Effects of basicity and temperature on mineralogy and reductionbehaviors of high-chromium vanadium-titanium magnetite sinters
来源期刊:中南大学学报(英文版)2019年第1期
论文作者:薛向欣 汤卫东 杨松陶 张立恒 黄壮 杨合
文章页码:132 - 145
Key words:basicity; high-chromium vanadium-titanium magnetite; sintering pot test; mineralogy; reduction behavior
Abstract: The effects of basicity and temperature on the reduction process of Hongge high-chromium vanadium-titanium magnetite (HCVTM) sinter were investigated in this work. The main characterization methods of X-ray fluorescence (XRF), X-ray diffraction (XRD), scanning electron microscope (SEM), and metallographic microscope were employed in this study. In this work, the reduction of HCVTM sinter with different temperature and basicity were experimented. The Fe, FeO, and TiO in reductive samples increase with increasing basicity and temperatures. The increase of basicity and temperature is favorable to the reduction of HCVTM sinter. The Fe phase has out-migration tendency to the surface of sinter while the perovskite and silicate phases have in-migration tendency to the inside of sinter. The reduction degradation index (RDI) decreases while the reduction index (RI) increases with increasing basicity. The RI increases from 67.14% to 82.09% with increasing temperature from 1073 K to 1373 K.
Cite this article as: TANG Wei-dong, YANG Song-tao, ZHANG Li-heng, HUANG Zhuang, YANG He, XUE Xiang-xin. Effects of basicity and temperature on mineralogy and reduction behaviors of high-chromium vanadium- titanium magnetite sinters [J]. Journal of Central South University, 2019, 26(1): 132–145. DOI: https://doi.org/10.1007/ s11771-019-3988-8.

J. Cent. South Univ. (2019) 26: 132-145
DOI: https://doi.org/10.1007/s11771-019-3988-8

TANG Wei-dong(汤卫东)1, YANG Song-tao(杨松陶)1, 2, ZHANG Li-heng(张立恒)1,HUANG Zhuang(黄壮)1, YANG He(杨合)1, XUE Xiang-xin(薛向欣)1
1. School of Metallurgy, Northeastern University, Shenyang 110819, China;
2. School of Materials and Metallurgy, University of Science and Technology Liaoning,Anshan 114051, China
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2019
Abstract: The effects of basicity and temperature on the reduction process of Hongge high-chromium vanadium-titanium magnetite (HCVTM) sinter were investigated in this work. The main characterization methods of X-ray fluorescence (XRF), X-ray diffraction (XRD), scanning electron microscope (SEM), and metallographic microscope were employed in this study. In this work, the reduction of HCVTM sinter with different temperature and basicity were experimented. The Fe, FeO, and TiO in reductive samples increase with increasing basicity and temperatures. The increase of basicity and temperature is favorable to the reduction of HCVTM sinter. The Fe phase has out-migration tendency to the surface of sinter while the perovskite and silicate phases have in-migration tendency to the inside of sinter. The reduction degradation index (RDI) decreases while the reduction index (RI) increases with increasing basicity. The RI increases from 67.14% to 82.09% with increasing temperature from 1073 K to 1373 K.
Key words: basicity; high-chromium vanadium-titanium magnetite; sintering pot test; mineralogy; reduction behavior
Cite this article as: TANG Wei-dong, YANG Song-tao, ZHANG Li-heng, HUANG Zhuang, YANG He, XUE Xiang-xin. Effects of basicity and temperature on mineralogy and reduction behaviors of high-chromium vanadium- titanium magnetite sinters [J]. Journal of Central South University, 2019, 26(1): 132–145. DOI: https://doi.org/10.1007/ s11771-019-3988-8.
1 Introduction
High-chromium vanadium-titanium magnetite (HCVTM) iron ore containing Fe, Ti, Cr, and V is a special and valuable iron ore as known by the exploitation of vanadium-titanium magnetite (VTM), ilmenite, and chromite [1, 2]. As a kind of valuable iron ore resource, HCVTM is found in the Hongge region (Panzhihua, China). Similar types of ores are mainly distributed in Russia, Canada, Australia and other places [3, 4]. As it is known, many studies have been done for the ordinary VTM, while the studies on HCVTM with complex phase compositions and reduction behaviors have been scarce [5, 6]. Owing to the immature production technology in the blast furnace (BF) process, the abundant quantity of such HCVTM has not been exploited and efficiently used on a large scale. Sinters, as the main furnace burden for smelting in the BF, play an essential role in the ironmaking process in Asia. Meanwhile, there is a massive gap between HCVTM sinter and ordinary sinter in the quality and smelting of sinter. Hence, the study on mineralogy and reduction behaviors of HCVTM sinter is both necessary and meaningful, and the quantity of HCVTM is adequate for industrial production in southwest China [7–14].
The basicity and temperature are essential factors in mineralogy and reduction behaviors of sinter. YANG et al [15] researched that mineralogy and quality of VTM sinter improve with increasing basicity, and Ti and V elements mainly existed in perovskite and silicate phases respectively. The RI of titanomagnetite sinter increases from 72.55% to 83.55% with increasing basicity, which shows that the high basicity sinter can easily be reduced in BF [16]. The adverse effect of TiO2 on titanomagnetite sinter can be restrained by high basicity due to the formation of melted phase SFCA. PIMENTA [17, 18] studied that the temperature had a significant influence on the microstructure of the sinter during low-temperature reduction. However, the research of the basicity and temperature on the mineralogy and reduction behavior of HCVTM sinter is scarce and inexplicit according to the previous studies, and the effects of basicity and temperature on the mineralogy and reduction behaviors of sinter are still in need of exploration before the application of HCVTM in BF. Hence, it is of great necessity to carry out the present work for the mineralogy and reduction behaviors of HCVTM.
To better research the utilization technology of HCVTM, as well as to provide a theoretical and practical basis, the mineralogy and reduction behaviors of HCVTM sinters were studied in this work. First, the mineralogy of HCVTM sinters with different basicity was observed. The RDI and RI of HCVTM were then tested and calculated. Phase transformation characteristics of HCVTM sinter with different basicity and reduction temperature were analyzed and discussed. Finally, the microstructure characteristics of HCVTM sinter with different reduction temperatures were detected and the distribution of elements in HCVTM sinter was revealed.
2 Materials and experiment
2.1 Raw materials
HCVTM iron ore originates from Hongge region (Panzhihua, China). Ordinary magnetite, gas-ash, magnetic powder, lime, coke, and coal were supplied by Chengde Jianlong Iron and Steel Group Company (Liaoning, China). The chemical compositions of raw materials are shown in Table 1. The coke and coal breeze and chemical compositions are shown in Table 2. Table 3 shows the size distribution of HCVTM, and it indicates the particle size lower than 75 μm occupies 72.47%, which indicates HCVTM iron ore is appropriate for granulation.
Figure 1 shows the XRD pattern of HCVTM, and the HCVTM primarily consists of magnetite (Fe3O4), titanomagnetite (Fe2.75Ti0.25O4), ilmenite (FeTiO3), coulsonite (Fe2VO4) and chromite (FeCr2O4).
Table 1 Chemical compositions of raw materials (mass fraction, %)
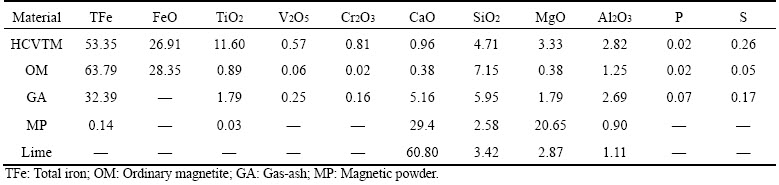
Table 2 Industrial analysis of coke and coal breeze and chemical compositions of ash (mass fraction, %)

Table 3 Size distribution of HCVTM (mass fraction, %)


Figure 1 XRD pattern of HTCVM ore
2.2 Sintering pot test
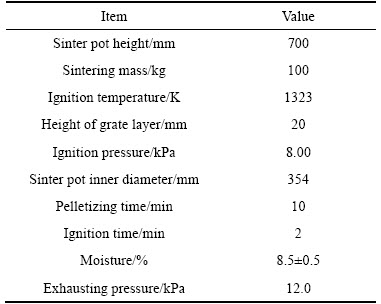
Table 4 shows experimental scheme and ingredient of sinter materials and basicity changes with the addition of lime. Firstly, 100 kg raw materials with 4.05% C (coke:coal=1) are mixed in a mixer and granulated in a granulator for 10 min, and rotation angle of granulator is in the range of ±45° at the process of granulation. Then pelletized sinter materials are filled in sinter pot with a diameter of 354 mm and a height of 700 mm, and 20 g coke is sprinkled evenly on the surface of sinter materials for ignition. Then, liquefied natural gas is lit and used to ignite the sinter materials at an ignition temperature of 1323 K for 2 min, and ignition suction is 8.00 kPa. At the end of ignition, extraction fan was started up to make sinter suction at 12.00 kPa. Finally, the temperature of flue gas reaches the peak value, and sinter temperature begins downturn. When the temperature of flue gas is below 373 K, sintering pot test finishes, and dust extraction fan starts up to eliminate the dust in sinter broken process. The parameters of sintering pot test are listed in Table 5.
The broken sinter is dropped from 2 m with three times. Then, HCVTM sinter is screened into five particle sizes (> 40 mm, 25–40 mm, 10–25 mm, 5–10 mm, and <5 mm) with a standard sieve system. After the sieving, HCVTM sinter is crushed into particles with a size distribution of 10–12.5 mm to do the reduction experiment. Each experiment was carried out in three repetitions.
2.3 Reduction experiment
Figure 2 shows the reduction equipment of HCVTM sinters. Firstly, 500 g HCVTM sinter with a size distribution of 10–12.5 mm is placed in a reactor with a diameter of 75 mm and a height of 215 mm. The electrical cabinet controls heating schedule. Meanwhile, mass change of HCVTM sinters is recorded by electronic balance and data transmits to the computer. The temperature of HCVTM sinter detects via a type-K thermocouple, and the reactor is heated by a heating furnace. Then, HCVTM sinters are heated from ambient temperature to reaction temperature, and the change process of gas is as follows: in the first step, N2 flow is 2 L/min when the temperature is below reaction temperature. In the second stage, when the temperature reaches to the reaction temperature, N2 is switched to the reaction gas. At the end of reduction experiment, the reactor is taken out from the heating furnace and cooled to below 373 K under a 1 L/min flow of N2. The reaction temperature of RDI is (773±10) K, and the composition of the reaction gas is CO, CO2, and N2 with a proportion of 20%, 20% and 60% under a (15±1) L/min total gas flow. The reaction temperature of RI is from (1073±10) K to (1373± 10) K, and the composition of the reaction gas is CO and N2 with a proportion of 30% and 70% under a (15±1) L/min total gas flow.
Table 4 Experimental scheme and ingredient of sinter materials

Table 5 Parameters of HCVTM sintering pot test
The RDI is a measure of disintegration property of iron ore sinter on the reduction reaction with CO at a temperature of 773 K. The RDI reflects the BF in the upper stack regions where it is mildly reducing, and temperatures are low [19]. The particle sizes (>3.15 mm) of sinter after reduction are used to calculate RDI. Referred to the standard of GB/T13242-91, the equation of RDI is given in Eq. (1):
 (1)
(1)
where mD0 is the mass of sinter after the reduction (g), mD1 is the mass of sinter on the 6.3 mm sieve (g), mD2 is the mass of sinter on the 3.15 mm sieve (g).
The reduction index (RI) of sinter is based on the standard GB/T 13241-1991, and it shows the reduction conditions of sinter in BF at 1173 K. The RI of sinter is calculated via Eq. (2):
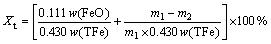 (2)
(2)
where Xt is RI of HCVTM sinter at time t (%), w(FeO) and w(TFe) are the mass of FeO and total iron of HCVTM sinter before reduction (%); m1 and m2 are the mass of HCVTM sinter before reduction (g). The constant 0.111 is a conversion ratio between unit FeO and unit Fe2O3, and constant 0.430 is a conversion ratio between unit Fe and unit Fe2O3.
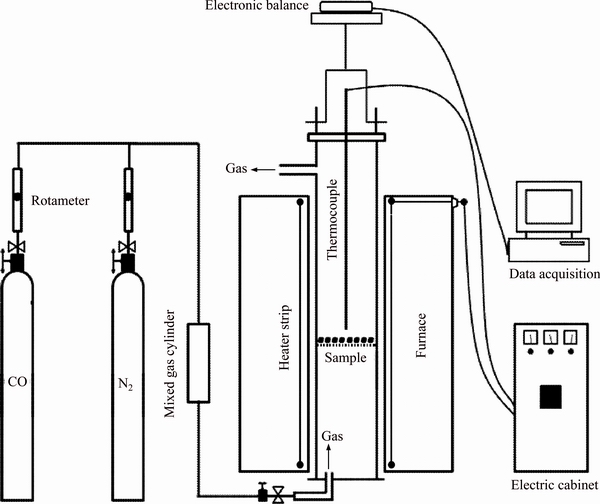
Figure 2 Schematic of reduction equipment
2.4 Characterization methods
The X-ray fluorescence (XRF, ZSXPrimus II; Rigaku, Japan) was used to test the chemical compositions of raw materials including CVTM, ordinary magnetite, ilmenite, gas-ash, and magnetic powder. The X-ray diffraction (XRD, X’ Pert Pro; PANalytical, Almelo, Netherlands) with Cu Kα radiation (wavelength 1.5406  ) at a setting of 40 kV and 40 mA was used to analyze the mineral phases of CVTM iron ore and CVTM sinters with different TiO2 content. The scanned range was 2q= 5°–90° with a step of 2q=0.17° and 1 s/step. All of the tested powders were ground lower than 200 μm. The scanning electron microscope (SEM, Ultra Plus; Carl Zeiss GmbH, Jena, Germany) was used to detect mineral phase structure and microstructure of reduced CVTM sinters, and backscattering detector (BSE) was used to investigate sinter surface, and energy disperse spectroscopy (EDS) was used to acquire mineral compositions. Sinter sample was heat mounted in resin and polished by mirror finish for microstructure and mineralogy analysis. A metallographic microscope (Leica DM1750M; Cambridge, England) was used to detect the mineralogy of CVTM sinter.
) at a setting of 40 kV and 40 mA was used to analyze the mineral phases of CVTM iron ore and CVTM sinters with different TiO2 content. The scanned range was 2q= 5°–90° with a step of 2q=0.17° and 1 s/step. All of the tested powders were ground lower than 200 μm. The scanning electron microscope (SEM, Ultra Plus; Carl Zeiss GmbH, Jena, Germany) was used to detect mineral phase structure and microstructure of reduced CVTM sinters, and backscattering detector (BSE) was used to investigate sinter surface, and energy disperse spectroscopy (EDS) was used to acquire mineral compositions. Sinter sample was heat mounted in resin and polished by mirror finish for microstructure and mineralogy analysis. A metallographic microscope (Leica DM1750M; Cambridge, England) was used to detect the mineralogy of CVTM sinter.
3 Results and discussion
3.1 Mineralogy of HCVTM sinters
Figure 3 shows the XRD pattern of HCVTM sinter with different basicities. The primary phases contain hematite (Fe2O3), magnetite (Fe3O4) and perovskite (CaTiO3) with basicity lower than 2.1. The peak intensity of Fe3O4 increases while the peak intensity of Fe2O3 decreases with basicity higher than 2.3. Moreover, the new phase peaks of magnesium ferrite (MgFe2O4) and wustite (FeO) generate with basicity higher than 2.1. Hence, Fe2O3 combines with MgO to generate MgFe2O4, and Fe2O3 is reduced to Fe3O4. Fe3O4 is then reduced to FeO due to the reduction reactions in the sintering process. Figure 4 shows the solid fuel consumption increases with increasing basicity. The solid fuel consumption is inversely proportional to the sinter yield, and the sinter yield of HCVTM decreases with increasing basicity. Hence, the solid fuel consumption increases with increasing basicity. The rise of solid fuel consumption means the increasing consumption of coal and coke under same carbon content of each HCVTM sinter pot test, which indicates the increasing heat transfer and reduction reactions in the sintering process. Hence, the FeO phase and magnetite melt increase with increasing basicity. MATSUNO et al [19] researched that coke reduction caused the formation of metallic iron and FeO phase during the heating process of iron ore sinter. LOO et al [20] studied that a significant reduction in pore area with high gangue level in the bonding phase of iron ore sinters.

Figure 3 XRD pattern of HCVTM sinter with different basicities

Figure 4 Solid fuel consumption of HCVTM sinter with different basicities
Figure 5 shows the mineralogy and microstructure of HCVTM sinter with different basicities. In Figure 5(a), hematite (Fe2O3) is the main mineral, while calcium ferrite (CF) is not observed. Small magnetite (Fe3O4) grains exist among hematite grains and perovskite (CaTiO3) grains. Long strip perovskite grains concentrate in the intervals of magnetite and silicate grains. When basicity reaches to 1.9, large hematite grains decompose to small block shape grains because a part of hematite reduces to magnetite and a part of other hematite phases surround block shape magnetite crystals. A part of perovskite phase exists as long strip shape, and CF phase exists as small dendrite shape in silicate grains as shown in Figure 5(b). When basicity of sinter reaches to 2.1, the small block shape hematite grains have an obvious demarcation line with block shape magnetite grains. Pores focus on the perovskite and silicate phases, and perovskite and silicate mix as shown in Figure 5(c). When basicity increases to 2.3 (Figure 5(d)), wustite (FeO) generates on the boundaries of magnetite grains due to the reduction reactions of coke and coal with magnetite in the sintering process. The small dendrite shape of CF and perovskite generate on silicate grains. Furthermore, the main mineralogical characteristic of HCVTM sinter is the grain refinement of hematite grains and the generation of CF with increasing basicity. HSIEH et al [21] researched that the amount of reoxidized hematite generated in the cooling process of sintering.
3.2 Reduction degradation index of HCVTM sinters
Figure 6 shows the RDI of HCVTM sinter. The RDI has a little variation with basicity lower than 2.1. Combined with the XRD pattern of HCVTM sinter with different basicity and the previous studies of our laboratory, the generation of magnesium ferrite has suppressing effect on the reduction of hematite. Hence, the RDI value increases when basicity changes from 1.9 to 2.1. However, the RDI decreases from 80.51% to 66.45% with increasing basicity. Hence, the basicity of HCVTM sinter in the range of 2.1 to 2.5 has a deteriorative effect on RDI. The appropriate basicity of HCVTM sinter is in the range of 1.7 to 2.1, which corresponds to the desirable basicity of ordinary sinters within the scope of 1.7 to 2.2. FU et al [22] considered that the increasing basicity of sinter improved the strength and metallurgical properties of sinter because of the intensified smelting and played an essential part in a substantial foundation. UMADEVI et al [23] deemed that the weakening and degradation of sinter are associated with volume increase due to the phase transformation of hematite to magnetite in sinter. With basicity higher than 2.1, more hematite is attainable, which led to more reduction of hematite to magnetite at low reduction temperature. Hence, less strain is formed in HCVTM sinter structure and leads to the decrease of RDI.

Figure 5 Microstructure of HCVTM sinter with different basicities (H: hematite; M: Magnetite; Ma: Magnesium ferrite; CF: Calcium ferrite; S: Silicate; Pe: Perovskite; P: Pore):
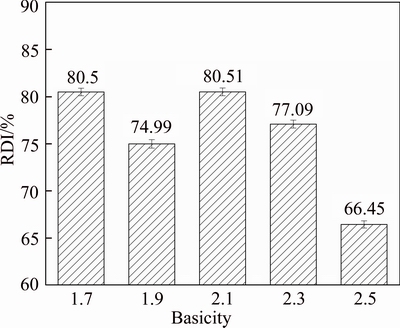
Figure 6 RDI of HTCVM sinters with different basicity
3.3 Reduction index of HCVTM sinters
Figure 7 shows the RI of HCVTM sinter with different temperatures. The RI increases from 67.14% to 82.09% with increasing temperature from 1073 K to 1373 K. The change rule of RI indicates the reduction rate of HCVTM sinter is the lowest at 1073 K while the reduction rate is the highest at 1373 K in the initial stage and decreases in the subsequent process of reduction reactions. Hence, the characteristic of high temperature on reduction reaction is more obviously than low temperature. Meanwhile, the increasing temperature promotes the diffusion of reducing gas. The RI regulation of HCVTM sinter illustrates that the RI enhances with increasing reduction temperature. HALIM et al [21] clarified that the reduction rate of iron oxide increased with increasing temperature. EL-GEASSY et al [25] considered that the reduction of iron ore increased gradually with increasing temperature and had a rapid increase in reduction rate when the temperature is higher than 1173 K.

Figure 7 RI of HTCVM sinters with different reduction temperatures
Figure 8 shows RI of HCVTM sinter with different basicity. The RI increases from 68.20% to 80.76% with increasing basicity from 1.7 to 2.5, which indicates that high basicity of HCVTM sinter is beneficial to be reduced in BF process. The suppressing effect of magnesium ferrite on the reduction of hematite leads to a slight decrease of RI when basicity is 2.1. With the rise of basicity, the reduction curves of HCVTM sinters with different basicities have an analogous tendency of each other, and the reduction rate increases with increasing basicity, as shown in Figure 9. Combing the mineralogy and microstructure of HCVTM sinter, the increasing CF promotes the reduction of sinter due to the easily reductive CF. The increasing basicity facilitates the reduction reaction of HCVTM sinters at the same temperature. Therefore, the positive effect of basicity on the RI of HCVTM sinter is attributed to the generation of CF. Meanwhile, the grain refinement of hematite grains increases the contact area by reducing gas and improves the reduction kinetics of HCVTM sinter. HALIM [21] clarified the contrast result that reduction rate decreased by increasing basicity due to the growth of grain and compact structure in the reductive pure compact.
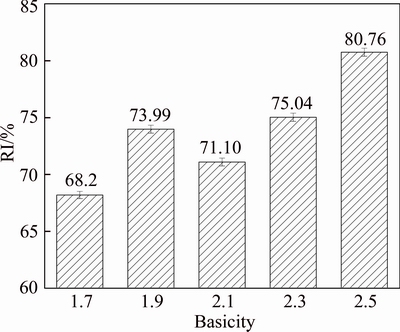
Figure 8 RI of HTCVM sinters with different basicity at 1173 K

Figure 9 Reduction curves of HTCVM sinters with different basicities
3.4 Phase transformation of reductive HCVTM sinters
Figures 10 and 11 show the XRD pattern of reductive HCVTM sinters to determine the phase variations of HCVTM sinter with different reduction temperatures and basicity. Compared with the unreduced and reductive HCVTM sinter with basicity of 1.9, the changes of primary phases are noticeable. The peak intensity of Fe2O3 and Fe3O4 become weak, and new phases of Fe, FeO, Fe2VO4, Cr-bearing Fe3O4 and MgFe2O4 generate with increasing reduction temperature from ambient temperature to 1073 K. The phenomenon indicates that Mg2+ combines Fe2+ to generate magnetite solid solution MgFe2O4 at the low sintering temperature. Hence, the peak intensity of Fe2O3 decreases in this situation. LV et al [26] studied that MgFe2O4 was a kind of spinel in the bonding phase of sinter. TANG et al [27] considered that stable MgFe2O4 generated during the roasting process of vanadium-titanium magnetite ore. The peak intensities of MgFe2O4, Cr-bearing Fe3O4 and Fe2VO4 decrease when the temperature rises to 1173 K. Meanwhile, titanium monoxide (TiO) generates from the reduction of titanic oxides. ANDERSSON et al [28] considered that the generation of TiO at 1173 K is stable to prepare TiOx. PHILIPP et al [29] studied that the carbon reduction of titanium oxides and TiO was the further reductive product in the range of 1173–1573 K. In Figure 10, the peaks intensity of MgFe2O4 and Fe2VO4 become weak while the peak intensity of akermanite (Ca2Mg(Si2O7)) and TiO become intense with the temperature rising to 1273 K. It is due to the migration of Mg element into silicate phase and the enhancement of reduction degree of titanium oxides. NECHKIN et al [30] considered that magnesium oxide could readily enter into low-melting silicates due to the formation of a synthetic substance in sintering flux. CHANADEE [31] researched the magnesium silicate phase formed in the rapid cooling rate. Furthermore, the peak intensities of TiO and FeO reach to the strongest at 1373 K, which indicates that the reduction of titanium oxides and iron oxides enhances with increasing reduction temperatures.
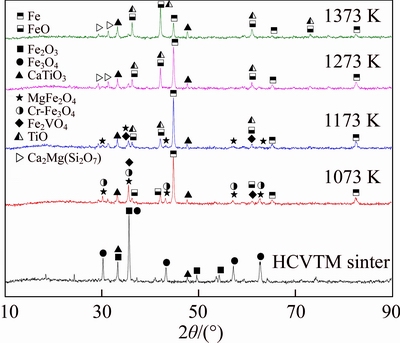
Figure 10 XRD patterns of reductive HCVTM sinter with different temperatures

Figure 11 XRD patterns of reductive HCVTM sinter with different basicities
The effect of basicity on phase transformation of reductive HCVTM sinter is not apparent than reduction temperatures. The peak intensities of Fe, FeO, and TiO increase with rising basicity, which indicates that basicity promotes the generation of the low-valence of titanium and iron oxides as the same tendency of reduction temperatures. Hence, the effect on the Fe phase in the reductive HCVTM sinter connects with the hindering of FeO and TiO. HALIM et al [21] considered that the reduction rate of iron oxide decreased by the increasing basicity value due to the presence of calcium silicate in doped compacts. MOUSA [32] researched that the formation of CF improved the reducibility of FeO in sinter and the formation of hard reduced silicate phase (Fe2SiO4 and (Ca0.5Fe0.5)SiO3) decreases the reduction rate of FeO.
3.5 Microstructure of reductive HCVTM sinters
Figure 12 shows the reduction characteristics of HCVTM sinter with increasing temperature. In Figure 12(a), Fe and FeO exist in the boundaries of hematite and magnetite grains in the outer layer of HCVTM sinter. The size dimension of iron-bearing grains in the reductive region is smaller than the iron-bearing grains in the unreduced region because the volume of iron oxide decreases with the removal of oxygen ion. What’s more, the silicate phase and perovskite phase have no apparent change in the reductive region and unreduced region of HCVTM sinter. The thickness of the reductive layer increases when reduction temperature rises to 1173 K. Meanwhile, the quantity of Fe and FeO increase because the reduction of HCVTM sinter improves with increasing temperature. The perovskite has a significant difference among different reduction regions. In the reductive area, perovskite decomposes to small and dendrite shape grains while the part of perovskite mixes with silicate phase in the unreduced region, as shown in Figure 12(b). Meanwhile, silicate and perovskite distribute on the intervals of Fe and FeO when the temperature rises to 1273 K, as shown in Figure 12(c). Furthermore, the Fe concentrates on the surface of HCVTM sinter, and unreduced FeO, silicate and perovskite phases exist in the inside of HCVTM sinter, as shown in Figure 12(d).
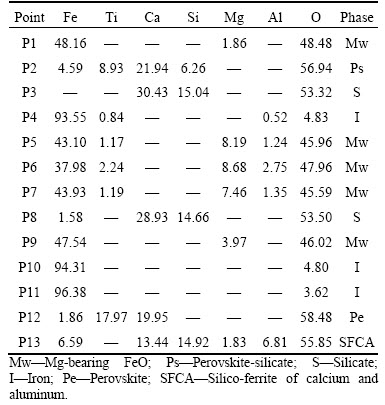
When the basicity of HCVTM sinter is 1.7, the mineralogy and microstructure of reductive HCVTM sinter are different with rising temperature, as shown in Figure 13. The Mg-bearing FeO occurs as euhedral crystal and subhedral crystal with increasing temperature. When the atom content of Mg on FeO is lower than 4%, the shape of Mg-bearing FeO is small particles. When the atom content of Mg on FeO is higher than 8%, the Mg-bearing FeO grains occupy the main part of FeO. The euhedral perovskite decomposes to small particle shape, while silicate phase has no apparent changes in HCVTM sinter. In Figure 13(a), the large grains of perovskite contain 6.26 at% Si, while the small grains of perovskite phase with no element Si as shown in Figure 13(d). Hence, the mineralogy of perovskite is affected by different reduction temperatures and Si contents. The compositions of main phase points are signed in Figure 13 and listed in Table 6.

Figure 12 BSE images of reductive HCVTM sinters with different temperatures at 500':

Figure 13 BSE images of reductive HCVTM sinters with different temperatures at 2000':
Figures 14 and 15 show the EDS mapping of reductive HCVTM sinter with 1073 K and 1373 K, respectively. Elements Ti, Ca and a part of Si overlap in the mapping while elements Fe and Ti separate. In the interspaces of the euhedral crystal of FeO, there are phases of SFCA and perovskite. BRISTOW et al [33] considered that the most of the element Ti existed in silica phase and glass phase. Hence, some perovskite exists in SFCA phase. What’s more, elements Fe and Mg overlap in the mapping due to the formation of MgFe2O4; the majority of elements Ca and Si bond together to generate calcium orthosilicate (Ca2SiO4), as shown in Figure 13. CLOUT et al [34] studied that SFCA phase precipitated from the Fe-rich melt at the cooling process from 1573 to 1513 K with basicity higher than 2.0.
Table 6 Compositions of main phases in HCVTM sinter detected by EDS (atomic fraction, %)
After reduction of HCVTM sinter at 1373 K for 2 h, the metallic iron spreads on the surface and outer layer of HCVTM sinter, and the inside of iron-bearing phase is FeO. In Fe-rich phases, Ti element bonds with Ca element as perovskite in the interspaces of metallic iron grains and FeO grains. In generally, perovskite phase disperses in the intervals of iron-bearing grains and silicate grains. A majority of element Mg exists in FeO rather than metallic iron, which indicates the affinity of Mg and O elements. Elements Ca, Si, Al, and O crystallize as glass phases which fill in the intervals of metallic iron grains and FeO grains, and SFCA phase exists on the grain boundaries of iron-bearing phases and glass phases. PANIGRAHY et al [35] considered that vitreous glass crystallized from the melt as silicates in the sintering process. MOUSA et al [36] researched that CF and SFCA filled the pore area between the metallic iron grains in the reduction process of iron ore sinter. HWSSIEN et al [37] studied that the formation of SFCA phase in sinter can improve the high-temperature reduction property due to the low slag content in sinter. The reduction degree of HCVTM sinter increases with high temperature because the favorable SFCA phases generate in high reduction temperature.

Figure 14 BSE image and EDS mapping of reductive HCVTM sinter at 1073 K
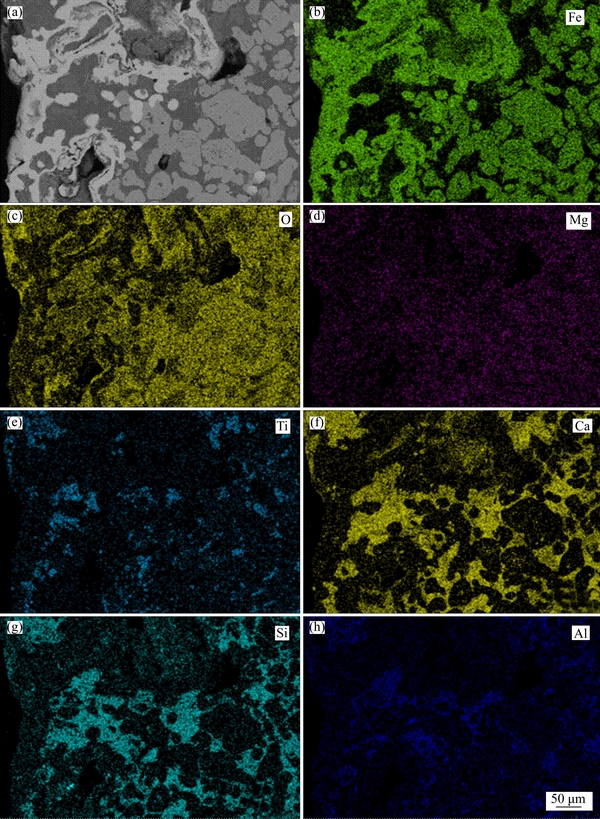
Figure 15 BSE image and EDS mapping of reductive HCVTM sinter at 1373 K
4 Conclusions
1) The quantity of Fe3O4 phase increases with increasing basicity. The FeO forms and local reduction happens while Fe2O3 phase disappears with basicity higher than 2.3.
2) The quantity of metallic iron and TiO phases increase with increasing temperature. The basicity has little influence on phase transformation of HCVTM sinters while temperature has an obvious effect on phase transformation of HCVTM sinter in the reduction process.
3) The iron oxide is reduced intentinonally with increasing temperature. The iron phases have out-migration tendency to the surface of HCVTM sinter while perovskite and silicate phases have in-migration tendency to the inside of HCVTM sinter. The mineralogy of perovskite is affected by different reduction temperatures and Si content.
4) The Mg-bearing FeO occurs as euhedral crystal and subhedral crystal with rising temperature. Element Mg migrates to iron-bearing oxides, and perovskite disperses and migrates to the intervals of iron-bearing grains and silicate grains at 1373 K.
5) The RDI has a little variation with basicity lower than 2.1. The RDI decreases from 80.51% to 66.45% with basicity increasing from 2.1 to 2.5. The RI increases from 67.14% to 82.09% with increasing temperatures from 1073 K to 1373 K. The RI increases from 68.20% to 80.76% with increasing basicity from 1.7 to 2.5.
References
[1] CHENG Gong-jin, GAO Zi-xian, LV Meng-yang, YANG He, XUE Xiang-xin. Coal-based reduction and magnetic separation behavior of low-grade vanadium-titanium magnetite pellets [J]. Minerals, 2017, 7(6): 86–99.
[2] LU Chang-yuan, ZOU Xing-li, LU Xiong-gang, XIE Xue-liang, ZHENG Kai, XIAO Wei, CHENG Hong-wei, LI Guang-shi. Reductive kinetics of panzhihua ilmenite with hydrogen [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(12): 3266–3273.
[3] CHENG Gong-jin, XUE Xiang-xin, GAO Zi-xian, JIANG Tao, YANG He, DUAN Pei-ning. Effect of Cr2O3 on the reduction and smelting mechanism of high-chromium vanadium-titanium magnetite pellets [J]. ISIJ International, 2016, 56(11): 1938–1947.
[4] LI Wei, FU Gui-qin, CHU Man-sheng, ZHU Miao-yong. Non-isothermal reduction behavior and mechanism of hongge vanadium titanomagnetite pellet with simulated shaft furnace gases [J]. ISIJ International, 2018, 58(3): 415–421.
[5] CHENG Gong-jin, GAO Zi-xian, YANG He, XUE Xiang-xin. Effect of calcium oxide on the crushing strength, reduction, and smelting performance of high-chromium vanadium–titanium magnetite pellets [J]. Metals, 2017, 7(6): 181.
[6] CHENG Gong-jin, XUE Xiang-xin, JIANG Tao, DUAN Pei-ning. Effect of TiO2 on the crushing strength and smelting mechanism of high-chromium vanadium-titanium magnetite pellets [J]. Metallurgical and Materials Transactions B, 2016, 47(3): 1713–1726.
[7] YANG Song-tao, TANG Wei-dong, ZHOU Mi, JIANG Tao, XUE Xiang-xin, ZHANG Wei-jun. Effects of dolomite on mineral compositions and metallurgical properties of chromium-bearing vanadium-titanium magnetite sinter [J]. Minerals, 2017, 7(11): 210–224.
[8] YANG Song-tao, ZHOU Mi, TANG Wei-dong, JIANG Tao, XUE Xiang-xin, ZHANG Wei-jun. Influence of coke ratio on the sintering behavior of high-chromium vanadium-titanium magnetite [J]. Minerals, 2017, 7(7): 107–120.
[9] YANG Song-tao, ZHOU Mi, JIANG Tao, XUE Xiang-xin. Isothermal reduction kinetics and mineral phase of chromium-bearing vanadium–titanium sinter reduced with CO gas at 873–1273 K [J]. International Journal of Minerals, Metallurgy, and Materials, 2018, 25(2): 145–152.
[10] CHENG Gong-jin, GAO Zi-xian, YANG He, XUE Xiang-xin. Effect of diboron trioxide on the crushing strength and smelting mechanism of high-chromium vanadium-titanium magnetite pellets [J]. International Journal of Minerals, Metallurgy, and Materials, 2017, 24(11): 1228–1240.
[11] GAN Min, JI Zhi-yun, FAN Xiao-hui, LV Wei, ZHENG Ru-yue, CHEN Xu-ling, LIU Shu, JIANG Tao. Preparing high-strength titanium pellets for ironmaking as furnace protector: Optimum route for ilmenite oxidation and consolidation [J]. Powder Technology, 2018, 333: 385–393.
[12] GAN Min, JI Zhi-yun, FAN Xiao-hui, CHEN Xu-ling, ZHENG Ru-yue, GAO Lu, WANG Guo-jiang, JIANG Tao. Value-added utilization of waste silica powder into high-quality chromite pellets preparation process [J]. Powder Technology, 2018, 328: 122–129.
[13] GAN min, FAN Xiao-hui, CHEN Xu-ling, JI Zhi-yun. High temperature mineralization behavior of mixtures during iron ore sintering and optimizing methods [J]. ISIJ international, 2015, 55(4): 742–750.
[14] TANG Wei-dong, YANG Song-tao, CHENG Gong-jin, GAO Zi-xian, YANG He, XUE Xiang-xin. Effect of TiO2 on the sintering behavior of chromium-bearing vanadium- titanium magnetite [J]. Minerals, 2018, 8(7): 263–275.
[15] YANG Song-tao, ZHOU Mi, JIANG Tao, XUE Xiang-xin, ZHANG Wei-jun. Effect of basicity on sintering behavior of low-titanium vanadium–titanium magnetite [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(6): 2087–2094.
[16] JIANG Tao, WANG Shuai, GUO Yu-feng, CHEN Feng, ZHENG Fu-qiang. Effects of basicity and MgO in slag on the behaviors of smelting vanadium titanomagnetite in the direct reduction-electric furnace process [J]. Metals, 2016, 6(5): 107–121.
[17] PIMENTA H P, SESHADRI V. Characterisation of structure of iron ore sinter and its behaviour during reduction at low temperatures [J]. Ironmaking & Steelmaking, 2002, 29(3): 169–174.
[18] PIMENTA H P, SESHADRI V. Influence of Al2O3 and TiO2 degradation behaviour of sinter and hematite at low temperatures on reduction [J]. Ironmaking & Steelmaking, 2002, 29(3): 175–179.
[19] MATSUNO F, HARADA T. Changes of mineral phases during the sintering of iron ore-lime stone systems [J]. Transactions of the Iron and Steel Institute of Japan, 1981, 21(5): 318–325.
[20] LOO C E, LEUNG W. Factors Influencing the bonding phase structure of iron ore sinters [J]. ISIJ International, 2003, 43(9): 1393–1402.
[21] HSIEH L H, JA W. Effect of raw material composition on the mineral phases in lime-fluxed iron ore sinter [J]. ISIJ International, 1993, 33(4): 462–473.
[22] FU Wei-guo, WEN Yong-cai, XIE Hong-en. Development of intensified technologies of vanadium-bearing titanomagnetite smelting [J]. Journal of Iron and Steel Research, International, 2011, 18(4): 7–18.
[23] UMADEVI T, NELSON K, MAHAPATRA P C, PRABHU M, RANJAN M. Influence of magnesia on iron ore sinter properties and productivity [J]. Ironmaking & Steelmaking, 2009, 36(7): 515–520.
[24] ABDEL HALIM K S, BAHGAT M, El-Kelesh H A. Metallic iron whisker formation and growth during iron oxide reduction: basicity effect [J]. Ironmaking & Steelmaking, 2009, 36(8): 631–640.
[25] EL-GEASSY A A, NASR M I, KHEDR M H, ABDEL-HALIM S. Reduction behaviour of iron ore fluxed pellets under load at 1023–1273 K [J]. ISIJ International, 2004, 44(3): 462–469.
[26] LV Xue-wei, BAI Cheng-guang, HE Sheng-ping, HUANG Qing-yun. Mineral change of Philippine and Indonesia nickel lateritic ore during sintering and mineralogy of their sinter [J]. ISIJ International, 2010, 50(3): 380–385.
[27] TANG Jue, CHU Man-sheng, XUE Xiang-xin. Optimized use of MgO flux in the agglomeration of high-chromium vanadium-titanium magnetite [J]. International Journal of Minerals, Metallurgy, and Materials, 2015, 22(4): 371–380.
[28] ANDERSSON S, COLLEN B, KUYLENSTIERNA U, MAGNELI A. Phase analysis studies on the titanium-oxygen system [J]. Acta Chemica Scandinavica, 1957, 11(10): 1641–1652.
[29] SCHLENDER P, ADAM A E W. Combined carboreduction– iodination reaction of TiO2 and FeTiO3 as the basic step toward a shortened titanium production process [J]. Industrial & Engineering Chemistry Research, 2017, 56(23): 6572–6578.
[30] NECHKIN G A, KOBELEV V A, CHERNAVIN A Y, CHERNAVIN D A. Effect of oxides of magnesium and manganese and the basicity of the iron-ore-bearing materials on the ability of the smelting products to filter through the coke column in blast furnaces [J]. Metallurgist, 2016, 59(11, 12): 1035–1039.
[31] CHANADEE T. Experimental studies on self-propagating high-temperature synthesis of Si-SiC composite from reactants of SiO2 derived from corn cob ash/C/Mg [J]. Journal of the Australian Ceramic Society, 2017, 53(1): 245–252.
[32] MOUSA E A. Effect of basicity on wüstite sinter reducibility under simulated blast furnace conditions [J]. Ironmaking & Steelmaking, 2013, 41(6): 418–429.
[33] BRISTOW N J, LOO C E. Sintering properties of iron ore mixes containing titanium [J]. ISIJ international, 1992, 32(7): 819–828.
[34] CLOUT J M F, MANUEL J R. Fundamental investigations of differences in bonding mechanisms in iron ore sinter formed from magnetite concentrates and hematite ores [J]. Powder Technology, 2003, 130(1–3): 393–399.
[35] PANIGRAHY S, VERSTRAETEN P, DILEWIJNS J. Influence of MgO addition on mineralogy of iron ore sinter [J]. Metallurgical Transactions B, 1984, 15(1): 23–32.
[36] MOUSA E, SENK D, BABICH A, GUGENAU H W. Influence of nut coke on iron ore sinter reducibility under simulated blast furnace conditions [J]. Ironmaking & Steelmaking, 2010, 37(3): 219–228.
[37] HESSIEN M, KASHIWAYA Y, ISHII K, NASR M I. Sintering and heating reduction processes of alumina containing iron ore samples [J]. Ironmaking & Steelmaking, 2008, 35(3): 191–204.
(Edited by HE Yun-bin)
中文导读
碱度和温度对高铬型钒钛烧结矿矿物学和还原行为的影响
摘要:在本工作中,研究了碱度和温度对红格高铬钒钛烧结矿还原过程的影响。本研究主要采用了XRF,XRD,SEM和金相显微镜的表征方法。在本文中,研究了不同温度和碱度对高铬钒钛烧结矿的还原行为。还原烧结矿中的Fe,FeO和TiO含量随着碱度和温度的升高而增加。结果表明升高碱度和温度有利于高铬钒钛烧结矿的还原。其中,Fe相有向烧结矿表面迁移的趋势,而钙钛矿和硅酸盐相有向烧结矿内部迁移的趋势。随着碱度的增加,低温还原粉化指数(RDI)降低,而还原指数(RI)增加。当温度从1073 K升高到1373 K时,RI从67.14%增加到82.09%。
关键词:碱度;高铬型钒钛磁铁矿;烧结杯实验;矿物学;还原行为
Foundation item: Project(2013CB632603) supported by the National Basic Research Program of China; Project(2015BAB19B02) supported by the National Key Technology R&D Program of China; Projects(51674084, 51174051, 51574082) supported by National Natural Science Foundation of China
Received date: 2017-11-17; Accepted date: 2018-07-16
Corresponding author: XUE Xiang-xin, PhD, Professor; Tel: +86-24-83681711; E-mail: xuexx@mail.neu.edu.cn;

