
Purification mechanism of copper electrolyte by As(Ⅲ)
XIAO Fa-xin(肖发新)1, 2, ZHENG Ya-jie(郑雅杰)1, WANG Yong(王 勇)1,
JIAN Hong-sheng(简洪生)1, HUANG Xing-guan(黄兴远)2, MA Yu-tian(马玉天)1
1. School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China;
2. School of Materials Science and Engineering, Henan University of Science and Technology, Luoyang 471003, China
Received 17 October 2007; accepted 25 February 2008
Abstract: A new kind of precipitate, antimony arsantimonate, was found during the precipitation reactions in acidic solution containing As(Ⅲ), Sb(Ⅲ) and Sb(Ⅴ) by means of chemical analysis, SEM, XRD and IR spectrometry. The results show that the As content in antimony arsantimonate increases with the increase of n(As(Ⅲ))/n(Sb) in solution and the content of component Sb(Ⅲ) and Sb(Ⅴ) remains almost constant with the variation of n(Sb(Ⅲ))/n(Sb(Ⅴ)) in solution. The antimony arsantimonate is a kind of floccules with size of 1-5 μm. The crystal performance of the compound gets better with the decrease of n(As(Ⅲ))/n(Sb), the cell parameter of which is near to 10.33×10-10 m under different n(As(Ⅲ))/n(Sb) and As atom locates on the surface, not in the inner of the grain. The chemical bonds of As—OH, Sb—OH, As—O—Sb, Sb—O—Sb and O—H of the precipitate are included in the precipitate. The chemical structure of precipitate is described as Sb(OH)2—O—[Sb(OH)3—(O—As(OH)—O—Sb(OH)3)3]—O—Sb(OH)2·xH2O. The structure analysis shows that the copper electrolyte can be purified by As(Ⅲ) because the antimony arsantimonate precipitate forms.
Key words: arsenic; purification; copper electrolyte; mechanism; antimony arsantimonate
1 Introduction
The laboratorial experiment indicated that Sb and Bi in the copper electrolyte could be removed effectively by adding appropriate arsenic[1], which has been proved in industrial experiments[2-3]. There is a viewpoint that the arsenate formed from As(Ⅴ), Sb(Ⅲ) and Bi(Ⅲ) is the reason of purification of electrolyte by arsenic[4-6]. As(Ⅴ) and Sb(Ⅴ) can form a series of arsenato- antimonic acids(AAAc) which can further react with As(Ⅲ), Sb(Ⅲ) and Bi(Ⅲ) to form arsenato-antimonates that is indissoluble even in the strongly acidic solution [7-8]. As(Ⅴ) is an indispensable element no matter in form of arsenate or arsenato-antimonate[9]. However, it was found that As(Ⅲ) is an essential element in purification of the copper electrolyte. Even for no As(Ⅴ) case, the purification performance is still good. Obviously, it can not be explained by the viewpoint of arsenate or arsenato-antimonate.
This work aims to find out the purification mechanism of electrolyte by As(Ⅲ) during the precipitate reactions in the solution containing H2SO4, As(Ⅲ), Sb, Bi, and the characterized structure of the precipitate.
2 Experimental
The analytically pure reagents, As2O3, Sb2O3, Bi2O3, HNO3, HCl and H2SO4 were used. First, the high concentration solutions of As(Ⅲ), Sb(Ⅲ), Sb(Ⅴ) and Bi(Ⅲ) were prepared. A solution containing 10 g/L As(Ⅲ), 0.6 g/L Sb, 0.25 g/L Bi and 185 g/L H2SO4 synthesized by mixing these solutions were stirred mechanically (200-400 r/min) under 65 ℃ for 2 h, then leached to remove the precipitate. The components of the filtrate and precipitate were respectively determined. The composition of precipitate was determined by chemical method, the appearance was observed by SEM (JSM- 6360LV, JEOL Corp.), and the crystal phase and the cell parameters were analyzed by XRD (D/max–rA, Rigaku Corp., Japan). The vibration characteristics were inferred by IR spectrum (NExus670, Nicolet Corp.).
3 Results and discussion
3.1 Precipitation reactions of As(Ⅲ), Sb and Bi in acidic solution
As(Ⅲ), Sb(Ⅲ), Sb(Ⅴ) and Bi interact in H2SO4 solution and the results are listed in Table 1. It can be seen that the deposit in the acidic solution containing As(Ⅲ), Sb and Bi would form only in a kind of solution containing As(Ⅲ), Sb(Ⅲ) and Sb(Ⅴ). The concentra- tions of As and Sb decrease in that solution.
Table 1 Precipitation reactions of As(Ⅲ), Sb(Ⅲ), Sb(Ⅳ) and Bi in H2SO4 solution

3.2 Structure characterization of precipitate of As(Ⅲ), Sb(Ⅲ) and Sb(Ⅴ)
3.2.1 Influences of synthesis conditions on components of precipitate
It is shown that As(Ⅲ), Sb(Ⅲ) and Sb(Ⅴ) in the acidic solution can form precipitate. A study was made on the influences of different conditions on the components of the precipitate to explore their structure.
1) Influence of n(Sb(Ⅲ))/n(Sb(Ⅴ)) ratio
1 L solution containing 1.5 g/L As(Ⅲ), 2.4 g/L Sb and 185 g/L H2SO4 was stirred mechanically under 65 ℃ for 2 h, then was filtrated to gain the precipitate. The influence of n(Sb(Ⅲ))/n(Sb(Ⅴ)) on the components of precipitate is shown in Fig.1. It can be seen that the contents of As, Sb(Ⅲ) and Sb(Ⅴ) almost remain constant, being 13.95%, 15.46% and 30.89% approximately, under the different ratio of n(Sb(Ⅲ))/n(Sb(Ⅴ)). The ratio in the precipitate is nearly equal to 1?2. Obviously, n(Sb(Ⅲ))/n(Sb(Ⅴ)) in the acidic solution has little effect on the components of precipitate.
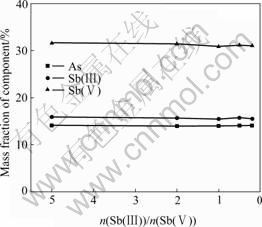
Fig.1 Influence of n(Sb(Ⅲ))/n(Sb(Ⅴ)) on components of precipitate
2) Influence of n(As(Ⅲ))/n(Sb) ratio
The influences of n(As(Ⅲ))/n(Sb) ratio in the acidic solution on the components of precipitate are shown in Fig.2 under n(Sb(Ⅲ))/n(Sb(Ⅴ))=1?1 and other conditions fixed. It can be seen from Fig.2 that the As content in the precipitate decreases with the decrease in the ratio down to n(AS(Ⅲ))/n(Sb)=0.5 and remains almost constant when it further decreases. The contents of Sb(Ⅲ) and Sb(Ⅴ) remain almost constant, being about 15.5% and 31% respectively, when the ratio varies. The n(Sb(Ⅲ))/n(Sb(Ⅴ)) of the precipitate is nearly equal to 1?2.
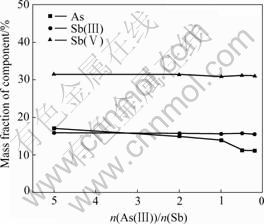
Fig.2 Influence of n(As(Ⅲ))/n(Sb) on components of preci- pitate
The results show that either n(Sb(Ⅲ))/n(Sb(Ⅴ)) or n(As(Ⅲ))/n(Sb) has some effects on the components of the precipitate. The As content in the precipitate increases with the increase of n(As(Ⅲ))/n(Sb) in the solution, the contents of Sb(Ⅲ) and Sb(Ⅴ) remain almost constant with the variation of n(As(Ⅲ))/n(Sb(Ⅴ)) in the solution and n(Sb(Ⅲ))/n(Sb(Ⅴ)) of solution has little effect on the component of precipitate. The ratio in the precipitate under different conditions is nearly 1?2.
3.2.2 SEM result of precipitate
The SEM result of the precipitate under ρ(Sb)=2.4 g/L, n(As(Ⅲ))?n(Sb(Ⅲ))?n(Sb(Ⅴ))=4?1?1 and ρ(H2SO4) =185 g/L is shown in Fig.3. It can be seen that the precipitate under above conditions is floccules with the size of 1-5 μm.
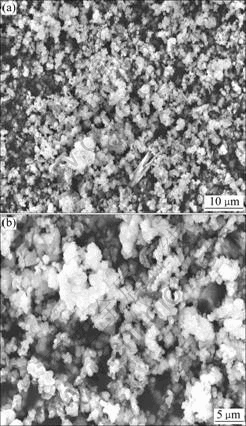
Fig.3 SEM images of precipitate under n(As(Ⅲ))?n(Sb(Ⅲ))? n(Sb(Ⅴ))=4?1?1
3.2.3 XRD results of precipitate
The precipitate under the different ratio of n(As(Ⅲ))/n(Sb) at ρ(Sb)T=2.4 g/L, n(Sb(Ⅲ))/n(Sb(Ⅴ))= 1?1 and ρ(H2SO4)=185 g/L were tested by XRD and the results are shown in Fig.4. It can be seen that the precipitate is crystal under different n(As(Ⅲ))/n(Sb) ratios, and the crystal performance gets better with the decrease of the ratio. To get the structure of the crystal, the XRD test with the low scanning rate of 0.5 (?)/min was carried out in the range of 42?-62?. The results of the precipitate at the ratios of n(As(Ⅲ))/n(Sb)=1?2 and 1?5 are shown in Fig.5 and Fig.6, respectively.
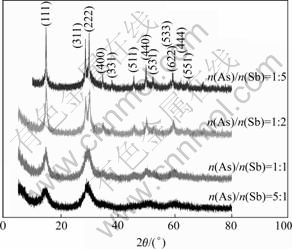
Fig.4 XRD patterns of precipitate
The crystal plane spacing can be calculated by reflection angle of θ according to Bragg’s Law:
d=λ/(2sin θ) (1)
The XRD result of precipitate is similar to that of the cubic antimonic acid[10], therefore, the crystal of the precipitate is considered as cubic phase and its cell parameter can be calculated according to the following formula:

According to Fig.5, the reflection angle of peak and gravity center of (511) crystal plane is 22.947 5? and 22.859?. The parameters of θ1θ2, λ=1.541 78×10-10 m (wavelength of Cu-target) were listed in Bragg’s law and the crystal plane spacing was calculated as: d1=1.977 2× 10-10 m, d2=1.984 4×10-10 m. The cell parameter of the precipitate can be calculated according to Eqn.(2) under h=5, k=1 and l=1 as a1=10.274×10-10 m and a2= 10.311 ×10-10 m.
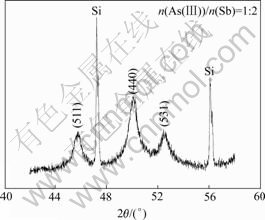
Fig.5 XRD pattern of precipitate under n(As(Ⅲ))/n(Sb)=1?2

Fig.6 XRD pattern of precipitate under n(As(Ⅲ))/n(Sb)=1?5
By the same way, the crystal plane spacing can be calculated as a3=10.292×10-10 m and a4=10.314×10-10 m for (440) plane, and a5=10.304×10-10 m and a6= 10.289×10-10 m for (531) plane. Thus, the cell parameter of the precipitate a=(10.274+10.311+10.292+ 10.314+10.304+10.289)/6=10.297×10-10 m. While for the precipitate of n(As(Ⅲ))/n(Sb)=1?5, the cell parameter is 10.33×10-10 m. Obviously, the cell parameter of the precipitate under different n(As(Ⅲ))/n(Sb) is almost a constant similar to that of cubic anionic acid ((10.30- 10.38)×10-10 m)[10], indicating that As atom locates on the surface, not in the inner of the crystal. This is the main reason why the content of Sb remains constant while the As content increases with the increase of n(As(Ⅲ))/n(Sb) in solution.
3.2.4 IR spectrum of precipitate
The IR spectrum of the precipitate at ρ(Sb)T=2.4 g/L, n(Sb(Ⅲ))/n(Sb(Ⅴ))=1?1 and ρ(H2SO4)=185 g/L is shown in Fig.7.
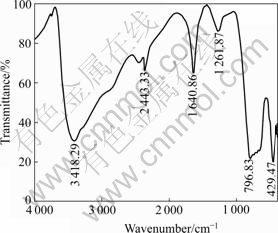
Fig.7 FT-IR spectrum of precipitate under n(As)?n(Sb(Ⅲ))? n(Sb(Ⅴ))=4?1?1
It can be seen from Fig.7 that the FTIR bands at 3 418.29 cm-1 and 1 640.86 cm-1 are the symmetrical and anti-symmetrical stretch vibration absorption spectra of O—H[11], the band at 1 261.87 cm-1 is the bending vibration absorption spectra of As—OH and Sb—OH [12-13], and the bands at 796.83 and 429.47 cm-1 are the anti-symmetrical stretch vibration absorption spectra of As—O—Sb and Sb—O—Y (Y=As, Sb) respectively [12,14]. Therefore, the valence bands of As—OH, Sb—OH, As—O—Sb, Sb—O—Sb and O—H are the parts in the chemical structure of the precipitate.
HUANG et al[15] synthesized the antimony thioanti- monate by Sb2O3 and Na5SbS4·9H2O. According to XRD analysis, the cell parameter of the precipitate is similar to that of cubic anionic acid. In addition, the n(As(Ⅲ))? n(Sb(Ⅲ))?n(Sb(Ⅴ)) in the precipitate is 3?2?4 according to chemical analysis. Therefore, the precipitate should be antimony arsantimonate and its chemical structure can be described as Sb(OH)2—O—[Sb(OH)3—(O—As(OH)—O—Sb(OH)3)3]—O—Sb(OH)2·xH2O.
3.3 Structure characterization of precipitate in industrial experiment
The copper arsenite was added into the copper electrolyte (provided by Daye Nonferrous Metal Ltd.) to purify the electrolyte in the industrial experiment. The components of the electrolyte before and after purification are listed in Table 2. The XRD and IR results of the precipitate in industrial experiment are shown in Fig.8 and Fig.9, respectively.
It can be seen from Table 2 that As(Ⅲ) has distinct effect on the purification of the electrolyte. The concentration of Sb and Bi in the electrolyte decreases greatly if As(Ⅲ) concentration gets up to a value.
Table 2 Components of electrolyte before and after purification (g·L-1)
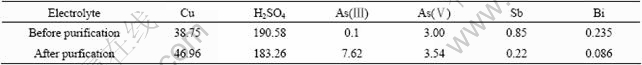
Fig.8 and Fig.9 all show that the precipitate by purification in industrial experimental contains antimony arsantimonate. The XRD peak is nearly the same as that of antimony arsantimonate of n(As)/n(Sb)=5?1. The ratio is high according to Table 2, so the crystal performance of the precipitate of industrial experiment is not so good.

Fig.8 XRD patterns of precipitate in industrial experiment
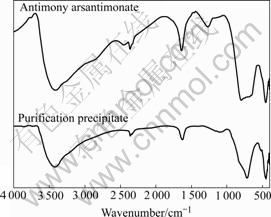
Fig.9 IR spectra of precipitate in industrial experiment
As(Ⅲ) reacts with Sb(Ⅲ), Sb(Ⅴ) to form antimony arsantimonate when adding copper arsenite in the electrolyte, making Sb concentration in the electrolyte decrease greatly. Obviously, this discovery during the purification indicates that the antimony arsantimonate is the main cause of the copper electrolyte being purified, which provides a new approach for purification of copper electrolyte.
4 Conclusions
1) In the acidic solution, As(Ⅲ) reacts with Sb(Ⅲ) and Sb(Ⅴ) to form antimony arsantimonate. As content of antimony arsantimonate increases with the increase of n(As(Ⅲ))/n(Sb) in solution and remains almost constant with the variation of n(Sb(Ⅲ))/n(Sb(Ⅴ)). The n(Sb(Ⅲ))/n(Sb(Ⅴ)) in the precipitate is nearly 1?2 under different conditions.
2) The SEM result shows that antimony arsantimonate is a kind of floccules with size of 1-5 μm. The XRD results show that the crystal performance of the floccules gets better with the decrease of n(As(Ⅲ))/ n(Sb). Its crystal is cubic under different n(As(Ⅲ))/n(Sb) with the cell parameter about 10.33×10-10 m, and As atom is located at the surface not in the inner of the crystal grain. The IR spectrum analyses show that the chemical bonds of As—OH, Sb—OH, As—O—Sb, Sb—O—Sb and O—H are included in the precipitate of antimony arsantimonate, the structure of which is determined as Sb(OH)2—O—[Sb(OH)3—(O—As(OH)—O—Sb(OH)3)3]—O—Sb(OH)2·xH2O.
3) The results of XRD and IR of the precipitate in industrial experiments show that the antimony arsantimonate is the main cause of the purification of copper electrolyte by As(Ⅲ).
References
[1] XIAO Fa-xin, ZHENG Ya-jie, WANG Yong. Novel technology of purification of copper electrolyte [J]. Trans Nonferrous Met Soc China, 2007, 17(5): 1069-1074.
[2] ZHENG Ya-jie, XIAO Fa-xia, WANG Yong, LI Chun-hua, XU Wei, JIAN Hong-sheng, MA Yu-tian. Industrial experiment of copper electrolyte purification with copper arsenite[J]. Journal of Central South University of Technology, 2008, 15(2): 204-208.
[3] XIAO Fa-xin, ZHENG Ya-jie, WANG Yong, JIAN Hong-sheng, LI Chun-hua, XU Wei, MA Yu-tian. Preparation technique of copper arsenite and its application in the purification of copper electrolyte [J]. Trans Nonferrous Met Soc China, 2008, 18(2): 474-479.
[4] XIAO Bing-rui, SU Zhong-fu, LI Yi-huang, LONG Zi-ping, HUANG Ming-jin. A new purification method of copper electrolyte. CN 02129694.4 [P]. 2003-03-05. (in Chinese)
[5] YANNOPOULOS J C, AGARWAL J C. Extractive metallurgy of copper: Pyrometallurgy and electrolytic refining [M]. New York: The Metallurgical Society of AIME, 1977: 512-524.
[6] ZHU Zhu-zhi, HE Jia-qi. Modern copper metallurgy science [M]. Beijing: Science Press, 2003: 491-492. (in Chinese)
[7] WANG Xue-wen, CHEN Qi-yuan, YIN Zhou-lan, ZHANG Ping-min, LONG Zi-ping, SU Zhong-fu. Removal of impurities from copper electrolyte with adsorbent containing antimony [J]. Hydrometallurgy, 2003, 69(1/3): 39-44.
[8] WANG Xue-wen, CHEN Qi-yuan, YIN Zhou-lan, XIAO Lian-sheng. Identification of arsenato antimonates in copper anode slimes [J]. Hydrometallurgy, 2006, 84(3/4): 211-217.
[9] WANG Xue-wen, CHEN Qi-yuan, LONG Zi-ping, SU Zhong-fu, YIN Zhou-lan, ZHANG Ping-min. Application of antimony in purification of copper electrolyte [J]. The Chinese Journal of Nonferrous Metals, 2002, 12(6): 1277-1280. (in Chinese)
[10] AMARILLA J M, ROJAS J M. Antimonic acid and sulfonated polystyrene proton-conducting polymeric composites [J]. Solid State Ionics, 2000, 127: 133-139.
[11] NAILI H, MHIRI T. X-ray structural, vibrational and calorimetric studies of a new rubidium pentahydrogen arsenate RbH5(AsO4)2 [J]. J Alloys Comp, 2001, 315: 143-149.
[12] QURESHI M, KUMAR V. Synthesis and IR, X-ray and ion-exchange studies of some amorphous and semi-crystalline phases of titanium antimonate: Separation of VO2+ from various metal ions [J]. Journal of Chromatography A, 1971, 62(3): 431-438.
[13] MYNENI S C, TRANINA S J, WAYCHUNAS G A. Experimental and theoretical vibrational spectroscopic evaluation of arsenate coordination in aqueous solutions, solids and at mineral-water interfaces [J]. Geochimica et Cosmochimica Acta, 1998, 62: 3285-3300.
[14] COLOMBAN P H, DOREMIEUX-MORIN C D, PIFFARD Y. Equilibrium between protonic species and conductivity mechanism in antimonic acid H2Sb4O11·nH2O [J]. J Mol Struct, 1989, 213: 83-96.
[15] HUANG Chun-lin, LI De-chang, LUO Gui-xin. Synthesis of antimony thioantimonate [J]. Modern Chemical Industry, 1999, 19(7): 24-26. (in Chinese)
Corresponding author: ZHENG Ya-jie, Tel: +86-731-8836285; E-mail: csuxfx@yahoo.com.cn
(Edited by YUAN Sai-qian)