添加碳酸钙促进镍渣碳热还原
来源期刊:中国有色金属学报(英文版)2019年第12期
论文作者:李小明 闻震宇 李怡 杨海博 邢相栋
文章页码:2658 - 2666
关键词:镍渣;铁橄榄石;碳热还原;碳酸钙
Key words:nickel slag; fayalite; carbothermic reduction; CaCO3
摘 要:研究添加碳酸钙对镍渣碳热还原过程的影响,并分析其机理。结果表明,随着原料中碳酸钙添加量从0增加到8%(质量分数),还原反应初始温度和达到最大反应速率所需的温度分别从1100和1150 °C降低到1000和1100 °C,镍渣的还原率从58%增加到88%;还原后的渣中铁粒发生粗化,金属铁的衍射峰强度增加,表明添加碳酸钙有利于促进镍渣中铁化合物的还原回收。
Abstract: The effect of CaCO3 addition on the carbothermic reduction of nickel slag was studied, and the mechanism of CaCO3 in improving the reduction was analyzed. The results showed that when the CaCO3 content added to the slag was increased from 0 to 8 wt.%, initiation temperature of the carbothermic reaction decreased from 1100 to 1000 °C, the temperature reaching the maximum reduction rate decreased from 1150 to 1100 °C, and the reduction degree of the nickel slag increased from 58% to 88%. The iron particles in the reduced nickel slag were coarsened and the X-ray diffraction intensity of metallic iron peaks increased, confirming that the addition of CaCO3 was beneficial to the reduction of nickel slag and recovery of iron.
Trans. Nonferrous Met. Soc. China 29(2019) 2658-2666
Xiao-ming LI1,2, Zhen-yu WEN1, Yi LI1, Hai-bo YANG1, Xiang-dong XING1,2
1. School of Metallurgical Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, China;
2. Shaanxi Engineering Research Center of Metallurgy, Xi’an University of Architecture and Technology, Xi’an 710055, China
Received 25 February 2019; accepted 28 October 2019
Abstract: The effect of CaCO3 addition on the carbothermic reduction of nickel slag was studied, and the mechanism of CaCO3 in improving the reduction was analyzed. The results showed that when the CaCO3 content added to the slag was increased from 0 to 8 wt.%, initiation temperature of the carbothermic reaction decreased from 1100 to 1000 °C, the temperature reaching the maximum reduction rate decreased from 1150 to 1100 °C, and the reduction degree of the nickel slag increased from 58% to 88%. The iron particles in the reduced nickel slag were coarsened and the X-ray diffraction intensity of metallic iron peaks increased, confirming that the addition of CaCO3 was beneficial to the reduction of nickel slag and recovery of iron.
Key words: nickel slag; fayalite; carbothermic reduction; CaCO3
1 Introduction
Rapid development of the iron and steel industry has increased the demand for iron sources. Nickel slag, a by-product of nickel flash-smelting and oxygen-enriched top-blowing processes [1], contains a high iron content and different quantities of valuable metals, such as nickel, copper, cobalt, gold, and silver [2]. It is a potential resource for the iron and steel industry. About 40 million tons of nickel slag has accumulated in China and about two million tons of new nickel slags are manufactured each year [3]. Most of this slag remains stockpiled, which occupies land and wastes metal resources. Development and utilization of nickel slag can meet the requirement for comprehensive utilization of secondary resources, with its associated environmental, economic, and social benefits.
Many processes for utilization of nickel slag have been developed in the past few decades. The processes are classified into three categories: extraction of valuable metal elements by leaching processes, preparation of ferroalloys, corrosion-resistant steel or nickel matte by smelting reduction, and production of concentrated iron powder by direct reduction followed by magnetic separation. In the leaching processes, iron enters the tailings for further processing and produces secondary pollution, such as waste acid, waste water, and residue containing heavy metal ions. In the smelting reduction process, iron is primarily present in nickel slag in the form of silicates, so it is difficult to reduce, separate and concentrate, and it is necessary to raise the slag smelting temperature, which leads to high energy consumption, a large amount of secondary residue, and strict production control conditions. The direct reduction process is generally preferred because of its low smelting temperature and stable processing technology [4-6]. Nickel slag is difficult to reduce because of its complex mineral composition, so improving its reduction to enable extraction of valuable metal elements has become an urgent issue.
There have been many advances in the reduction of iron-containing materials. Addition of Na2CO3 promoted the carbothermic reduction of high-phosphorus limonite and siderite ores [7,8]. Increased CaCO3 content was shown to be beneficial to the direct reduction and melting of a low-alkalinity vanadium–titanomagnetite concentrate [9]. The metallurgical properties of a titanium-bearing blast-furnace slag were improved by adding CaO [10,11]. A small amount of Na2CO3 was beneficial to the reduction of iron oxide during a redox process and increased its reduction degree [12,13]. Although many experiments have been performed to promote the reduction process with additives, the enhanced reduction of nickel slag has not been systematically studied.
In this work, the mechanism of the improved reduction with CaCO3 during carbothermic reduction of nickel slag was investigated. The effects of reduction conditions on the carbothermic reduction of the slag, including reduction temperature and CaCO3 content, were studied. The method proposed in this work provides a good technical and theoretical basis for comprehensive utilization of nickel slag and other similar smelting slags.
2 Experimental
2.1 Raw materials
The nickel slag was a by-product of nickel smelting in a flash furnace, and it was collected from a plant in China. The chemical composition of the nickel slag is given in Table 1. The contents of total Fe, SiO2, and CaO were 39.40%, 32.80%, and 1.20%, respectively. X-ray diffraction (XRD), shown in Fig. 1, confirmed that the main phases of the nickel slag were Fe2SiO4 and Mg2SiO4. The slag was ground to a size less than 0.074 mm before use in experiments. High-purity graphite powder was used as the reducing agent, and CaCO3 was used to promote the reaction.
Table 1 Chemical composition of nickel slag (wt.%)

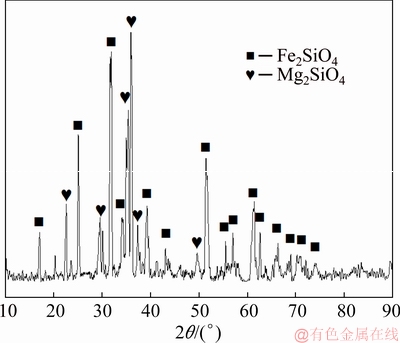
Fig. 1 X-ray diffraction pattern of nickel slag
Scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS) analysis results of the nickel slag is shown in Fig. 2. The EDS analysis showed that the slag was primarily composed of Fe, O, Si, Mg and S. The O and Si formed SiO2 that was uniformly distributed in the slag. Fe was primarily distributed in the gaps among large particles. The large particles were mainly solid solutions formed by Mg and silicate. S existed in the slag in the form of small particles, indicating that the sulfide and silicate did not form a solid solution.
2.2 Experiment
Thermogravimetry (TG), which provides a direct measurement of mass loss during a reaction as a function of time, is an effective technique to study the reaction processes. TG (SETARAM SETSYS Evolution, France) was used to study the carbothermic reduction of the nickel slag. Approximately 5 mg of sample was used for each experiment, placed in a circular crucible with a height of 8 mm and a diameter of 5 mm. The mass loss of the sample was recorded from room temperature to 1200 °C at a heating rate of 20 °C/min. To ensure the reproducibility of the results, each test was repeated three times.
Reduction experiments were performed in a vertical high-temperature tube furnace. A schematic of the experimental apparatus is shown in Fig. 3. The main section of the apparatus was a vertical tube furnace equipped with an automatic balance that had a detection precision of 1 mg. The temperature of the furnace was controlled by a temperature controller. The accuracy of the temperature measurement was ±1 K. The balance and temperature controller were connected to a computer that was used to collect the experimental data. Argon gas (99.999% in purity) was used as a shielding gas [14].
To ensure complete reaction of the sample, the ratio of the actual carbon content added to the theoretical carbon required was 1.2:1 (i.e., 20% stoichiometric excess). The nickel slag was uniformly mixed with the graphite powder and CaCO3, and then pressed into a cylinder with diameter of 8 mm and mass of about 5 g. Polyvinyl alcohol (2 wt.%) was used as a binder. The briquette was placed in a corundum crucible, which was placed into the furnace under a flowing argon atmosphere (0.8 L/min) and heated. When the temperature of non-isothermal carbothermic reduction reached the set value (600, 800, 1000, 1100 and 1200 °C), the crucible was quickly removed and cooled in an argon atmosphere (0.8 L/min). The reduced nickel slag was crushed and milled to less than 0.074 mm to determine the phase composition and morphology.
The crystalline phases of the raw material and reduced samples were identified by XRD (PANalytical, Netherlands) using Cu Kα radiation at 40 kV and 40 mA, and a scan speed of 4 (°)/min with a step of 0.026°. The morphology of the samples was characterized by SEM (Phenom-World, Netherlands) equipped with an EDS using an acceleration voltage of 15 kV.
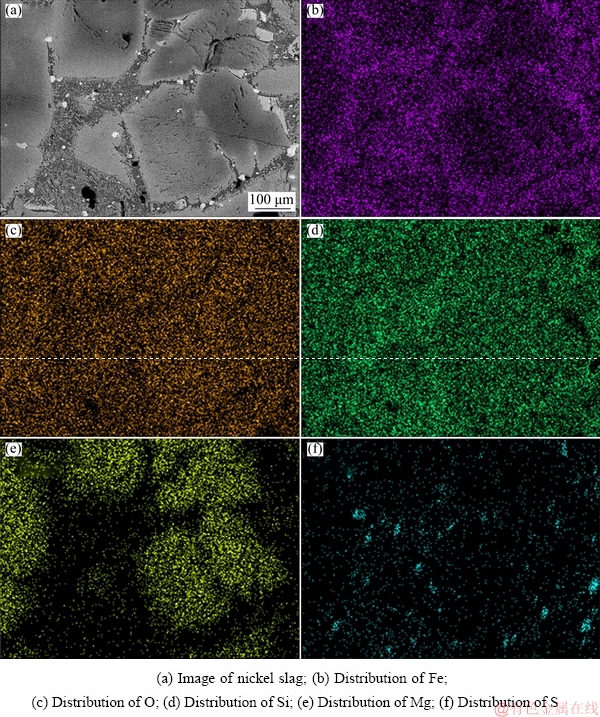
Fig. 2 Scanning electron micrographs and energy-dispersive spectra of nickel slag
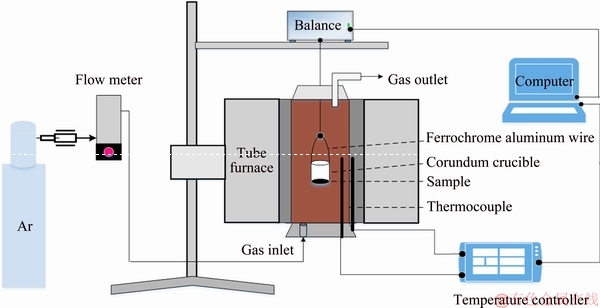
Fig. 3 Schematic of experimental apparatus
2.3 Determination of reduction degree
Reduction of oxide in the nickel slag caused a mass loss because oxygen in the crystal structure of oxide is released during the reduction process. The reduction degree (α) of the nickel slag at any time t is defined as the ratio of the loss of gas in an experiment to the theoretical maximum gas loss:
α=(Δmt /m0)×100% (1)
where Δmt is the mass loss of gas at reduction time t, and m0 is the theoretical mass loss.
2.4 Thermodynamic calculations
Thermodynamics of the reactions between carbon and the main components in the nickel slag were calculated by FactSage software. The basic principle is to minimize the Gibbs free energy of the constant- temperature and constant-pressure system by satisfying the material balance equation. The calculated temperatures were 25-1200 °C and the pressure of the system was 101.325 kPa. The main reaction and Gibbs free energy changes are given in Table 2 [15]. The calculated amounts of substances at different temperatures are shown in Fig. 4.
Table 2 Main reactions and standard Gibbs free energies
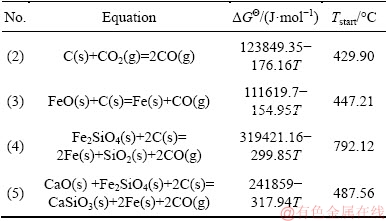
Table 2 shows that CO2 which was dissociated from CaCO3 underwent a Boudouard reaction with C, which promoted the carbon gasification reaction and increased the CO content of the gaseous reducing agent. Mass and heat transfer during the reducing reaction can be optimized by CO2 [16,17]. The Gibbs free energy change of Eq. (5) was significantly lower than that of Eq. (4), indicating that the former was more likely to occur. CaO which was dissociated from CaCO3 could replace FeO in fayalite, which could increase the reducing activity of FeO and promote the reduction of nickel slag [18].
The initial reactants of nickel slag contained 0.5 mol FeO, 0.5 mol Fe2SiO4 and 1.2 mol C, and the initial reactants of nickel slag with 8 wt.% CaCO3 (Fig. 4(b)) contained 0.5 mol FeO, 0.5 mol Fe2SiO4, 1.2 mol C and 0.17 mol CaCO3. By comparing Fig. 4(a) with 4(b), the addition of CaCO3 caused changes in the amount and composition of substances in carbothermic reduction products of nickel slag at different temperatures. In the equilibrium compositions with CaCO3 addition (Fig. 4(b)), the content of iron in the reduction product was significantly higher than that without adding CaCO3 (Fig. 4(a)). In addition, CaSiO3 appeared in the slag, while the content of Fe2SiO4 decreased and the content of FeO increased. The results showed that CaO, the decomposition product of added CaSiO3, reacted with Fe2SiO4 to form CaSiO3, and FeO was dissociated and then was further reduced to iron. The thermodynamic calculation diagram clearly showed that the addition of CaCO3 can promote the carbothermic reduction of nickel slag.
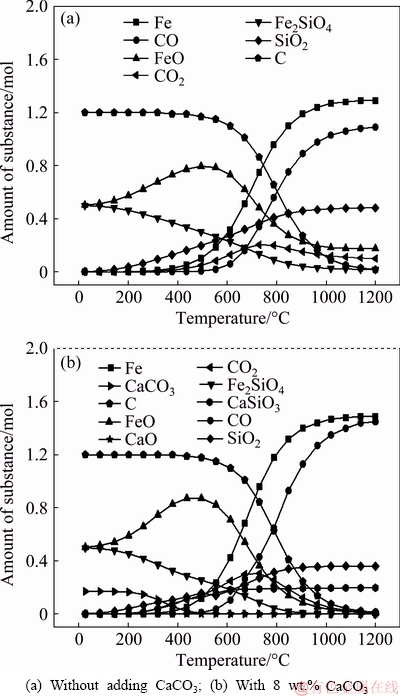
Fig. 4 Calculated amounts of substances at different temperatures
3 Results and discussion
3.1 Effect of CaCO3 content on reduction degree
The effect of CaCO3 content on the degree of iron reduction in the nickel slag at a heating rate of 20 °C/min is shown in Fig. 5. The amount of CaCO3 added had a significant effect. At a given reduction temperature and time, the reduction degree of the iron increased with increasing CaCO3 content of the raw material. The longer the reduction time, the more obvious the trend was.
After 90 min reduction, the reduction degree of the iron in the samples without adding CaCO3 was 58%; this value was increased to 70%, 80%, 85% and 88% when the CaCO3 added contents were 2, 4, 6 and 8 wt.%, respectively. The reduction degrees of the iron were increased by 12%, 22%, 27% and 30%, respectively. It was indicated that CaCO3 had a positive effect of CaCO3 on the reduction of nickel slag as the CaCO3 content was increased from 0 to 8 wt.%.
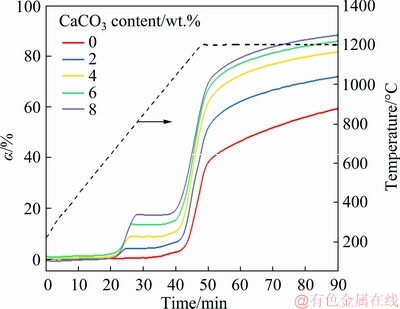
Fig. 5 Effect of CaCO3 content on reduction degree
The effects of reduction temperature and CaCO3 content on the reaction rate are shown in Fig. 6. The reaction rate remained unchanged at temperatures below 600 °C. As the temperature was increased, the reaction rate increased. The reaction rate reached the maximum rate of reduction between 1100 and 1150 °C. As the ferrous oxide content in the slag and consumption of graphite decreased, the reaction rate decreased.
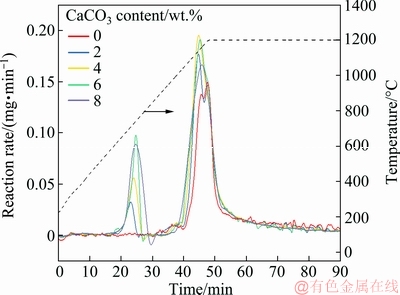
Fig. 6 Effects of reduction temperature and CaCO3 content on reaction rate
The reaction rate increased with the addition of CaCO3. When the temperature reached 1000 °C, the reduction of ferrous oxide in the raw material was initiated. The reaction rate of the sample with adding CaCO3 was significantly higher than that without adding CaCO3, indicating that the presence of CaCO3 could increase the reaction rate and promote the reduction of iron in the nickel slag. The reaction reached its peak rate within 40-50 min. The reaction rate of the sample without adding CaCO3 was 0.143 mg/min; when the CaCO3 additions were 2, 4, 6 and 8 wt.%, the reaction rates were 0.168, 0.191, 0.189 and 0.177 mg/min, respectively. The peak reaction rate occurred at an earlier time for samples with adding CaCO3 and the peak reaction temperature was lower. Addition of CaCO3 reduced both the time and temperature required for the reduction rate to reach its peak value.
The optimal parameters for nickel slag reduction were 8 wt.% CaCO3 added, a reduction temperature of 1200 °C, and a retention time of 45 min.
3.2 Characteristics of reduced nickel slag
3.2.1 Effect of CaCO3 content
SEM images of nickel slag added with different contents of CaCO3 reduced at 1100 °C are shown in Fig. 7.
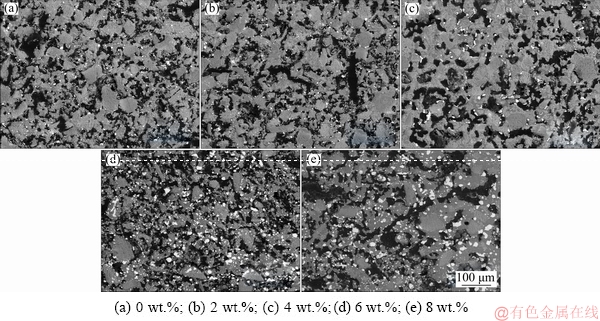
Fig. 7 SEM images of nickel slag added with different contents of CaCO3 reduced at 1100 °C
As the added CaCO3 content increased, the amounts of iron particles increased (bright white areas) and their size was coarsened. The sizes of the iron particles shown in Figs. 7(a) and 7(e) were measured using Image-Pro Plus software. The results showed that the average size of the iron particles was 6 μm after reduction of the slag without adding CaCO3, but increased to 21 μm when 6 wt.% CaCO3 was added. The structure of the product without the CaCO3 was compact, which made its growth and accumulation difficult; by adding CaCO3, cracks and pores formed during the reaction, which provided channels for the reducing gas and accelerated the reduction process.
XRD patterns of the nickel slag added with different contents of CaCO3 reduced at 1100 °C are shown in Fig. 8. The peak intensities of Fe2SiO4 decreased as the CaCO3 content increased, due to its reaction with Mg and CaO to form Mg2SiO4 and Fe. As a result, the peak intensity of Mg2SiO4 and Fe gradually increased and that of CaFeSi2O6 decreased. The chemical reactions are as follows:
Fe2SiO4+2MgO+2C=Mg2SiO4+2Fe+2CO (6)
Fe2SiO4+CaO+2C=2Fe+CaSiO3+2CO (7)
CaFeSi2O6+CaO+C=Fe+2CaSiO3+CO (8)
3.2.2 Effect of temperature
SEM images and EDS data of the nickel slag added with 8 wt.% CaCO3 reduced at different temperatures are shown in Fig. 9 and Table 3, respectively. The SEM images showed that only few iron grains (bright white areas) were observed at 600 and 800 °C. The amount and size of the iron grains increased with the temperature increasing. The structure of the product was relatively loose at low temperature and became denser with the increase of temperature.
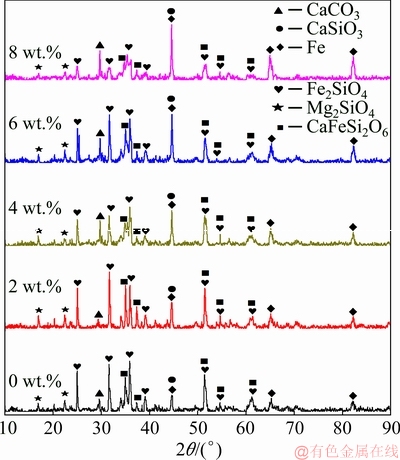
Fig. 8 X-ray diffraction patterns of nickel slag added with different contents of CaCO3 reduced at 1100 °C
The results of the EDS analysis in Table 3 showed that the reduced samples were composed of Fe, Mg, Si, Ca, C, O and a small amount of S. Iron present in the whole region, but calcium, magnesium, silicon and oxygen only present in the slag phase.
XRD patterns of the reduced products at different temperatures are shown in Fig. 10.
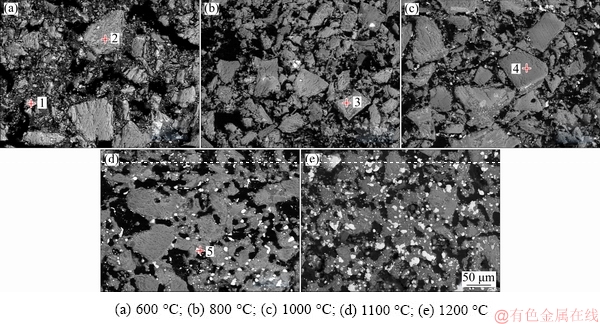
Fig. 9 SEM images of reduced nickel slag added with 8 wt.% CaCO3 at different temperatures
At 600 °C, the intensities of the CaCO3 diffraction peaks were higher than those at other temperatures because no decomposition occurred at this temperature; the diffraction peak intensities decreased at 800 °C because some CaCO3 was decomposed. The main chemical reaction is expressed by
CaCO3=CaO+CO2 (9)
Table 3 EDS data of nickel slag added with 8 wt.% CaCO3 at different temperatures
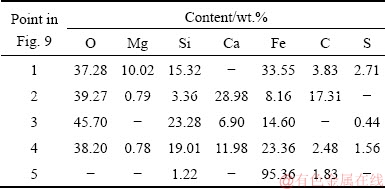
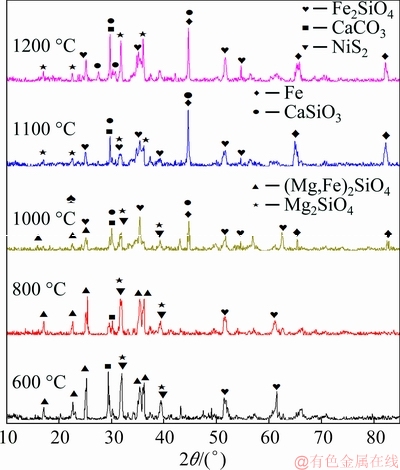
Fig. 10 X-ray diffraction patterns of reduced products with 8 wt.% CaCO3 at different temperatures
As the reaction temperature increased, the structure of slag gradually changed from amorphous to crystalline and iron appeared as a result of the reaction of fayalite with CaO and carbon. The main chemical reaction is given by
Fe2SiO4+CaO+2C=2Fe+CaSiO3+2CO (10)
Fe diffraction peaks appeared at 1000 °C. As the temperature increased, the intensity of the diffraction peaks of Fe2SiO4 gradually decreased and diffraction peaks of CaSiO3 began to appear. (Mg,Fe)2SiO4 gradually disappeared as the temperature increased, which was mainly due to the reaction between MgO and Fe2SiO4 to form Mg2SiO4. The main chemical reaction is expressed by
Fe2SiO4+2MgO+2C=Mg2SiO4+2Fe+2CO (11)
4 Mechanism analysis
XRD analysis showed that Fe in the sample present as Fe2SiO4 primarily. During reduction, C had difficulty in directly reacting with fayalite. After adding CaCO3, the Ca2+ ions were used as the carrier of 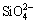 in olivine. The proposed reaction mechanism is shown in Fig. 11.
in olivine. The proposed reaction mechanism is shown in Fig. 11.
When CaCO3 came into contact with Fe2SiO4, the presence of Ca2+ weakened the bond between 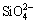 and Fe2+. Since the binding abilities of Ca2+ and
and Fe2+. Since the binding abilities of Ca2+ and 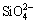 were strong, the chemical bond was broken under reducing conditions to form FeO and Ca2SiO4. FeO was further reduced to iron by the carbon added to the nickel slag. The main chemical reactions are expressed by
were strong, the chemical bond was broken under reducing conditions to form FeO and Ca2SiO4. FeO was further reduced to iron by the carbon added to the nickel slag. The main chemical reactions are expressed by
CaO+Fe2SiO4=2FeO+CaSiO3 (12)
FeO+C=Fe+CO (13)
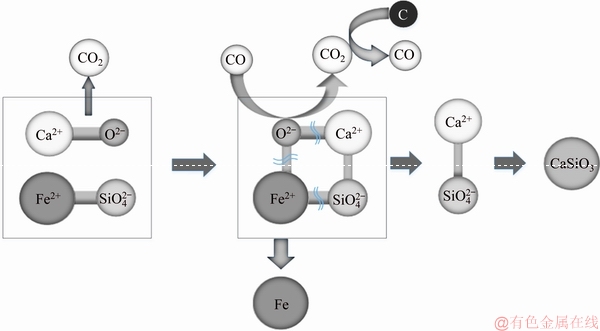
Fig. 11 Mechanism of acceleration of iron reduction from nickel slag by CaCO3
It is worth noting that when the reaction temperature was high, the nickel slag underwent some sintering during reduction and the reduced FeO combined with SiO2 to form liquid-phase FeO·SiO2, which seriously deteriorated the reduction kinetics and hindered the reduction reaction [19]. The CaO activity generated by pyrolysis of CaCO3 was high and the binding ability of CaO and SiO2 was stronger than that of FeO. CaO combined with SiO2 to form CaO·SiO2, which hindered the formation of liquid-phase FeO·SiO2 and improved the reduction conditions. As the reduction reaction proceeded, the resulting slag phase hindered the contact of the carbon with iron oxide to a certain extent and the chemical reaction transformed to a gas-based reaction. CO2 generated by the decomposition of CaCO3 at high temperatures accelerated the melting loss of carbon [20] and the reduction reaction increased the CO content of the gaseous reductant, thereby improving the reduction of nickel slag.
Previous studies have shown that the amount of CaCO3 added is limited. The reason is that CaCO3 will decompose to produce CaO at high temperature, which affects the basicity and melting point of the slag.
The phase diagram of the CaO-SiO2-MgO system is shown in Fig. 12. When the contents of SiO2 and MgO are constant and a small amount of CaCO3 is added, the calcium oxide content and basicity of the nickel slag are lower. The melting point of the slag is higher under low-basicity conditions. As the added CaCO3 content increases, the basicity of the sample gradually increases. This decreases the melting point of the slag and more slag will melt during the reduction process [21,22]. The impurity of the sample increases, which would affect the reduction process to some extent. Molten slag blocks the porosity of the sample, which decreases the porosity of the nickel slag and hinders the reduction.
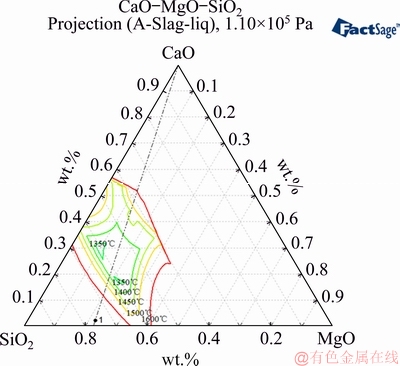
Fig. 12 Phase diagram of CaO-SiO2-MgO system
5 Conclusions
(1) Adding CaCO3 increased the reaction rate and decreased the temperature of the reduction reaction. Carbothermic reduction of nickel slag began at 1000 °C. The higher the temperature, the larger the reaction rate was. In the absence of CaCO3, the reduction rate reached a maximum of 0.143 mg/min between 1100 and 1150 °C. When the CaCO3 content was increased to 8 wt.%, the initial temperature of carbothermic reduction reaction of nickel slag was reduced to 1000 °C, and the temperature reaching the maximum reaction rate was reduced to 1100 °C.
(2) After 90 min reduction, the reduction degree of iron in the sample without adding CaCO3 was 58%. The reduction degree of iron increased to 70%, 80%, 85% and 88% when the added CaCO3 content was 2, 4, 6 and 8 wt.%, respectively, which indicated that CaCO3 had a positive effect on the reduction of nickel slag.
(3) The average size of the iron particles in reduced nickel slag without adding CaCO3 was 6 μm, but increased to 21 μm when 6 wt.% CaCO3 was added.
(4) A feasible process to recover iron from nickel slag by carbothermic reduction was proposed. The optimum parameters were 8 wt.% CaCO3 added, reduction temperature 1200 ℃, and reduction time 45 min.
References
[1] ZHANG Chuan-fu, LIU Hai-xia, ZHONG Da-long. Thermodynamical analysis of smelting process of nickel sulfides concentrate [J]. The Chinese Journal of Nonferrous Metals, 1999, 9: 805-810. (in Chinese)
[2] LI Xiao-ming, SHEN Miao, WANG Chong, CUI Ya-ru, ZHAO Jun-xue. Current situation and development of comprehensive utilization of nickel slag [J]. Materials Review, 2017, 31(5): 100-105. (in Chinese)
[3] PIATAK N M, PARSONS M B, SEAL R R. Characteristics and environmental aspects of slag: A review [J]. Applied Geochemistry, 2015, 57: 236-266.
[4] CHEN Wei-peng, LI Guang-wei, ZHAO Zeng-wu, LI Bao-wei, WU Wen-fei. Direct reduction of limonite with iron-rich coal slime as reducing agent [J]. Mining and Metallurgical Engineering, 2017, 37(1): 68-72. (in Chinese)
[5] PANG Jian-ming, GUO Pei-min, ZHAO Pei. New technology of low-temperature reduction and grain growth of copper slag [J]. Non-ferrous Metals (Extractive Metallurgy), 2013(3): 51-53, 57. (in Chinese)
[6] ZHAO Zhi-long, TANG Hui-qing, GUO Zhan-cheng. Influences of CaO and MgO on precipitation micro-morphology of metallic iron under CO atmosphere [J]. Mining and Metallurgical Engineering, 2012, 32(5): 105-109, 112. (in Chinese)
[7] BAI Shao-jun, WEN Shu-ming, LIU Dian-wen, ZHANG Wen-bin, CAO Qin-bo. Beneficiation of high phosphorus limonite ore by sodium-carbonate-added carbothermic reduction [J]. ISIJ International, 2012, 52(10): 1757-1763.
[8] BAI Shao-jun, WEN Shu-ming, LIU Dian-wen, ZHANG Wen-bin, XIAN Yong-jun. Catalyzing carbothermic reduction of siderite ore with high content of phosphorus by adding sodium carbonate [J]. ISIJ International, 2011, 51(10): 1601-1607.
[9] DING Shan, XUE Qing-guo, SHE Xue-feng, WANG Guang, NING Xiao-yu, WANG Jing-song. Effect of CaCO3 on direct reduction-smelting separation of vanadium-bearing titanomagnetite concentrate [J]. Iron and Steel, 2014, 49(8): 15-20. (in Chinese)
[10] KIM H, SOHN I. Effect of CaF2 and Li2O additives on the viscosity of CaO-SiO2-Na2O slags [J]. ISIJ International, 2011, 51: 1-8.
[11] KIM H, MATSUURA H, TSUKIHASHI F. Effect of Al2O3 and CaO/SiO2 on the viscosity of calcium-silicate–based slags containing 10 mass Pct MgO [J]. Metall Mater Trans B, 2013, 44: 5-12.
[12] BASUMALLICK A. Influence of CaO and Na2CO3 as additive on the reduction of hematite-lignite mixed pellets [J]. Transactions of the Iron & Steel Institute of Japan, 2007, 35(9): 1050-1053.
[13] XU Yan, SUN Ti-chang, LIU Zhi-guo, XU Cheng-yan. Dephosphorization effects of sodium carbonate in the process of direct reduction roasting of high phosphorous oolitic hematite [J]. Journal of Northeastern University (Natural Science Edition), 2014, 35(7): 1028-1032. (in Chinese)
[14] SUN Yong-sheng, HAN Yue-xin, GAO Peng, WEI Xin-chao, LI Guo-feng. Thermogravimetric study of coal-based reduction of oolitic iron ore: Kinetics and mechanisms [J]. International Journal of Mineral Processing, 2015, 143: 87-97.
[15] CAO Yu-xin, WANG Heng-hui, MA Jiang-hua, ZHAO Dong, LI Guang-qiang. Effect of additives on the preparation of reduced iron powder from iron concentrate [J]. The Chinese Journal of Process Engineering, 2018, 18(1): 133-139. (in Chinese)
[16] LI Guang-hui, ZHOU Tai-hua, LIU Mu-dan, JIANG Tao, FAN Xiao-hui. Novel process and mechanisms of aluminum-iron separation of high-aluminum limonite ore [J]. The Chinese Journal of Nonferrous Metals, 2008, 18: 2087-2093. (in Chinese)
[17] JIANG Tao, LIU Mu-dan, LI Guang-Hui, SUN Na, ZENG Jing-hua, QIU Guang-zhou. Novel process for treatment of high-aluminum limonite ore by reduction roasting with addition of sodium salts [J]. The Chinese Journal of Nonferrous Metals, 2010, 20: 565-571. (in Chinese)
[18] HUANG Bin-fang, LIU Bin, YANG Lei, LI Y, CHENG M, HUANG D, WANG H, ZHANG X, ZHENG J, LI Q, JI W, ZHOU Y, LU J. Functional genetic variants of c-Jun and their interaction with smoking and drinking increase the susceptibility to lung cancer in southern and eastern Chinese [J]. International Journal of Cancer, 2012, 131(5): E744-E758.
[19] ZHANG Jian-liang, WANG Chun-long, LIU Zheng-jian, WANG Zhe, CAO Ming-ming. Influencing factors of the reduction of vanadium titano-magnetite carbon composite pellets [J]. Journal of University of Science and Technology Beijing, 2012, 34(5): 512-518. (in Chinese)
[20] AN Xiu-wei, WANG Jing-song, SHE Xue-feng, DING Yin-gui, XU Qing-guo. New technologies of energy saving and low CO2 emission for iron making [J]. Advanced Materials Research, 2012, 463-464: 957-961.
[21] YU Hai-yan, PAN Xiao-lin, DONG Kai-wei, WU Yan. Effect of P addition on mineral transition of CaO-Al2O3-SiO2 system during high-temperature sintering [J]. Transactions of Nonferrous Metals Society of China, 2019, 29(3): 650-656.
[22] YU Wen-zhou, MA Wen-hui, ZHENG Zhong, JIANG Wei-yan, LI Jie, Mao-hong TIAN. Effects of melt viscosity on enrichment and separation of primary silicon from Al-Si melt [J]. Transactions of Nonferrous Metals Society of China, 2017, 27(2): 467-474.
李小明1,2,闻震宇1,李 怡1,杨海博1,邢相栋1,2
1. 西安建筑科技大学 冶金工程学院,西安 710055;
2. 西安建筑科技大学 陕西省冶金工程技术研究中心,西安 710055
摘 要:研究添加碳酸钙对镍渣碳热还原过程的影响,并分析其机理。结果表明,随着原料中碳酸钙添加量从0增加到8%(质量分数),还原反应初始温度和达到最大反应速率所需的温度分别从1100和1150 °C降低到1000和1100 °C,镍渣的还原率从58%增加到88%;还原后的渣中铁粒发生粗化,金属铁的衍射峰强度增加,表明添加碳酸钙有利于促进镍渣中铁化合物的还原回收。
关键词:镍渣;铁橄榄石;碳热还原;碳酸钙
(Edited by Bing YANG)
Foundation item: Projects (51774224, 51574189) supported by the National Natural Science Foundation of China
Corresponding author: Xiang-dong XIANG, Tel: +86-29-82202923, E-mail: xaxingxiangdong@163.com;
Xiao-ming LI, Tel: +86-82202931, E-mail: xmli@xauat.edu.cn
DOI: 10.1016/S1003-6326(19)65172-1

