
[15] MATTER A E, KOZHUKHAROV S, MACHKOVA M, KOZHUKHAROV V. Comparison between the inhibition efficiencies of Ce(III) and Ce(IV) ammonium nitrates against corrosion of AA2024 aluminum alloy in solutions of low chloride concentration [J]. Corrosion Science, 2012, 65: 22-33.
[16] MACHKOVA M, MATTER A E, KOZHUKHAROV S, KOZHUKHAROV V. Effect of the anionic part of various Ce(III) salts on the corrosion inhibition efficiency of AA2024 aluminium alloy [J]. Corrosion Science, 2013, 69: 396-405.
[17] PAUSSA L, ANDREATTA F, de FELICIS D, BEMPORAD E, FEDRIZZI L. Investigation of AA2024-T3 surfaces modified by cerium compounds: A localized approach [J]. Corrosion Science, 2014, 78: 215-222.
[18] PAUSSA L, ANDREATTA F, ROSERO NAVARRO C N, DURAN A, FEDRIZZI L. Study of the effect of cerium nitrate on AA2024-T3 by means of electrochemical micro-cell technique [J]. Electrochimica Acta, 2012, 70: 25-33.
[19] RODIC P, MILOSEV I. Corrosion inhibition of pure aluminium and alloys AA2024-T3 and AA7075-T6 by cerium(III) and cerium(IV) salts [J]. Journal of Electrochemical Society, 2016, 163: C85-C93.
[20] UHART A, LEDEUIL B J, GONBEAU D, DUPIN C J, BONINO P J, ANSART F, ESTEBAN J. An auger and XPS survey of cerium active corrosion protection for AA2024-T3 aluminum alloy [J]. Applied Surface Science, 2016, 390: 751-759.
[21] SAEEDIKHANI M, JAVIDI M, VAFAKHAH S. Anodising of 2024-T3 aluminium alloy in electrolyte of sulphuric-boric-phosphoric mixed acid containing cerium salt as corrosion inhibitor [J]. Transactions of Nonferrous Metals Society of China, 2017, 27: 711-721.
[22] HU T, SHI H, HOU D, WEI T, FAN S, LIU F, HAN E H. A localized approach to study corrosion inhibition of intermetallic phases of AA 2024-T3 by cerium malate [J]. Applied Surface Science, 2019, 467-468: 1011-1032.
[23] SHI H, HAN E H, LIU F. Corrosion protection of aluminium alloy 2024-T3 in 0.05 M NaCl by cerium cinnamate [J]. Corrosion Science, 2011, 53: 2374-2384.
[24] FOX G P, LEWIS G, BODEN J P. Some chemical aspects of the corrosion inhibition of copper by benztriazole [J]. Corrosion Science, 1979, 19: 457-467.
[25] FINSGAR M, MILOSEV I. Inhibition of copper corrosion by 1,2,3-benzotriazole: A review [J]. Corrosion Science, 2010, 52: 2737-2749.
[26] ZHAO R H, XU L Y, CHEN K C, CHEN Y, LIU W Y, YANG N Z. New aspects of copper corrosion in a neutral NaCl solution in the presence of benzotriazole [J]. Corrosion, 2018, 74: 613-622.
[27] ZHELUDKEVICH L M, YASAKAU A K, POZNYAK K S, FERREIRA S G M. Triazole and thiazole derivatives as corrosion inhibitors for AA2024 aluminium alloy [J]. Corrosion Science, 2005, 47: 3368-3383.
[28] WILLIAMS G, COLEMAN J A, McMURRAY N H. Inhibition of aluminium alloy AA2024-T3 pitting corrosion by copper complexing compounds [J]. Electrochimica Acta, 2010, 55: 5947-5958.
[29] RECLOUX I, ANDREATTA F, DRUART M E, COELHO L B, CEPEK C, COSSEMENT D, FEDRIZZI L, OLIVIER M G. Stability of benzotriazole-based films against AA2024 aluminium alloy corrosion process in neutral chloride electrolyte [J]. Journal of Alloys and Compounds, 2018, 735: 2512-2522.
[30] COELHO B L, COSSEMENT D, OLIVIER M G. Benzotriazole and cerium chloride as corrosion inhibitors for AA2024-T3: An EIS investigation supported by SVET and ToF-SIMS analysis [J].Corrosion Science, 2018, 130: 177-189.
[31] COELHO B L, MOUANGA M, DRUART M E, RECLOUX I, COSSEMENT D, OLIVIER M G. A SVET study of the inhibitive effects of benzotriazole and ceriumchloride solely and combined on an aluminium/copper galvanic coupling model [J]. Corrosion Science, 2016, 110: 143-156.
[32] BIRBILIS N, BUCHHEIT G R. Electrochemical characteristics of intermetallic phases in aluminum alloys—An experimental survey and discussion [J]. Journal of Electrochemical Society, 2005, 152: B140-B151.
[33] LUO C. Role of microstructure on corrosion control of AA2024-T3 aluminium alloy university of manchester [M]. Manchester: Faculty of Engineering and Physical Sciences, 2011.
[34] GHOSH S K, HILAL M, BOSE S. Corrosion behavior of 2024 Al-Cu-Mg alloy of various tempers [J]. Transactions of Nonferrous Metals Society of China, 2013, 23: 3215-3227.
[35] RALSTON D K, BIRBILIS N, CAVANAUGH K M, WEYLAND M, MUDDLE C B, MARCEAU W K R. Role of nanostructure in pitting of Al-Cu-Mg alloys [J]. Electrochimica Acta, 2010, 55: 7834-7842.
[36] BIRBILIS N, BUCHHEIT G R. Investigation and discussion of characteristics for intermetallic phases common to aluminum alloys as a function of solution pH [J]. Journal of Electrochemical Society, 2008, 155: C117-C126.
[37] YOON Y, BUCHHEIT G R. Dissolution behavior of Al2CuMg (S Phase) in chloride and chromate conversion coating solutions [J]. Journal of Electrochemical Society, 2006, 153: C151-C155.
[38] CAVANAUGH K M, LI J C, BIRBILIS N,BUCHHEIT G R. Electrochemical characterization of intermetallic phases common to aluminum alloys as a function of solution temperature [J]. Journal of Electrochemical Society, 2014, 161: C535-C543.
[39] QINZAND F Y, WANG Q S. Ab-initio study of the role of Mg2Si and Al2CuMg phases in electrochemical corrosion of Al alloys [J]. Journal of Electrochemical Society, 2015, 162: C503-C508.
[40] LI J, HURLEY B, BUCHHEIT R. Effect of temperature on the localized corrosion of AA2024-T3 and the Electrochemistry of intermetallic compounds during exposure to a dilute NaCl solution [J]. Corrosion, 2016, 72: 1281-1291.
[41] BOAG A, TAYLOR J R, MUSTER H T, GOODMAN N, McCULLOCH D, RYAN C, ROUT B, JAMIESON D, HUGHES E A. Stable pit formation on AA2024-T3 in a NaCl environment [J]. Corrosion Science, 2010, 52: 90-103.
[42] BRUNNER G J, BIRBILIS N, RALSTON D K, VIRTANEN S. Impact of ultrafine-grained microstructure on the corrosion of aluminium alloy AA2024 [J]. Corrosion Science, 2012, 57: 209-214.
[43] LI J, BIRBILIS N, BUCHHEIT G R. Electrochemical assessment of interfacial characteristics of intermetallic phases present in aluminium alloy 2024-T3 [J]. Corrosion Science, 2015, 101: 155-164.
[44] SHI H, HAN H E, LIU F, WEI T, ZHU Z, XU D. Study of corrosion inhibition of coupled Al2Cu-Al and Al3Fe-Al by cerium cinnamate using scanning vibrating electrode technique and scanning ion-selective electrode technique [J]. Corrosion Science, 2015, 98: 150-162.
[45] HASHIMOTO T, ZHANG X, ZHOU X, SKELDON P, HAIGH J S, THOMPSON E G. Investigation of dealloying of S phase (Al2CuMg) in AA 2024-T3 aluminium alloy using high resolution 2D and 3D electron imaging [J]. Corrosion Science, 2016, 103: 157-164.
[46] RALSTON D K, BIRBILIS N, CAVANAUGH K M, WEYLAND M, MUDDLE C B, MARCEAU W K R. Role of nanostructure in pitting of Al-Cu-Mg alloys [J]. Electrochimica Acta, 2010, 55: 7834-7842.
[47] LI J, HURLEY B, BUCHHEIT R. The effect of CeCl3 as an inhibitor on the localized corrosion of AA2024-T3 as a function of temperature [J]. Journal of Electrochemical Society, 2016, 163: C845-C852.
[48] LI J, HURLEY B, BUCHHEIT R. Microelectrochemical characterization of the effect of rare earth inhibitors on the localized corrosion of AA2024-T3 [J]. Journal of Electrochemical Society, 2015, 162: C563-C571.
[49] JAIN S, LIM C L M, HUDSON L J, SCULLY R J. Spreading of intergranular corrosion on the surface of sensitized Al-4.4Mg alloys: A general finding [J]. Corrosion Science, 2012, 59: 136-147.
[50] JEGDIC B, BOBIC B, LINIC S. Corrosion behaviour of AA2024 aluminium alloy in different tempers in NaCl solution and with the CeCl3 corrosion inhibitor [J]. Materials and Corrosion, 2019, DOI: 10.1002/maco.201911219.
[51] JEGDIC B, BOBIC B, STEVANOVIC M, MIHAILOVIC M, DANICIC D, KOVACINA J, RADOJKOVIC B. Resistance to pit formation and pit growth for different tempers of AA2024 aluminium alloy in presence of benzotriazole [J]. Metals and Materials International, 2019, DOI 10.1007/s12540-019-00451-8.
[52] MANSFELD F. Electrochemical impedance spectroscopy [M]//Analytical methods in corrosion science and engineering. London, New York, Singapore: Taylor & Francis Group, 2006.
[53] FRANKEL S G, LI T, SCULLY R J. Localized corrosion: Passive film breakdown vs pit growth stability [J]. Journal of Electrochemical Society, 2017, 164: C180-C181.
[54] ZHU Y, SUN K, FRANKEL S G. Intermetallic phases in aluminum alloys and their roles in localized corrosion [J]. Journal of Electrochemical Society, 2018, 165: C807-C820.
Bore JEGDIC, Biljana BOBIC, Bojana RADOJKOVIC, Jovanka KOVACINA, Dunja MARUNKIC
University of Belgrade, Institute for Chemistry, Technology and Metallurgy, Belgrade, Serbia
摘 要:分析自然时效和人工时效AA2024铝合金在0.5 mol/dm3 NaCl溶液中的腐蚀行为,溶液中分别加入环境友好的缓蚀剂:10 mmol/dm3 CeCl3,10 mmol/dm3 BTA和5 mmol/dm3 CeCl3 + 5 mmol/dm3 BTA混合缓蚀剂。本研究的目的是确定混合缓蚀剂的协同效应水平,并解释这种效应的本质。采用电化学阻抗谱(EIS)研究缓蚀剂层的腐蚀性能,通过动电位极化试验研究其抑制点蚀形成和点蚀生长的能力。扫描电子显微镜(SEM/EDS)的结果表明,自然时效铝合金中形成的点蚀尺寸小于人工时效合金中形成的点蚀尺寸。在自然时效合金测试的96 h内以及在人工时效合金测试的后期,均观察到混合缓蚀剂对腐蚀性能的协同效应,但没有发现混合缓蚀剂对点蚀形成和点蚀生长的协同效应。
关键词:铝合金;点蚀;缓蚀剂;铈;苯并三唑
(Edited by Xiang-qun LI)
Corresponding author: Bore JEGDIC; E-mail: borejegdic@yahoo.com
DOI: 10.1016/S1003-6326(20)65312-2

