采用TOPS-99和Cyanex 272钠盐从镍红土矿细菌浸出液中萃取锌、锰、钴和镍
来源期刊:中国有色金属学报(英文版)2016年第1期
论文作者:R. K. MISHRA P. C. ROUT K. SARANGI K. C. NATHSARMA
文章页码:301 - 309
关键词:镍红土矿;锰;镍;TOPS-99;Cyanex 272
Key words:nickel laterite; manganese; nickel; TOPS-99; Cyanex 272
摘 要:在煤油中采用TOPS-99和Cyanex 272钠盐从镍红土矿细菌浸出液中提取与分离锌、锰、钴和镍。采用沉淀法去除不需要的金属离子,使用溶剂萃取提取/分离锌、锰、钴和镍。生物浸出铬铁矿表土样品得到的镍红土矿浸出液中含有3.72 g/L Fe, 2.08 g/L Al, 0.44 g/L Ni, 0.02 g/L Co, 0.13 g/L Mn, 0.14 g/L Zn 和0.22 g/L Cr。在pH 4时采用CaCO3沉积去除100% Fe, 96.98% Al 和 70.42% Cr,随后在pH 5.4时采用50%氨沉积,溶液中剩有Al 和 Cr。沉积后,采用0.1 mol/L TOPS-99从无铁、铝和铬的浸出液中提取锌,随后采用0.04 mol/L NaTOPS-99提取锰。锌和锰的产率分别是97.77% 和 95.63%。提取锰后,再采用0.0125 mol/L NaCyanex 272从浸取液去除钴,最后采用0.12 mol/L NaTOPS-99提取镍,其产率达99.84%。采用稀硫酸去除浸出液中的有机相。
Abstract: The extraction and separation of zinc, manganese, cobalt and nickel from nickel laterite bacteria leach liquor were carried out using sodium salts of TOPS-99 and Cyanex 272 in kerosene. The unwanted metal ions were removed by precipitation method and solvent extraction was used to extract/separate Zn, Mn, Co and Ni. The nickel laterite leach liquor which was obtained from bioleaching of chromite overburden samples contained 3.72 g/L Fe, 2.08 g/L Al, 0.44 g/L Ni, 0.02 g/L Co, 0.13 g/L Mn, 0.14 g/L Zn and 0.22 g/L Cr. From this leach liquor, 100% Fe, 96.98% Al and 70.42% Cr were removed by precipitation with CaCO3 at pH 4.4 followed by precipitation of remaining Al and Cr with 50% ammonia at pH 5.4. After precipitation, the extraction of Zn from the Fe, Al and Cr free leach liquor was carried out with 0.1 mol/L TOPS-99 followed by Mn extraction with 0.04 mol/L NaTOPS-99. The yields of Zn and Mn were 97.77% and 95.63%, respectively. After Mn extraction, cobalt was removed from the leach liquor using 0.0125 mol/L NaCyanex 272 and finally nickel extraction was carried out using 0.12 mol/L NaTOPS-99 with 99.84% yield. The stripping of loaded organic (LO) phases were achieved with dilute H2SO4.
R. K. MISHRA1, P. C. ROUT1,2, K. SARANGI1,2, K. C. NATHSARMA1
1. Council of Scientific and Industrial Research, Institute of Minerals and Materials Technology,Bhubaneswar 751 013, Odisha, India;
2. Academy of Scientific and Innovative Research, Chennai 600 113, Tamilnadu, India
Received 5 February 2015; accepted 17 November 2015
Abstract: The extraction and separation of zinc, manganese, cobalt and nickel from nickel laterite bacteria leach liquor were carried out using sodium salts of TOPS-99 and Cyanex 272 in kerosene. The unwanted metal ions were removed by precipitation method and solvent extraction was used to extract/separate Zn, Mn, Co and Ni. The nickel laterite leach liquor which was obtained from bioleaching of chromite overburden samples contained 3.72 g/L Fe, 2.08 g/L Al, 0.44 g/L Ni, 0.02 g/L Co, 0.13 g/L Mn, 0.14 g/L Zn and 0.22 g/L Cr. From this leach liquor, 100% Fe, 96.98% Al and 70.42% Cr were removed by precipitation with CaCO3 at pH 4.4 followed by precipitation of remaining Al and Cr with 50% ammonia at pH 5.4. After precipitation, the extraction of Zn from the Fe, Al and Cr free leach liquor was carried out with 0.1 mol/L TOPS-99 followed by Mn extraction with 0.04 mol/L NaTOPS-99. The yields of Zn and Mn were 97.77% and 95.63%, respectively. After Mn extraction, cobalt was removed from the leach liquor using 0.0125 mol/L NaCyanex 272 and finally nickel extraction was carried out using 0.12 mol/L NaTOPS-99 with 99.84% yield. The stripping of loaded organic (LO) phases were achieved with dilute H2SO4.
Key words: nickel laterite; manganese; nickel; TOPS-99; Cyanex 272
1 Introduction
The requirement of cobalt and nickel in the present day world is too much for which a lot of work is being under taken around the world to recover these valuable metals even from very low grade ores. In India, there is no primary source for nickel, but the chromite overburden of Sukinda, Odisha, India, contains 0.3%-0.9% Ni. To extract the metals from this chromite overburden, bacterial leaching was carried out [1] and the leach liquor contained Fe, Al, Cr, Zn, Mn, Co and Ni. So, it is required to separate/remove other metal ions to get pure nickel solution. The separation of metal ions such as Fe, Cu, Co, Ni, Zn and Mn can be achieved by various methods like selective precipitation, ion exchange resin, adsorption and solvent extraction, etc. Among all these methods, solvent extraction is the most suitable technique for separation of metal ions and has been used since a long period for separation of metal values. Separation of manganese, zinc and nickel from leaching solution of nickel-metal hydride spent batteries has been carried out by solvent extraction [2]. The spent Ni-MH battery was leached and the nickel leach liquor was separated from manganese and zinc by liquid-liquid extraction using D2EHPA. Both D2EHPA and Cyanex 272 were investigated for separation of Zn and Mn and from some preliminary tests, it was found that D2EHPA was more efficient to separate Mn and Zn. D2EHPA, Cyanex 272 and TBP were used for separation of zinc and manganese from sulfate solution [3]. By mixing the two extractants, the extraction efficiency increased.Increasing the D2EHPA to Cyanex 272 ratio in the organic phase caused a right shifting of extraction isotherms of manganese and zinc, and the manganese shifting curve was more than that of zinc. The manganese curve had considerable right shifting with 5% D2EHPA and 15% Cyanex 272. TBP did not affect the zinc and manganese extraction.
The separation of Zn, Mn, Co from a solution containing Zn, Mn, Co, Cd and Ni was investigated using D2EHPA, Cyanex 272 and Cyanex 302 [4]. Addition of Cyanex 302 indicated a left-shifting-effect on the extraction curve of zinc, a right-shifting-effect on the extraction curve of manganese and no effect on the extraction of cobalt. Addition of Cyanex 272 shifted all three curves to the right.The most suitable extractant for separation of zinc from manganese was 0.3:0.3 mixture of D2EHPA and Cyanex 302. The separation of manganese and cobalt was achieved by pure D2EHPA. A solvent extraction process was developed for the recovery of zinc and manganese from spent zinc-carbon battery leach solution [5]. Between the two systems considered, D2EHPA and Ionquest 801, the latter is a better choice in terms of metal separation and stripping.The separation of Zn and Mn [6], and Co and Ni [7] from sulphate solutions have been carried out including the synergism study [8] of Cyanex 302 and Cyanex 272 with D2EHPA in the separation of Co and Ni. The extraction of zinc from the sulfuric acid leaching of cold purification filter cake of Angouran concentrate was studied by BALESINI et al [9] using D2EHPA. Zinc separation from Cd and Ni was 98.8% with 40% (volume fraction) D2EHPA at pH 2.5-3. LEE and NAM [10] have also studied the extraction of Zn from strong hydrochloric acid solution up to 10 mol/L in which Alamine 336 was used as extractant. Similar study for the extraction of Mn from strong hydrochloric acid medium was carried out by LEE and FILIZ [11]. BISWAS and RAHMAN [12] investigated that Mn can be extracted by Cyanex 272 from sulphate-acetate medium. Also, the separation of Mn(II) from its binary mixtures with Sc(III), Ti(IV), V(IV), V(V), Fe(III), Ni(II), Co(II), Cr(III), Zn(II) and Gd(III) was carried out.
The separation of Zn(II) and Mn(II) from a bioleach liquor of spent alkaline and Zn-C batteries using Cyanex 272 and DEHPA was studied by FALCO et al [13], and they observed that the performance of Cyanex 272 was better than D2EHPA. The extraction of Co(II) from a synthetic sulphate solution (having same composition as Ni laterite ore) bearing Co, Ni and high content of Mg was carried out by LEE et al [14] using D2EHPA, PC88A, Cyanex 272, Alamine 336 and their combinations. The extraction of Ni was negligible with the cationic extractants, but the highest separation factor was observed between Co(II) and Mg(II) with saponified Cyanex 272 whereas Alamine 336 extracted only Co(II) in the presence of MgCl2. In another study, REDDY et al [15] investigated the separation of Cd(II), Co(II) and Ni(II) from chloride leach liquor of spent Ni-Cd batteries. After extraction of Cd, the separation of Co from Ni using Cyanex 272 was achieved with a separation factor larger than 4700.
Above literature studies showed that D2EHPA and Cyanex 272 can be used for separation of Zn, Mn, Co and Ni. As these solvents are commercially available, they can be used for solvent extraction process development.The bacterial leach liquor (pH 1.3) of chromite overburden of Sukinda, Odisha, India, contains 3.72 g/L Fe, 2.08 g/L Al, 0.44 g/L Ni, 0.02 g/L Co,0.13 g/L Mn, 0.14 g/L Zn and 0.22 g/L Cr. To obtain pure Mn and Ni solutions from the above leach liquor using solvent extraction technique, a process has been developed and has been reported in this work.
2 Experimental
2.1 Chemicals and reagents
The commercial extractant TOPS-99 (Di(2- ethylhexyl) phosphoric acid) and bis(2, 4, 4-trimethyl pentyl) phosphinic acid (Cyanex 272) were supplied by Heavy Water Plant, Talcher, Odisha, India and Cytec Inc., USA, respectively and were used as such without any purification. Distilled kerosene (b.p. 180-240 °C) was used as the diluent. Sodium hydroxide solution (12.88 N) was used for neutralization of the leach liquor, and saponification of TOPS-99 and Cyanex 272. CaCO3 and ammonia solution were used for precipitation of metals. All chemicals used in the experiments were of AR grade and supplied by BDH, India. The overburden samples were obtained from the major deposits of chromite overburden of Sukinda Mines, Odisha, India.
2.2 Apparatus
Systronics pH meter Model 361 was used for measurement of aqueous phase pH, and Perkin Elmer atomic absorption spectrophotometer (AAS) Model AA 200 was used for the analysis of metal ions in solution.
2.3 Method
All the experiments were carried out at room temperature, i.e., (30±1) °C. Before starting the solvent extraction studies, the leach liquor was made free of Fe, Al and Cr by precipitation with CaCO3 and 50% ammonia. Desired volumes of the leach liquor and the extractants diluted in kerosene were equilibrated manually in separatory funnel for 5 min and after phase disengagement, the aqueous phase was separated followed by the measurement of equilibrium pH and determination of metal concentrations in the raffinate by atomic absorption spectrophotometer (AAS). The concentration of metal ions in the organic phase was calculated from the difference between the metal concentrations in the aqueous phase before and after extraction. Also when required, the organic phase was analyzed for metal ion concentration after filtering through 1PS phase separating paper and stripping with 1 mol/L HCl. For the construction of McCabe-Thiele plots for extraction and stripping studies, the organic:aqueous (O:A) ratio was varied keeping the total volume of the phase constant.
The extraction mechanism of divalent metal ions with phosphoric and phosphinic acid (designated as HA) can be written as
 (1)
(1)
3 Results and discussion
The chromite overburden of Sukinda Mines, Odisha, India, was leached using bacteria and the metals were recovered from the bacterial leach liquor (pH 1.3) having composition of 3.72 g/L Fe, 2.08 g/L Al, 0.44 g/L Ni, 0.02 g/L Co, 0.13 g/L Mn, 0.14 g/L Zn and 0.22 g/L Cr. The leach liquor was treated with CaCO3 at pH 4.4 to precipitate 100% Fe, 96.98% Al and 70.42% Cr. The remaining Al and Cr from the solution were precipitated by maintaining the pH of solution at 5.4 with 50% ammonia. After bulk precipitation from the leach liquor, there was reduction in volume. So, the concentration of metal ions in solution was changed to 0.64 g/L Ni, 0.03 g/L Co, 0.16 g/L Mn and 0.09 g/L Zn. This Fe, Al, Cr-free solution was treated with sodium salts of TOPS-99 and Cyanex 272 to separate the metals.
3.1 Extraction of zinc
Solvent extraction of Zn from the Fe, Al, Cr-free solution bearing 0.64 g/L Ni, 0.03 g/L Co, 0.16 g/L Mn and 0.09 g/L Zn was carried out with 0.1 mol/L TOPS-99 at 1:1 phase ratio. The initial pH of the solution was within 1.0 to 6.7 (equilibrium pH 0.9-2.5). The extraction of Zn increases from 5.43% to 81.52% with increasing equilibrium pH. The extraction of other metal ions within the pH range was zero. The experimental data for equilibrium pH versus zinc extraction rate are illustrated in Fig. 1.
To study the effect of TOPS-99 concentration on zinc extraction, the concentration of TOPS-99 was varied from 0.01 to 0.15 mol/L. The pH of the aqueous phase was maintained at 6.7. The extraction rate of zinc was plotted against TOPS-99 concentration in Fig. 2. The extraction of zinc increased from 31.1% to 81% with increasing extractant concentration from 0.01 to 0.1 mol/L and increased slowly thereafter up to 81.7% with further increase of extractant concentration up to 0.15 mol/L.

Fig. 1 Effect of equilibrium pH on extraction of Zn with 0.1 mol/L TOPS-99 (Experimental condition: 0.09 g/L Zn, 0.1 mol/L TOPS-99, O:A= 1:1)

Fig. 2 Effect of [TOPS-99] on extraction of Zn (Experimental condition: 0.09 g/L Zn, pH: 6.7, O:A= 1:1)
The McCabe-Thiele plot was constructed (Fig. 3) for extraction of Zn with 0.1 mol/L TOPS-99 within O:A ratio of 1:5 to 5:1. The diagram indicated 0.012 g/L Zn in raffinate after 3-stage of extraction at O:A ratio of 1:3. To confirm the theoretical prediction, a 3-stage counter-current simulation (CCS) study was carried out at O:A ratio of 1:3 to generate the loaded organic (LO) (0.264 g/L Zn) and to show 90.8%, 96.4% and 97.77% extraction in first, second and third stages of extraction, respectively.
Stripping of Zn from the LO was carried out with 0.5-20 g/L H2SO4 by equilibrating the phases at O:A ratio of 1:1. The plot (Fig. 4) of zinc stripping rate vs [H2SO4] increased from 2.62% to 98.84% with increasing acid concentration. The zinc loaded organic was stripped with 15 g/L H2SO4 and after stripping, the spent organic contained 0.003 g/L Zn.

Fig. 3 Extraction isotherm of zinc with 0.1 mol/L TOPS-99 (Experimental condition: 0.09 g/L Zn, 0.1 mol/L TOPS-99,pH 6.7)

Fig. 4 Effect of [H2SO4] on stripping of Zn, Mn and Ni-LO
3.2 Extraction of manganese
The separation of manganese from the Fe, Cr, Al and Zn-free nickel laterite leach liquor bearing 0.64 g/L Ni, 0.03 g/L Co and 0.16 g/L Mn solution was carried out. To study the effect of pH on extraction of manganese from the leach liquor, the initial pH of the solution was varied within the range of 1.5-3.5 while keeping the concentration of NaTOPS-99 constant at 0.025 mol/L. The corresponding equilibrium pH was in the range of 1.7-6.1. The equilibrium pH values were plotted against the extraction rate of Mn in Fig. 5 which indicated an increase in extraction of manganese from 8% to 71.82% with increase in the equilibrium pH from 1.7 to 6.1. The co-extraction of cobalt and nickel with manganese increased from 0 to 44.84% and from 0.16% to 2.43%, respectively with the increase of equilibrium pH from 1.7 to 6.1.

Fig. 5 Effect of equilibrium pH on extraction of metal ions with 0.025 mol/L NaTOPS-99
The manganese extraction was carried out with different concentrations (0.005-0.05 mol/L) of neutral TOPS-99 at 1:1 phase ratio. The initial pH of the solution was 2.5. The extraction rates for manganese, cobalt and nickel versus different concentrations of NaTOPS-99 were plotted in Fig. 6. Figure 6 showed an increase in manganese extraction from 6.11% to 93.06% with increase in extractant concentration from 0.005 to 0.05 mol/L NaTOPS-99. Also, there was co-extraction of cobalt and nickel. The co-extraction of cobalt increased from 7.48% to 67.59% and that of nickel increased from 0.16% to 7.14% with increasing NaTOPS-99 concentration.

Fig. 6 Effect of [NaTOPS-99] on extraction of metal ions
At solution pH of 2.5, the extraction of manganese was 87.29% and the co-extraction rates of nickel and cobalt were 3.45% and 24.52%, respectively with 0.04 mol/L NaTOPS-99. So, the extraction isotherm for manganese was constructed with above condition to know the number of stages of extraction required at a chosen phase ratio. The McCabe-Thiele plot (Fig. 7) showed quantitative extraction of manganese in two stages at 1:1 phase ratio. To confirm the predictions of the extraction isotherm, a two-stage countercurrent simulation study was carried out with 0.04 mol/L NaTOPS-99 at 1:1 phase ratio. The raffinates and loaded organic samples were collected and analyzed for concentration of metal ions, but after five cycles onwards the concentration of metal ions in the loaded organic and raffinate samples were separately identical. The raffinate samples were analyzed to contain 0.007 g/L Mn indicating 95.63% extraction. The loaded organic was analyzed to contain 0.153 g/L manganese. There was no co-extraction of cobalt and nickel with manganese. A sufficient quantity of Mn loaded organic was generated to carry out the stripping studies.

Fig. 7 Extraction isotherm for Mn with 0.04 mol/L NaTOPS-99 (Experimental condition: [Mn] 0.16 g/L, pH 2.5, 0.04 mol/L NaTOPS-99)
The Mn loaded TOPS-99 was stripped with different concentrations of H2SO4 within the range of 0.2-2.5 g/L at equal phase ratio. The experimental data for manganese stripping rate versus different concentrations of H2SO4 as shown in Fig. 4 illustrated an increase in stripping from 7.8% to 99.43% with increase in H2SO4 concentration from 0.2 to 1.5 g/L. With further increase in acid concentration to 2.5 g/L, the stripping rate increased to 99.68%. Thus, 1.5 g/L H2SO4 was chosen for stripping of the Mn loaded organic phase.
The loaded organic phase contained 0.153 g/L manganese. To know the number of stages required for stripping of manganese, the manganese loaded organic was contacted with 1.5 g/L H2SO4 at O:A ratio of 1:10 to 10:1. The isotherm (Fig. 8) indicated 2-stage at O:A ratio of 9:1 for quantitative stripping. To confirm this, a 2- stage counter-current simulation study was carried out with 1.5 g/L H2SO4 at O:A ratio of 9:1. The strip solution and the spent organic contained 1.341 and 0.004 g/L Mn leading to 97.38% stripping.
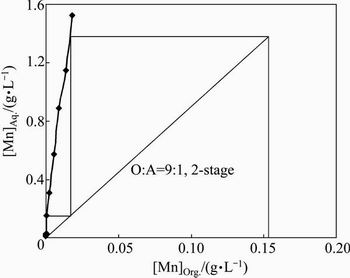
Fig. 8 Stripping isotherm for Mn-LO with 1.5 g/L H2SO4 (Experimental condition: [Mn]LO 0.153 g/L, [H2SO4] 1.5 g/L)
3.3 Removal of cobalt
The separation of Co from the Fe, Cr, Al, Zn and Mn-free nickel laterite leach liquor bearing 0.64 g/L Ni and 0.03 g/L Co was carried out. To study the effect of pH on extraction of cobalt from the leach liquor, the initial pH of the solution was varied within the range of 1.0-5.6. The concentration of NaCyanex 272 was kept constant at 0.005 mol/L. The equilibrium pH of raffinate samples was measured to be within the range of 1.1-7.2. The plot of equilibrium pH versus cobalt extraction rate is shown in Fig. 9. The plot showed that there was no extraction of Co and Ni up to equilibrium pH of 1.9.The extraction starts from equilibrium pH 2.8. The cobalt extraction increased from 6.3% to 74.15% with increasing equilibrium pH from 2.8 to 7.2. The co-extraction of nickel also increased from 0.46% to 14.3% with increasing equilibrium pH.

Fig. 9 Effect of equilibrium pH on extraction of cobalt and nickel with 0.005 mol/L NaCyanex 272
The Mn-free nickel laterite leach liquor was used to extract cobalt with different concentrations (0.005- 0.0125 mol/L) of neutral Cyanex 272 at 1:1 phase ratio. The initial pH of the leach liquor was 3.4. The experimental data for cobalt and nickel extraction rate versus different concentrations of NaCyanex 272 plotted in Fig. 10 showed an increase in cobalt extraction from 15.23% to 90.75% with increase in extractant concentration from 0.001 to 0.0125 mol/L. The co-extraction of nickel increased from 0.12% to 6.44% with increase of NaCyanex 272 concentration.
The extraction isotherm for cobalt was constructed to know the number of stages of extraction at a chosen phase ratio for which the Mn-free nickel laterite leach liquor (pH 3.4) and the neutral 0.0125 mol/L NaCyanex 272 in kerosene were contacted at different O:A ratios within 1:5 to 5:1 for 5 min followed by phase separation and analysis of the phases. The McCabe-Thiele plot (Fig. 11) showed quantitative extraction of cobalt in 2-stage at 1:1 phase ratio. To confirm the predictions of the extraction isotherm, a 2-stage countercurrent simulation study was carried out with 0.0125 mol/L NaCyanex 272 at 1:1 phase ratio. The raffinates and loaded organic samples were collected and analyzed for metal ion concentration. The raffinate samples contained 0.002 g/L cobalt indicating 93.3% extraction rate. The loaded organic was analyzed to contain 0.028 and 0.02 g/L cobalt and nickel, respectively. The loaded organic was stripped with 1 g/L H2SO4 to free NaCyanex 272 for reuse.

Fig. 10 Effect of [NaCyanex 272] on extraction of cobalt and nickel (Experimental condition: [Ni] 0.64 g/L, [Co] 0.03 g/L, pH 3.4, O:A= 1:1)

Fig. 11 Extraction isotherm for cobalt with 0.0125 mol/L NaCyanex 272 (Experimental condition: [Co] 0.03 g/L, pH 3.4, 0.0125 mol/L NaCyanex 272)
3.4 Extraction of nickel
The extraction of nickel was carried out from the Fe, Cr, Al, Zn, Mn and Co-free nickel laterite leach liquor bearing 0.62 g/L Ni. To study the effect of pH on extraction of nickel from the leach liquor, the initial pH of the solution was varied within the range of 2.0-5.0. The concentration of NaTOPS-99 was kept constant at 0.06 mol/L. The equilibrium pH of raffinate samples was measured to be 5.6-7.2. The equilibrium pH values plotted against the extraction rate (Fig. 12) indicated an increase in extraction of nickel from 24.59% to 80.37% with increase in equilibrium pH.
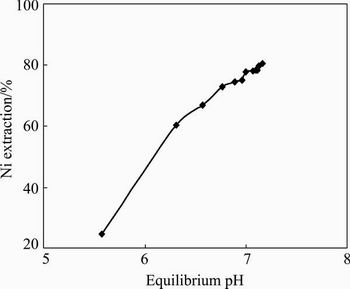
Fig. 12 Effect of equilibrium pH on extraction of Ni with 0.06 mol/L NaTOPS-99 (Experimental condition: [Ni] 0.62 g/L, 0.06 mol/L NaTOPS-99, O:A= 1:1)
The leach liquor (pH 2.5) was used to extract nickel with different concentrations (0.02-0.12 mol/L) of neutral TOPS-99 at 1:1 phase ratio. The experimental data for nickel extraction rate versus different concentrations of NaTOPS-99 plotted in Fig. 13 showed an increase in nickel extraction from 1.25% to 99.12% with increase in extractant concentration.
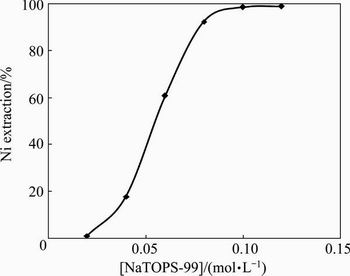
Fig. 13 Effect of [NaTOPS-99] on extraction of Ni (Experimental condition: [Ni] 0.62 g/L, pH 2.5, O:A= 1:1)
The extraction isotherm for nickel was constructed to know the required number of stages of extraction at a chosen phase ratio for which the Fe, Cr, Al, Zn, Mn and Co-free nickel laterite leach liquor (pH 2.5) and 0.12 mol/L neutral TOPS-99 in kerosene were contacted for 5 min at different O:A ratios within 1:5 to 5:1 followed by phase separation and analysis of the phases. The McCabe-Thiele plot (Fig. 14) showed quantitative extraction of nickel in two stages at phase ratio of O:A=2:3. To confirm the theoretical predictions for the extraction isotherm, a 2-stage counter-current simulation study with 0.12 mol/L NaTOPS-99 at 2:3 phase ratio (O:A) was carried out. The raffinates and loaded organic samples were collected and analyzed for metal ion concentration. The raffinate samples were analyzed to contain 0.001 g/L nickel indicating 99.84% extraction rate. The loaded organic was analyzed to contain 0.96 g/L of nickel. A sufficient quantity of nickel loaded organic was generated to carry out the stripping studies.

Fig. 14 Extraction isotherm for Ni with 0.12 mol/L NaTOPS-99 (Experimental condition: [Ni] 0.62 g/L, pH 2.5, 0.12 mol/L NaTOPS-99)
The nickel loaded TOPS-99 was stripped with different concentrations of H2SO4 (0.5 to 20 g/L) at equal phase ratio. The samples were equilibrated for 5 min and the strip solution was diluted and analyzed for metal values.The experimental data for nickel stripping rate versus different concentrations of H2SO4 as shown in Fig. 4 illustrated an increase in stripping from 8.82% to 75.58% with the increase in H2SO4 concentration from 0.5 to 2.0 g/L. With further increase in acid concentrations, the quantum of stripping increased and at 20 g/L H2SO4, the stripping rate attained was 99.34%.
The loaded organic phase contained 0.96 g/L nickel and it was required to strip out metal values from the loaded organic phase. To know the number of stages required for stripping of nickel, the Ni-LO was contacted with 20 g/L H2SO4 at O:A ratios within 1:10 to 10:1. The isotherm (Fig. 15) indicated 2-stage at O:A ratio of 8:1 for quantitative stripping. To confirm this, a 2-stage counter-current simulation study was carried out with the LO and 20 g/L H2SO4 solution at O:A ratio of 8:1. The strip solution and the spent organic contained 7.59 and 0.011 g/L nickel, respectively, leading to 98.85% stripping efficiency.
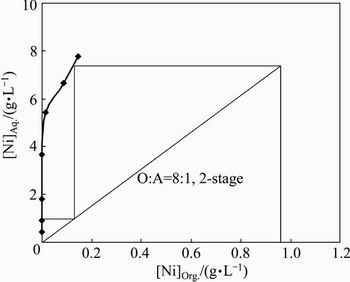
Fig. 15 Stripping isotherm for Ni-LO with 20 g/L H2SO4 (Experimental condition: [Ni]LO 0.96 g/L, H2SO4 20 g/L)
Based on the above results, a flow sheet was developed as shown in Fig. 16 for extraction of Mn and Ni from the nickel laterite bacterial leach liquor. The flow sheet described precipitation of Fe, Al and Cr from the leach liquor and separation of Zn, Mn, Co and Ni to get respective metal strip solutions.
4 Conclusions
The Ni-laterite bacterial leach liquor bearing 3.72 g/L Fe, 2.08 g/L Al, 0.44 g/L Ni, 0.02 g/L Co, 0.13 g/L Mn, 0.14 g/L Zn and 0.22 g/L Cr was processed for metal separation. Treatment with CaCO3 at pH 4.4 precipitated 100% Fe, 96.98% Al, and 70.42% Cr. The remaining Al and Cr from the solution were precipitated with 50% ammonia solution by maintaining the pH of solution at 5.4 leading to quantitative precipitation of Al and Cr. Extraction of Zn from the leach liquor was carried out with 0.1 mol/L TOPS-99 in 3-stage at O:A ratio of 1:3 followed by extraction of Mn with 0.04 mol/L NaTOPS-99 in 2-stage at 1:1 phase ratio. The extraction yields for Zn and Mn were 97.77% and 95.63%, respectively. Removal of cobalt from the Mn-free solution was then carried out with 0.0125 mol/L Cyanex 272 in 2-stage at 1:1 phase ratio with 93.3% yield. Finally, the extraction of Ni from the Co-free solution was carried out using 0.12 mol/L NaTOPS-99 in 2-stage at O:A ratio of 2:3 with resultant yield of 99.84% to generate the LO containing 0.96 g/L Ni. The Ni-LO was stripped with 20 g/L H2SO4 in 2-stage at O:A ratio of 8:1 to produce a strip solution bearing 7.59 g/L Ni leading to 98.85% stripping.
Acknowledgements
The authors wish to thank Prof. B. K. MISHRA, Director, Institute of Minerals & Materials Technology, Bhubaneswar and Dr. I. N. BHATTACHARYA, HOD, Hydro & Electrometallurgy Department for their kind permission to publish this paper.This work was supported by Orissa Mining Corporation, Odisha.

Fig. 16 Flow sheet for extraction of Mn and Ni from nickel laterite bacterial leach liquor (SX: solvent extraction)
References
[1] MOHAPATRA S, BOHIDHAR S, PRADHAN N, KAR R N, SUKLA L B. Microbial extraction of nickel from Sukinda chromite overburden by Acidithiobacillus ferrooxidans and Aspergillus strains [J]. Hydrometallurgy, 2007, 85: 1-8.
[2] INNOCENZI V, VEGLIO F. Separation of manganese, zinc and nickel from leaching solution of nickel-metal hydride spent batteries by solvent extraction [J]. Hydrometallurgy, 2012, 129-130: 50-58.
[3] AHMADIPOUR M, RASHCHI F, GHAFARIZADEH B, MOSTOUFI N. Synergistic effect of D2EHPA and Cyanex 272 on separation of zinc and manganese by solvent extraction [J]. Sep Sci Technol, 2011, 46(15): 2305-2312.
[4] DARVISHI D, HAGHSHENAS D F, ALAMDARI E K, SADRNEZHAAD S K. Extraction of Zn, Mn and Co from Zn-Mn-Co-Cd-Ni containing solution using D2EHPA, Cyanex 272 and Cyanex 302 [J]. Int J Eng Trans B: Applications, 2011, 24(2): 183-192.
[5] LEE J Y, PRANOLO Y, CHENG C Y, ZHANG Z W. The recovery of zinc and manganese from synthetic spent-battery leach solutions by solvent extraction [J]. Solvent Extr Ion Exch, 2010, 28(1): 73-84.
[6] NATHSARMA K C, DEVI N. Separation of Zn(II) and Mn(II) from sulphate solutions using sodium salts of D2EHPA, PC 88A and Cyanex 272 [J]. Hydrometallurgy, 2006, 84: 149-154.
[7] SARANGI K, PARHI P K, PADHAN E, PALAI A K, NATHSARMA K C, PARK K H. Separation of iron(III), copper (II) and zinc (II) from a mixed sulphate/chloride solution using TBP, LIX 84I and Cyanex 923 [J]. Sep Purif Technol, 2007, 55: 44-49.
[8] SATO T, HORIE J, UEDA M. Liquid-liquid extraction of divalent manganese, copper and zinc from hydrochloric acid solutions by 2-ethylhexyl phosphonic acid [J]. Solvent Extr Res Dev, 2005, 12: 189-201.
[9] BALESINI A A, RAZAVIZADEH H, ZAKERI A. Solvent extraction of zinc from acidic solution obtained from cold purification filter cake of Angouran mine concentrate using D2EHPA [J]. Iranian Journal of Chemical Engineering, 2011, 8(3): 43-47.
[10] LEE M S, NAM S H. Solvent extraction of zinc from strong hydrochloric acid solution with alamine336 [J]. Bull Korean Chem Soc, 2009, 30(7): 1526-1530.
[11] LEE M S, FILIZ M. Solvent extraction of Mn(II) from strong hydrochloric acid solutions by alamine336 [J]. Mater Trans, 2008, 49(11): 2642-2647.
[12] BISWAS R K, RAHMAN M S. Solvent extraction of manganese from sulphate-acetate medium with Cyanex 272 [J]. Indian J Chem Technol, 2011, 18: 372-380.
[13] FALCO L, QUINA M J, GANDO-FERREIRA L M, THOMAS H, CURUTCHET G. Solvent extraction studies for separation of Zn(II) and Mn(II) from spent batteries leach solutions [J]. Sep Sci Technol, 2014, 49: 398-409.
[14] LEE M, KIM S, CHOI Y, CHAE J. Solvent extraction separation of Co(II) from synthetic leaching sulfate solution of nickel laterite ore with high magnesium content [J]. Mater Trans, 2011, 52(6): 1211-1215.
[15] REDDY B R, NEELA PRIYA D, RAO S V, RADHIKA P. Solvent extraction and separation of Cd(II), Ni(II) and Co(II) from chloride leach liquors of spent Ni-Cd batteries using commercial organo-phosphorus extractants [J]. Hydrometallurgy, 2005, 77: 253-261.
R. K. MISHRA1, P. C. ROUT1,2, K. SARANGI1,2, K. C. NATHSARMA1
1. Council of Scientific and Industrial Research, Institute of Minerals and Materials Technology,Bhubaneswar 751 013, Odisha, India;
2. Academy of Scientific and Innovative Research, Chennai 600 113, Tamilnadu, India
摘 要:在煤油中采用TOPS-99和Cyanex 272钠盐从镍红土矿细菌浸出液中提取与分离锌、锰、钴和镍。采用沉淀法去除不需要的金属离子,使用溶剂萃取提取/分离锌、锰、钴和镍。生物浸出铬铁矿表土样品得到的镍红土矿浸出液中含有3.72 g/L Fe, 2.08 g/L Al, 0.44 g/L Ni, 0.02 g/L Co, 0.13 g/L Mn, 0.14 g/L Zn 和 0.22 g/L Cr。在pH 4时采用CaCO3沉积去除100% Fe, 96.98% Al 和 70.42% Cr,随后在pH 5.4时采用50%氨沉积,溶液中剩有Al 和 Cr。沉积后,采用0.1 mol/L TOPS-99从无铁、铝和铬的浸出液中提取锌,随后采用0.04 mol/L NaTOPS-99提取锰。锌和锰的产率分别是97.77% 和 95.63%。提取锰后,再采用0.0125 mol/L NaCyanex 272从浸取液去除钴,最后采用0.12 mol/L NaTOPS-99提取镍,其产率达99.84%。采用稀硫酸去除浸出液中的有机相。
关键词:镍红土矿;锰;镍;TOPS-99;Cyanex 272
(Edited by Xiang-qun LI)
Corresponding author: P. C. ROUT; Tel: +91-9861204567; E-mail: pradeep2005_chem@yahoo.co.in
DOI: 10.1016/S1003-6326(16)64119-5

