
Optical properties of nanosized ZnO films prepared by sol-gel process
LOU Xiao-bo (楼晓波), SHEN Hong-lie (沈鸿烈), ZHANG Hui (张 惠), LI Bin-bin (李斌斌)
College of Materials Science and Technology, Nanjing University of Aeronautics and Astronautics, Nanjing 210016, China
Received 15 July 2007; accepted 10 September 2007
Abstract:Nanosized ZnO films were prepared by sol-gel process on quartz substrates. The effects of sol concentration and annealing temperature on the surface morphology, microstructure and optical properties of the films were investigated. The results show that the sols remain stable and usable for spin-coating within 7 d. The ZnO films have a homogeneous and dense surface with grain size about 30 nm. The ZnO thin film annealed at 500 ℃ for 1 h from the sol with Zn concentration of 0.8 mol/L shows an average transmittance of 94% in visible wavelength range. The optical band gaps in ZnO films by various annealing temperatures are from 3.265 eV to 3.293 eV. The violet emission located at 438 nm is probably due to the recombination transitions relating to the interface traps at the grain boundaries.
Keywords: ZnO film; sol-gel process; transparence; nanostructure
1 Introduce
ZnO is a widely used functional material with wide and direct band gap, large exciton binding energy, and excellent chemical and thermal stability[1]. ZnO is a semi-conducting material widely used as transparent electrodes in solar cells[2], chemical and gas sensors[3], spintronic devices[4], and light emitting diodes[5]. Different types of techniques for deposition of ZnO films have been reported, such as sputtering[6], spray pyrolysis[7], chemical vapour deposition[8] and sol-gel method[9-14]. Nowadays, the sol-gel method has been extensively used to obtain various kinds of functional oxide films due to its simplicity and low cost. It has been found that post-annealing plays an important role on the properties of ZnO films[10], and the optical transmittance and photoluminescence are very sensitive to the quality of crystal structure and to the presence of defects[11].
In this paper, ZnO films were obtained by spin coating using sol-gel process. The transmittance spectra and UV-fluorescence spectra were measured as function of Zn concentration and annealing temperature. The crystalline quality of the films was judged from XRD patterns and surface morphology.
2 Experimental
ZnO films were prepared by sol-gel method on quartz substrate. Zinc acetate dihydrate was dissolved in a solution of isopropanol and diethanolamine (DEA). The molar ratio of DEA to zinc acetate was maintained at 1.0 and the concentrations of zinc acetate were 0.6 mol/L, 0.8 mol/L and 1.0 mol/L, respectively. The solutions were stirred at 60 ℃ for 1 h by a magnetic stirrer to yield a clear and homogeneous solution. All of the solutions were then aged at room temperature for 2 d before coating.
The substrates were cleaned by acetone, alcohol and finally with deionized water before spinning-coating (3 000 r/min for 30 s). Each newly coated layer was dried at 200 ℃ for 15 min to evaporate the solvent and remove the organic residuals. The process was repeated 20 times to obtain a desired thickness. The films were finally put into a tube furnace and annealed in ambient atmosphere at selected temperatures for 1 h. The experimental conditions for the preparation of ZnO films are summarized in Table 1.
Table 1 Experimental condition for preparation of ZnO samples

The structure of ZnO films was analyzed by X-ray diffractometry (BRUKER D8Advance), with Cu Kα radiation. Surface morphology was observed by scanning electron microscopy (LEO 1530VP) and atomic force microscopy (AFM, SPA 300 HV). The optical transmittance of ZnO films was measured at room temperature by UV-vi spectrometry (SHIMADZU UV2550) and the optical band gap energy was calculated. UV-fluorescence (VARIAN CARY100) was used to study the luminescence property at RT.
3 Results and discussion
XRD patterns of sample B series annealed at 400 ℃, 500 ℃ and 600 ℃ respectively were firstly investigated. The peaks identified as (100), (002), (101), (102) and (110) diffractions for wurtzite structure of ZnO were found. The XRD results indicate that all the films are polycrystalline and the c-axis preferred orientation is not very significant. The full width at half maximum (FWHM) of the (002) diffraction peak decreases when the annealing temperature increases up to 600 ℃, demonstrating that the crystalline quality of the film gets better as the annealing temperature becomes higher. The crystallite size could be calculated from XRD data using Scherrer’s formula. The calculated average crystallite sizes of the undoped ZnO thin films annealed at 400, 500 and 600 ℃ are 17.3, 29.5 and 31.3 nm, respectively. The results imply that the grain size of sol-gel produced films grows with the increasing annealing temperature, which can be understood by considering the merging process induced in the thermal treatment.
Fig.1 shows the surface micrographs of SEM and AFM from sample B2 preheated at 200 ℃ and post-heated at 500 ℃ for 1 h. The grain size of the film is about 30 nm and uniform, and dense microstructure is observed. The result is almost the same as XRD. There exist some pores in the surface, which are partly formed by the coalescence of microvoids[12]. This indicates that the pores in the surface are related with the decomposition reaction of the precursor and the evaporation of residual organics in gel films. The surface roughness of the film over a 1 μm×1 μm area was measured by AFM. The surface roughness mean square (RMS) of the film is 25 nm, which demonstrates that the surface morphology is smooth.
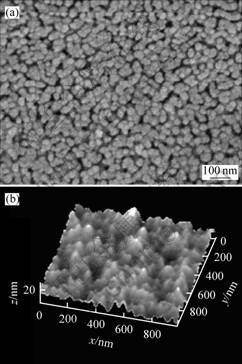
Fig.1 SEM (a) and AFM (b) micrographs of sample B2 preheated at 200 ℃ and post-heated at 500 ℃ for 1 h in air
The effect of aging time on sol stability was investigated by optical transmittance, as shown in Fig.2, using the sol prepared for sample B series which was aged at RT from 1 d to 8 d. It’s observed that the intensity of absorption increases with extending aging time. It’s mainly because that the crystal forms and grows gradually in the sol. Within 7 d the absorption edge and intensity of the transmittance spectrum keep almost the same, indicating that the sol is stable, but it changes obviously at the eighth day (insert figure in Fig.2). It probably means that the sol quality is changed after 7 d, and the sol is able to be used before 8 d.

Fig.2 Optical transmittance of sols kept for various days
Fig.3 shows the optical transmittance spectra from samples with different Zn2+ concentrations and annealed at 500 ℃ for 1 h in air. The transmittance within the visible and the near infrared wavelength region is always higher than 90% and is slightly larger than that of quartz substrate, which reveals the superior optical properties in our ZnO thin films produced by the sol-gel method. For all the samples, the ZnO thin film annealed at 500 ℃ for 1 h from the sol with a Zn concentration of 0.8 mol/L shows a transmittance of 94% on average in visible wavelength range. And all the samples present a sharp absorption edge in the UV region at wavelength of about 370 nm due to the onset of fundamental absorption. It can be seen that optical transmittance is almost the same in the visible region, but changes obviously in the UV region (regionⅠ) where it decreases with increasing Zn2+ concentration. It is attributed to the film thickness which increases with the Zn concentration. The thicker the film is, the larger the optical absorption is.

Fig.3 Curves of optical transmittance vs wavelength for quartz substrate and samples A, B2 and C after annealing at 500 ℃ for 1 h in air
The effect of annealing temperature on the optical transmittance for sample B series was investigated from 400 ℃ to 600 ℃. A slight decrease in average transmission is observed with the increase of annealing temperature and is attributed to the increase of surface roughness. The optical absorption edge has a trend to shift to a higher wavelength with increasing annealing temperature.
It is well known that the optical absorption determines the optical band gap and ZnO films have a direct band gap. The optical band gap Eg for direct band gap semiconductor is given by[13]
αhν=C(hν-Eg)1/2 (1)
where hν is the photon energy, C is a constant for a direct transition, and α is the optical absorption coefficient determined by α=(1/d)ln(1/T), where d is the film thickness and T is the optical transmittance[14]. Fig.4 shows (αhν)2 as a function of hν for obtaining optical band gap, which was derived from transmittance spectrum. According to Eqn.(1), the energy gap Eg was obtained by extrapolating the linear absorption edge part of the curve to the intersection with energy axis, as shown in Fig.4. The optical band gap of ZnO films was found to decrease from 3.293, 3.284 to 3.265 eV with the increase of annealing temperature. The decrease in band gap of ZnO films may be attributed to the improvement in the crystalline quality of the films along with reduction in porosity and increase of grain size.
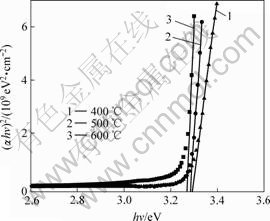
Fig.4 Plots of (αhν)2 vs hν for ZnO films with various annealing temperatures
Fig.5 shows the RT fluorescence spectrum of ZnO films excited by 340 nm wavelength. It is obvious that the spectrum presents a violet emission band centered at around 438 nm, similar to the emission band reported by JIN et al[15]. The energy level of the interface states within the depletion regions located at the

Fig.5 RT fluorescence spectrum of sample B2 annealed at 500 ℃ for 1 h
ZnO-ZnO boundaries has been found to be 0.33 eV below the conduction-band edge. Therefore, the violet emission is probably due to the recombination transitions between the interface traps existing at the grain boundaries and the valence band since the energy of this band (2.83 eV) is well below the band gap energy (3.284 eV).
4 Conclusions
1) Nanosized ZnO thin films were prepared by sol-gel method. The sols remain stable and were usable for spin-coating within 7 d.
2) ZnO films have a homogeneous and dense surface with the crystalline structure of hexagonal wurtzite. The ZnO grain size is about 30 nm and the surface roughness is 25 nm.
3) The ZnO thin film annealed at 500 ℃ for 1 h using Zn concentration of 0.8 mol/L in sol has a largest transmittance of 94% on average in visible wavelength range.
4) The optical band gaps in ZnO films by various annealing temperatures are from 3.265 eV to 3.293 eV.
5) The violet emission located at 438 nm is probably due to the recombination transitions related to the interface traps existing at the grain boundaries.
References
[1] NORTON D P, HEO Y W, IVILL M P, IP K, PEARTON S J, CHISHOLM M F, STEINER T. ZnO: growth, doping & processing[J]. Materials Today,2004, 7( 6): 34-40.
[2] FORTUNATO E, BARQUINHA P, PIMENTEL A, GONCALVES A, MARQUES A, PEREIRA l, MARTINS R. Recent advances in ZnO transparent thin film transistors[J]. Thin Solid Films,2005, 487(1/2): 205-211.
[3] RAO B B. Zinc oxide ceramic semi-conductor gas sensor for ethanol vapour[J]. Materials Chemistry and Physics,2000, 64(1): 62-65.
[4] LIU C, YUN F, MORKOC H. Ferromagnetism of ZnO and GaN: A Review[J]. Journal of Materials Science: Materials in Electronics, 2005, 16: 555-597.
[5] MATSUBARA K, FONS P, IWATA K, YAMADA A, SAKURAI K, TAMPO H, NIKI S. ZnO transparent conducting films deposited by pulsed laser deposition for solar cell applications [J]. Thin Solid Films, 2003, 431-432: 369-372.
[6] ZHOU Y, KELLY P J, POSTILL A, ABU-ZEID O, ALNAJJAR A A. The characteristics of aluminium-doped zinc oxide films prepared by pulsed magnetron sputtering from powder targets [J]. Thin Solid Films, 2004, 447-448: 33-39.
[7] LEE J H, PARK B O. Characteristics of Al-doped ZnO thin films obtained by ultrasonic spray pyrolysis: effects of Al doping and an annealing treatment [J]. Mater. Sci. Eng. B, 2004, 106(3): 242-245.
[8] BARNES T M, LEAF J, FRY C, WOLDEN C A. Room temperature chemical vapor deposition of c-axis ZnO [J]. J. Cryst. Growth, 2005, 274(3/4): 412-417.
[9] LEE J H, KO K H, PARK B O. Electrical and optical properties of Zn O transparent conducting films by the sol–gel method[J]. Journal of Crystal Growth, 2003, 247: 119-125.
[10] GHOSH R, PAUL G K, BASAK D. Effect of thermal annealing treatment on structural, electrical and optical properties of transparent sol–gel ZnO thin films [J]. Materials Research Bulletin, 2005, 40(11): 1905-1914.
[11] SAGARA P, SHISHODIAA P K, MEHRA R M, OKADA H, WAKAHARA A, YOSHIDA A. Photoluminescence and absorption in sol–gel-derived ZnO films [J]. Journal of Luminescence, 2007, 126(2): 800-806.
[12] KIM Y S, TAI W P. Electrical and optical properties of Al-doped ZnO thin films by sol–gel process [J]. Applied Surface Science 2007, 253(11): 4911-4916.
[13] XU Z Q, DENG H, LI Y, GUO Q H, LI Y R. Characteristics of Al-doped c-axis orientation ZnO thin films prepared by the sol–gel method [J]. Materials Research Bulletin, 2006, 41(2): 354-358.
[14] WANG M S, LEE K E, HAHN S H, KIM E J, KIM S, CHUNG J S, SHIN E W, PARK C. Optical and photoluminescence properties of sol-gel Al-doped ZnO thin films [J]. Materials Letters, 2007, 61(4/5): 1118-1121.
[15] JIN B J, IM S, LEE S Y. Violet and UV luminescence emitted from ZnO thin films grown on sapphire by pulsed laser deposition [J]. Thin Solid Films,2000, 366(1/2): 107-110.
(Edited by LONG Huai-zhong)
Foundation item: National 863 High Tech Project (2006AA03Z219); NUAA Foundation (S0417061); PCSIRT(IRT0534)
Corresponding author: SHEN Hong-lie; Tel: +86-25-52112626; E-mail: hlshen@nuaa.edu.cn