查尔酮缩氨基硫脲类希夫碱的合成与表征
潘春跃1,蒋呈奎1,唐新村1,于 扬1,金 乐1,刘宗耀2
(1. 中南大学 化学化工学院,湖南 长沙,410083;
2. 湖南大学 环境科学与工程系,湖南 长沙,410082)
摘要:由苯甲醛、对二甲氨基苯甲醛和苯乙酮通过Claisen-Schmidt缩合反应合成反应中间体查尔酮和对二甲氨基查尔酮。用查尔酮和对二甲氨基查尔酮与氨基硫脲为原料,在弱酸性条件下经过羟醛缩合反应合成查尔酮缩氨基硫脲希夫碱和对二甲氨基查尔酮缩氨基硫脲希夫碱。通过元素分析、红外光谱、气相色谱/质谱联机分析及核磁共振氢谱对所合成的化合物进行表征。研究结果表明:C == C的伸缩红外光谱吸收峰和== CH的伸缩及面外振动红外光谱吸收峰分别出现在波数为1 644~1 651,3 020~3 034,988~998 cm-1处,表明所合成的希夫碱为反式异构体。C== S的红外光谱吸收峰在1 219~1 221 cm-1处,低于特征频率1 230~1 253 cm-1;且伯胺上的质子的化学位移相对于仲胺大幅度向低场移动;推测希夫碱之间存在分子间氢键作用,且C== S主要以酮式存在。
关键词:查尔酮;对二甲氨基查尔酮;缩氨基硫脲希夫碱;反式异构体;合成;表征
中图分类号:O621 文献标识码:A 文章编号:1672-7207(2007)01-0093-05
Synthesis and characterization of Schiff bases derived from
chalcone and thiosemicarbazide
PAN Chun-yue1, JIANG Cheng-kui1, TANG Xin-cun1, YU Yang1, JIN Le1, LIU Zong-yao2
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Department of Environmental Science and Engineering, Hunan University, Changsha 410082, China)
Abstract: The intermediates including chalcone and 4-dimethylaminochalcone were synthesized from benzaldchyde, 4-dimethylamino-benzaldchyde and hypnone by Claisen-Schmidt reaction. And then using chalcone and 4-dimethyla- minochalcone with thiosemicarbazide as raw material, two novel Schiff bases comprising thiosemicarbazone were synthesized by aldol condensation under the faintly acid condition. The compounds were characterized by elemental analysis, infrared, GC-MS and 1HNMR. The results show that in the infrared spectra of C== C and == CH there are absorption peaks at 1 644-1 651, 3 020-3 034, 988-998 cm-1, respectively, indicating that the two Schiff bases are trans-isomerization. The infrared spectra of C== S there are absorption peaks at 1 219-1 221 cm-1, lower than its characteristic frequency at 1 230-1 253 cm-1, and the chemical shifts of primary amine to low magnetic field greatly in reference to that of secondary amine, which indicate the molecules is combined with intermolecular hydrogen bonds and conclude C== S existing mainly in the form of keto tautomer.
Key words: chalcone; 4-dimethylaminochalcone; Schiff base containning thiosemicarbazone; trans- isomerization; synthesis; characterization
含硫希夫碱因其结构中含有S和N杂原子而具有较强的配位能力,可与Cu2+,Fe3+,Co2+和Ni2+等金属离子形成稳定的配合物,近年来又发现这些配合物具有较强的生理活性,可以作为抗菌、抗癌、抗结核等药物[1-6]。查尔酮及其衍生物是重要的有机合成中间体,该类化合物具有较大的柔性,能与不同的生物受体结合[7-8],一些含羟基的查尔酮表现出多种药理作用。为此,本文作者对2种新型查尔酮类缩氨基硫脲希夫碱的合成及表征进行研究。
1 实 验
1.1 仪器与试剂
a. 仪器为:德国耶拿公司生产的multi N/C UV HS湿化学法总有机碳分析仪;Nicolet公司生产的AVATAR360型红外光谱仪;日本岛津公司生产的GC-MS-QP2010型气相色谱/质谱联用仪;瑞士布鲁克拜厄斯宾公司生产的AVANCE800全数字化核磁共振波谱仪。
b. 所有试剂均为分析纯。
1.2 试样合成
1.2.1 查尔酮的制备
在装有搅拌器、温度计和液滴漏斗的三颈瓶中加入6 g(5.8 mL)苯乙酮,15 mL无水乙醇和25 mL 10%氢氧化钠溶液,控制温度在5 ℃以下,然后,在搅拌下滴加5.3 g(5 mL)苯甲醛,控制滴加速度保持反应温度在25~30 ℃[9-10]。滴加完毕后,继续保持此温度搅拌0.5 h,而后在室温下反应6~8 h,过滤、水洗、干燥。将粗产品用无水乙醇重结晶,得浅黄色片状结晶,室温晾干。产率为85%,熔点为55~56 ℃(文献值为56~57 ℃[9])。
1.2.2 查尔酮缩氨基硫脲希夫碱的制备
将查尔酮与等量的氨基硫脲(0.02 mL)加入三颈瓶中,再加入200 mL甲醇,然后滴加2~5 mL冰醋酸调节pH值至4~6。在80 ℃左右水浴搅拌回流3~6 h后,将回流装置改为蒸馏装置,收集65 ℃左右馏分,蒸出部分甲醇后,将烧瓶置于冰水浴中冷却15~30 min,有白色固体粉末析出[11-12]。产率为66%,熔点为152~153 ℃。
1.2.3 对二甲氨基查尔酮的制备
在装有搅拌器,温度计和液滴漏斗的三颈瓶中加入9.26 g(9 mL)苯乙酮,44 mL无水乙醇和84 mL 10%氢氧化钠溶液,控制温度在5 ℃以下。然后,在搅拌下加入11.5 g对二甲氨基苯甲醛。控制加入速度保持反应温度在25~30℃。加入完毕后,继续保持此温度搅拌0.5 h,而后在室温下反应8~10 h,过滤、水洗、干燥。将粗产品用无水乙醇重结晶,得橘黄色晶体,于室温晾干。产率为82%,熔点为103~104 ℃。
1.2.4 对二甲氨基查尔酮缩氨基硫脲希夫碱的制备
制备方法与查尔酮缩氨基硫脲希夫碱的合成方法相同。最后得橘红色固体粉末。产率为62%,熔点为186~190 ℃。
2 结果与讨论
2.1 元素分析
各化合物的元素分析结果如表1所示。由表1可以推断出查尔酮(CLN),查尔酮缩氨基硫脲希夫碱(CLN-TCZ),对二甲氨基查酮(4-DTA-CLN),对二甲氨基查尔酮缩氨基硫脲希夫碱(4-DTA-CLN-TCZ)的化学组成分别为C15H12O,C16H15N3S,C17H17ON和C18H2ON4S。溶解性试验结果表明,2种希夫碱能溶于DMF、DMSO和丙酮乙醇及乙醇等有机溶剂。
2.2 IR分析
用KBr压片法在400~4 000 cm-1范围内测定所有化合物的IR光谱,结果如表2所示。1 619和1 613 cm-1
表1 化合物元素分析
Table 1 Elemental analyses of compounds w/%

注:括号的数据为理论值。
表2 化合物的IR光谱数据
Table 2 Infrared spectral data of compounds 波数/cm-1

分别为CLN和4-DTA-CLN的C== O伸缩振动峰,在进行缩合反应后,C== O伸缩振动峰消失。同时,反应后出现C== N、伯胺及仲胺的振动吸收峰[11-14]。表明进行缩合反应生成了CLN-TCZ和4-DTA-CLN- TCZ。在1 532和647 cm-1分别为CLN及其希夫碱芳环C== C双键的伸缩振动峰和面内振动峰。4-DTA-CLN及其希夫碱芳环的振动峰向高频移动,分别为1 562和651 cm-1。这是因为二甲氨基上的N原子与苯环形成π-p共轭,使得4-DTA-CLN及其希夫碱的化学键力常数增大。化合物烯烃双键的伸缩振动峰在1 644~1 651 cm-1处,烯烃碳氢键的伸缩振动峰在3 020~3 034 cm-1处。988~998 cm-1处的吸收可认为是烯烃C—H键的面外振动所致[13-15],据此可推断所合成化合物C== C双键均为反式取代[16]。化合物以反式存在使得空间位阻减小,有利于化合物的稳定。vC=S出现在1 219~1 221 cm-1处,说明C== S以酮式存在[17],但此处的特征频率低于1 230~1 253 cm-1处的特征频率,这可能是S原子参与氢键的形成所致[18]。
2.3 GC-MS分析
对2种配合物进行GC-MS分析,观察它们的气相色谱,在飞行时间为0~18 min时,CLN-TCZ和4-DTA-CLN-TCZ分别仅在保留时间为3 min和3.7 min左右有1个峰,表明所合成的配合物样品为纯物质。分别在3 min和3.7 min进行MS谱分析(见图1),它的主要碎片离子峰数据如表3和表4所示。
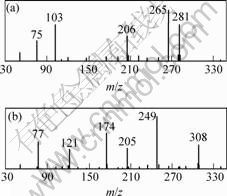
图1 CLN-TCZ(a)和4-DTA-CLN-TCZ(b)质谱图
Fig.1 Mass spectrum of CLN-TCZ (a) and
4-DTA-CLN-TCZ (b)
2.4 1HNMR分析
以DMSO作溶剂,TMS作内标,在0~15×10-6范围内测定CLN-TCZ和4-DTA-CLN-TCZ样品的核磁共振谱(见图2),各质子的化学位移δ及其归属见图3、图4、表5和表6所示。数据表明主要基团质子的化学位移与预期的位移基本相符。值得关注的是2种希夫碱的伯胺上质子化学位移相对于仲胺大幅度向低场移动,其原因是:一方面,伯胺的连接基团较少;
表3 CLN-TCZ的质谱数据
Table 3 Mass spectrum data of CLN-TCZ
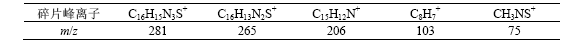
表4 4-DTA-CLN-TCZ的质谱数据
Table 4 Mass spectrum date of 4-DTA-CLN-TCZ

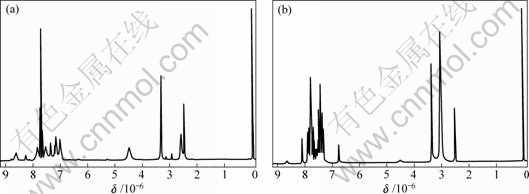
图2 CLN-TCZ(a)和4-DTA-CLN-TCZ(b)的1HNMR谱图
Fig.2 1HNMR spectrum of CLN-TCZ (a) and 4-DTA-CLN-TCZ (b)
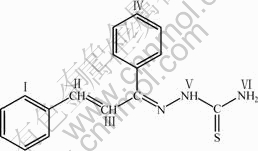
图3 CLN-TCZ的结构式
Fig.3 Structure of CLN-TCZ
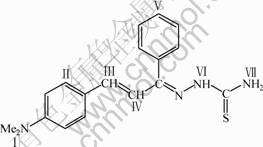
图4 4-DTA-CLN-TCZ的结构式
Fig.4 Structure of 4-DTA-CLN-TCZ
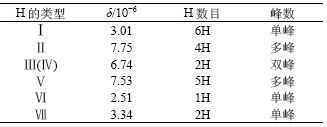
表5 CLN-TCZ的1HNMR数据
Table 5 1HNMR data of CLN-TCZ

表6 4-DTA-CLN-TCZ的1HMNR数据
Table 6 1HNMR Date of 4-DTA-CLN-TC
另一方面,可能是因为伯胺上H原子与C== S上的S形成氢键。C== S键的红外光谱向低波数移动也证实了这一事实[13, 15, 19]。由于原子共价半径及空间结构的限制,推测应为分子间氢键。
3 结论
a. 由苯甲醛、对二甲氨基苯甲醛和苯乙酮通过Claisen-Schmidt缩合合成反应中间体查尔酮和对二甲氨基查尔酮。用查尔酮和对二甲氨基查尔酮与氨基硫脲为原料,在弱酸性条件下经过羟醛缩合反应合成查尔酮缩氨基硫脲希夫碱和对二甲氨基查尔酮缩氨基硫脲希夫碱。结合元素分析、红外光谱、气-质联用谱及核磁共振氢谱等分析,证实所合成的化合物就是目标产物。
b. C== C的伸缩红外光谱吸收峰、== CH的伸缩及面外振动红外光谱吸收峰分别出现在波数为1 644~ 1 651,3 020~3 034和988~998 cm-1处,表明所合成的希夫碱为反式异构体。
c. C== S键红外光谱的吸收峰在1 219~1 221 cm-1处,低于其特征波数1 230~1 253 cm-1;且伯胺上的质子的化学位移相对于仲胺大幅度向低场移动。推测希夫碱之间存在分子间氢键,且C== S键主要以酮式存在。
参考文献:
[1] Bastos M B R, Moreira J C, Farias P A M. Adsorptive stripping voltammetric behaviour of UO2 complexed with the Schiff base N, N′-ethylenebis(salicylidenimine) in aqueous 4-(2- hydroxyethyl)-1-piperazine ethanesulfonic acid medium[J]. Analytica Chimica Acta, 2000, 408: 83-88.
[2] Ma H, Chen S H, Niu L, et al. Studies on electrochemical behavior of copper in aerated NaBr solutions with Schiff base[J]. Journal of Electrochemical Society, 2001, 148(5): 208-216.
[3] Yong L, Huanh J L, Lian B, et al,Synthesis of titanium(IV) complexes with Schiff bases ligand and their catalytic activities for polymerization of ethylene and etyrene[J]. Chinese Journal of Chemistry, 2001, 19(4): 429-432.
[4] Baseer M A, Jadhav V D, Phule R M. Synthesis and antibacterial activity of some new Schiff bases[J]. Oriental Journal of Chemistry, 2000, 16(3): 553-556.
[5] 张建民, 李瑞芳, 刘树祥. 过渡金属配合物的稳定性及其杀菌活性[J]. 无机化学学报, 1999, 15(4): 493-496.
ZHANG Jian-min, LI Rui-fang, LIU Shu-xiang. A relation between stability of transition metal Schiff base complexes and the IR disinfectivity[J]. Chinese Journal of Inorganic Chemistry, 1999, 15(4): 493-496.
[6] ZHU Xin-de, WANG Cheng-gang, LE Zhi-feng. Synthesis, characterization and scavenger eddect on O2- of copper (Ⅱ) and zinc (Ⅱ) complexes derived from thiosemicarbazide[J]. Synth React Inorg, Met-Org Chem, 1991, 21(9): 1365-1373.
[7] Carolyn N, Lemieux C, Jorgensen R. Introduction of a chimetic chalcone synthase gene into Petunia results in reversible co-suppression of homologous genes in trans[J]. Plant Cell, 1990, 23: 279-289.
[8] Jorgensen R A, Cluster P D. Chalcone synthase cosuppression phenotypes in petunia flowers: comparison of sense vs. antisense constructs and single-copy vs. complex T DNA sequences[J]. Plant Mol Biol , 1996, 31(5): 957-973.
[9] 胡志国, 刘 军, 李工安. SOCl2/ EtOH催化查尔酮的合成[J]. 化学研究与应用, 2004, 16(4): 583-584.
HU Zhi-guo, LIU Jun, LI Gong-an. Synthesis of chalcones catalyzed by SOCl2/EtOH[J].Chemical Research and Application, 2004, 16(4): 583-584.
[10] 关丽萍, 尹秀梅, 全红梅. 羟基查尔酮类衍生物的合成[J]. 有机化学, 2004, 24(10): 1274-1277.
GUAN Li-ping, YIN Xiu-mei, QUAN Hong-mei. Synthesis of hydroxylated chalcones and related derivatives[J]. Chinese Journal of Organic Chemistry, 2004, 24(10): 1274-1277.
[11] 陆绍荣.含硫希夫碱的合成研究[J].合成化学, 2003, 10(4): 349-353.
LU Shao-rong. Synthetic study of Schiff base type of containing thiocarbaimide group[J]. Chinese Journal of Synthetic Chemistry, 2003, 10(4): 349-353.
[12] 李晓如, 杨 辉, 赵金尧, 等. 季铵盐催化合成β-硝基对氯苯乙烯[J]. 中南大学学报: 自然科学版, 2004, 35(5): 802-805.
LI Xiao-ru, YANG Hui, ZHAO Jin-yao, et al. Catalytic synthesis of β-nitro-p-chloro-phenylethylene with quaternary ammonium salt PTCA[J]. Journal of Central South University: Science and Technology, 2004, 35(5): 802-805.
[13] 唐瑞仁, 朱金娟, 严子耳, 等. 相转移催化法合成4-羟甲基二苯甲酮[J]. 中南大学学报: 自然科学版, 2005, 36(4): 594-598.
TANG Rui-ren, ZHU Jin-juan, YAN Zi-er, et al. Synthesis of 4-hydroxymethylbenzophenone by phase transfer catalytic method[J]. Journal of Central South University: Science and Technology, 2005, 36(4): 594-598.
[14] 李冬青, 谭明雄, 刘 星. 吡啶甲醛缩氨基硫脲类希夫碱的合成[J]. 化工时刊, 2004, 18(11): 36-40.
LI Dong-qing, TAN Ming-xiong, LIU Xing. Synthesis of Schiff bases derived from pyridinecarbaldehyde and thiosemicarba- zone[J]. Chemical Industry Times, 2004, 18(11): 36-40.
[15] 刘丰良, 周康根, 阳卫军. 3β-乙酰氧基雄甾-5, 15-二烯-17-酮的合成[J]. 中南大学学报: 自然科学版, 2004, 35(3): 396-401.
LIU Feng-liang, ZHOU Kang-gen, YANG Wei-jun. Synthesis of 3β-acetoxyandrosta-5, 15-dien-17-one[J]. Journal of Central South University: Science and Technology, 2004, 35(3): 396- 401.
[16] 常建华, 董绮功. 波谱原理及解析[M].北京: 科学出版社, 2001.
CHANG Jian-hua, DONG Qi-gong. Principle and explanation of spectrum[M]. Beijing: Science Press, 2001.
[17] 张安将, 张力学, 侯云龙. 双缩二氨基硫脲类化合物的合成与结构[J]. 有机化学, 2003, 23(3): 281-285.
ZHANG An-jiang, ZHANG Li-xue, HOU Yun-long. Synthesis and crystal structure of some dithiocarbohydrazones[J]. Chinese Journal of Organic Chemistry, 2003, 23(3): 281-285.
[18] 田作霖, 张 新. Schiff碱化合物的合成及IR、1HNMR波谱性质[J]. 长春光学精密仪器学院学报, 1995, 18(4): 42-45.
TIAN Zuo-lin, ZHANG Xin. Syntheses and IR, 1HNMR spectra of Schiff base compounds[J]. J Changchun Inst Opt & Fine Mech, 1995, 18(4): 42-45.
[19] 曹育才, 蒋玉仁, 薛玉兰, 等. 3, 5, 6-三氯吡啶-2-酚的合成与波谱特征[J]. 中南工业大学学报: 自然科学版, 2000, 31(1): 24-26.
CAO Yu-cai, JIANG Yu-ren, XUE Yu-lan, et al. The synthesis of 3, 5, 6-trichloropyridin-2-ol and its wave-spectrum characteristics[J]. Journal of Central South University: Science and Technology, 2000, 31(1): 24-26.
收稿日期:2006-06-24
基金项目:国家自然科学基金资助项目(20406024)
作者简介:潘春跃(1963-),男,湖南郴州人,教授,从事功能高分子材料研究
通讯作者:潘春跃,男,教授;电话:0731-8836848(O);E-mail:panchunyue@sina.com