


Trans. Nonferrous Met. Soc. China 22(2012) 1161-1168
Size-controlled preparation of hollow silica spheres and glyphosate release
LIU Chun1,2, YIN Heng-bo1, WANG Ai-li1, WU Zhan-ao3, WU Gang3,
JIANG Tao3, SHEN Yu-tang1, JIANG Ting-shun1
1. Faculty of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, China;
2. College of Chemistry and Biology, Beihua University, Jilin 132013, China;
3. Chinese PLA 359 Hospital, Zhenjiang 212001, China
Received 15 November 2011; accepted 16 April 2012
Abstract: Different-sized hollow SiO2 spheres of 249–1348 nm in diameter were successfully prepared by using Na2SiO3 as the precursor and using polystyrene and polystyrene-methyl acrylic acid latexes as the templates. The diameter and shell thickness of the hollow SiO2 spheres increase with increasing the latex template diameter at a given mass ratio of SiO2 to latex template. The diameter and shell thickness of the hollow SiO2 spheres also increase with increasing the mass ratios of SiO2 to latex template. The presence of carboxylic acid groups on the surfaces of polystyrene-methyl acrylic acid latex templates favors the formation of dense and uniform SiO2 shells. The hollow SiO2 sphere is constructed by mesoporous shell with large specific surface area. When glyphosate is used as a release model chemical, glyphosate release rate is tuned by varying the shell thickness.
Key words: hollow SiO2 sphere; polystyrene; polystyrene-methyl acrylic acid; glyphosate release
1 Introduction
Recently, preparation of inorganic hollow spheres, such as hollow SiO2 [1,2], Ni [3,4], Co/B [5], TiO2 [6-8], Pt/Ru/Pd [9], Co3O4 [10], Ni/Fe/P [11], ferrite [12], carbon [13], SnO2 [14], and calcium silicate [15] spheres, has attracted increasing research interest. The inorganic hollow spheres have potential application in many fields, such as catalysis [1,2,5,6], magnetics [3,11,12], microwave absorption [4], lithium ion battery [7,10], solar cell [8], electrocatalysis [9], hydrogen storage [13], gas sensor [14], and drug delivery [15] due to their high chemical and thermal stability, high specific surface area, high porosity, low density, and good biocompatibility.
Among the inorganic hollow spheres, hollow SiO2 spheres have potential application in catalysis, heat and sound insulation, adsorption, and drug delivery. Hollow SiO2 spheres are usually prepared by using Fe3O4 nanoparticles [16], poly(vinylpyrrolidone) [17], polystyrene [18], chitosan-polyacrylic acid [19], calcium carbonate nanoparticles [20], amine-functionalized polystyrene [21], and polystyrene-methyl acrylic acid [22,23] as templates. Tetraethoxysilane [16,17,20,23], SiO2 nanoparticles [18], colloidal silica [19], and sodium silicate [22] were used as starting materials. And poly(vinylalcohol) [16], cetyltrimethylammonium bromide [17,20,22,23], and poly-L-lysine [21] were used as shell structure-directing agents. In the previously reported works, more efforts were dedicated to the construction of hollow SiO2 spheres by using different types of templates, SiO2 precursors, and structure-directing agents. Although the diameter and thickness of hollow SiO2 spheres unavoidably affect their performances, the size-controlled preparation of hollow SiO2 spheres was seldom reported.
In the present work, the size-controlled hollow SiO2 spheres were prepared from Na2SiO3 using different-sized polystyrene and polystyrene-methyl acrylic acid latex as templates. Hollow SiO2 spheres of 249-1348 nm in diameter were prepared by calcination of SiO2-coated latex composites. The diameter and shell thickness of the hollow SiO2 spheres were tuned by varying the latex template diameter and SiO2 loading. To investigate the controlled release performance of hollow SiO2 spheres, the release rate of glyphosate from the hollow SiO2 spheres with different shell thicknesses was investigated. Glyphosate was selected as a model chemical because it is a widely used herbicide and the controlled release will increase its weeding capacity.
2 Experimental
2.1 Materials
The chemicals, such as sodium silicate (Na2SiO3·9H2O, 99%), cetyltrimethylammonium bromide (99%), styrene (99%), potassium persulfate (99.5%), methyl acrylic acid (98%), and sulfuric acid (98%) were purchased from China Chemical Reagent Co., Ltd. All the above-mentioned chemicals were of analytical grade. Styrene was used after distillation. Glyphosate (98%) was supplied by Zhenjiang Pesticide Co., Ltd. Distilled water was used throughout all of the experiments.
2.2 Preparation of polystyrene and polystyrene- methyl acrylic acid latex templates
Polystyrene-methyl acrylic acid latex spheres were prepared by an emulsifier-free emulsion polymerization method [22,23]. Given amounts of styrene, methyl acrylic acid, ethanol, and water were added into a 1000 mL four-necked flask equipped with mechanical stirrer, reflux condenser, nitrogen inlet and temperature controller. After deoxygenating the reaction mixture via bubbling nitrogen gas for 30 min, reaction temperature was raised to 70 °C and an aqueous solution of potassium persulfate (0.25 g in 10 mL of water) was added to start the polymerization process. The reaction was allowed to proceed for 24 h and polystyrene-methyl acrylic acid latex was obtained as a stable dispersion in water. The preparation method of polystyrene latex was similar to that of the polystyrene-methyl acrylic acid latex without methyl acrylic acid. The as-prepared latex templates were centrifugated and washed with ethanol and distilled water, respectively. The preparation conditions of the latex templates are listed in Table 1.
Table 1 Preparation conditions of polystyrene-methyl acrylic acid latex templates

2.3 Preparation of hollow silica spheres
To prepare different-sized hollow SiO2 spheres, the latex templates (T1-T5) were coated by SiO2 with a mass ratio of SiO2 to latex template of 80:100. Firstly, 2.5 g of polystyrene-methyl acrylic acid (or polystyrene) latex templates were ultrasonically treated in 200 mL of water for 20 min to obtain a well-dispersed suspension. The suspension was transferred into a 2000 mL flask and 20 mL of an aqueous solution of cetyltrimethyla- mmonium bromide (10%) was added and stirred for 1 h. The suspension was adjusted to pH value of 7 by adding a sodium hydroxide aqueous solution (10%). Then, 333 mL of sodium silicate aqueous solution (0.1 mol/L) and sulfuric acid (1%) were added to the above-mentioned suspension slowly with two pumps at pH value of 7 and stirred at 80 °C for 2 h. After being cooled to room temperature, the suspension was aged for 3 h under stirring. The precipitate was filtrated and washed with distilled water until the conductivity of the filtrate was less than 20 mS/m. The washed precipitate was dried in an electric oven at 120 °C for 4 h. Then the dried SiO2-coated latex core-shell composites were heated from room temperature to 550 °C at 1 °C/min and kept at 550 °C for 4 h under atmospheric condition in order to oxidize the organic cores completely.
To investigate the effect of SiO2 loading on shell thickness, different amounts of SiO2 were coated on the same polystyrene-methyl acrylic acid latex template (T4). The mass ratios of SiO2 to latex template were changed from 80:100 to 120:100, 160:100, and 200:100, respectively, by varying the volume of the sodium silicate aqueous solution.
2.4 Characterization
Scanning electron microscopy (SEM, JSM 7001F) and transmission electron microscopy (TEM, Philips Tecnnai-12, operating at 120 kV) were used to investigate the morphologies of the latex templates, the SiO2-coated latex core–shell composites, and the hollow silica samples. The samples for TEM inspection were dispersed in ethanol solution under ultrasonic treatment for 10 min. Then a few drops of the suspension were dropped onto a copper grid coated with a layer of amorphous carbon. The Fourier transform infrared spectra of the samples were obtained by the KBr pellet technique on a Fourier transform infrared spectrometer (Nicolet Nexus470) to determine the interfacial chemical bonding structure. The average pore size, pore volume, and specific surface area of the hollow silica samples were determined by nitrogen adsorption-desorption method on a Quntachrome Corporation, NOVA2000e. The sample was first degassed in vacuum at 300 °C for 1 h. Then the measurement was carried out at 77 K over a range of relative pressures (p/p0), where p0 is the saturated vapor pressure, from 0.1 to 1. The specific surface area and average pore size were calculated by BET and BJH methods, respectively.
2.5 Glyphosate release
Glyphosate was loaded into hollow SiO2 spheres by wetness impregnation method. The glyphosate loading was 7.9% of hollow SiO2 spheres. The glyphosate-loaded hollow SiO2 spheres were dried in an vacuum oven at 80 °C for 12 h. For glyphosate release, 3 g of the dried samples was added into 1000 mL of water at 20 °C under stirring at 100 r/min. 5 mL of the suspension was taken out for analysis at different time. After filtration and nitration, the concentration of glyphosate in the filtrate was analyzed on a UV-VIS spectrometer at 242 nm.
3 Results and discussion
3.1 Morphologies of latex templates, SiO2-coated latex composites, and hollow silica samples
Figure 1 shows the SEM images of the latex templates (T1-T5) prepared under different experimental conditions. The as-prepared latex templates were spherical and had smooth surface. The average diameters of the latex templates (T1-T5) were 1231, 580, 465, 362, and 224 nm, respectively (Table 2).

Fig. 1 SEM images of polystyrene and polystyrene-methyl acrylic acid latex templates prepared under different experimental conditions: (a) T1; (b) T2; (c) T3; (d) T4; (e) T5
Table 2 Preparation conditions and diameters of latex templates, SiO2-coated latex composites, and hollow SiO2 spheres

Figure 2 shows the SEM and TEM images of the SiO2-coated latex composites and the hollow SiO2 spheres. The SEM images show that when the latex templates (T1-T5) were coated by SiO2 with a mass ratio of SiO2 to template of 80:100, the average diameters of the SiO2-coated latex composites were 1342, 685, 522, 371, and 249 nm, respectively. After calcination, the average diameters of the resultant hollow SiO2 spheres were 1348, 660, 523, 368, and 249 nm, similar to those of the SiO2-coated latex composites. The results show that the diameter of the hollow SiO2 sphere was tuned by changing the diameter of the template. It is interesting that when polystyrene latex was used as the template, the shells of the as-prepared hollow SiO2 spheres were constructed by large-sized SiO2 nanoparticles with an average diameter of about 100 nm. While polystyrene- methyl acrylic acid latexes were used as the templates, dense and uniform SiO2 shells were formed. It can be explained that acidic groups on the surfaces of polystyrene-methyl acrylic acid latex templates favored the uniform deposition of hydrated silica gels on the surfaces of the latex templates. Furthermore, the TEM images show that the shell thicknesses of the hollow SiO2 spheres gradually decreased from 81 to 38, 37, 22, and 15 nm with the decrease in the diameters of latex templates (T1-T5) (Fig. 2, Table 2). The shell thickness was significantly affected by the latex template diameter at a fixed SiO2 load.
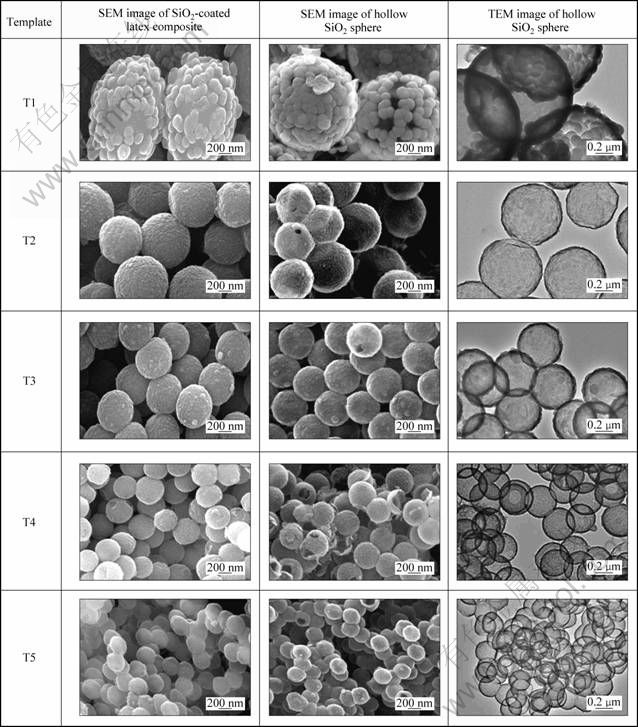
Fig. 2 SEM images of SiO2-coated latex composites prepared by using different-sized latex templates, and SEM and TEM images of hollow SiO2 spheres (Mass ratio of SiO2 to latex template was fixed at 80:100)
To investigate the effect of SiO2 load on the shell thickness, hollow SiO2 spheres were prepared with different mass ratios of SiO2 to latex template (T4). Figure 3 shows the SEM and TEM images of the SiO2-coated latex composites and the hollow SiO2 spheres. With increasing the mass ratios of SiO2 to latex template from 80:100 to 200:100, the diameters of the SiO2-coated latex composites increased from 371 to 410 nm (Table 2). The diameters of the resultant hollow SiO2 spheres increased from 368 to 407 nm. The shell thicknesses increased from 22 to 33 nm, indicating that the shell thickness was also tuned by varying the SiO2 loading.
3.2 FT-IR analysis
Figure 4 shows the FT-IR spectra of polystyrene latex, polystyrene-methyl acrylic acid latex, CTAB, SiO2-coated polystyrene-methyl acrylic acid latex composite, and hollow SiO2 spheres. For the polystyrene latex, strong bands appearing at 3026, 2921, 1600, 1492, 1450, 755, and 699 cm–1 were observed. For the polystyrene-methyl acrylic acid latex, a new band at 1699 cm–1 ascribed to carboxylic group was observed. The characteristic bands of CTAB appeared at 962 and 910 cm–1. For the SiO2-coated polystyrene-methyl acrylic acid latex composite, a broad band at 1089 cm–1 and two weak bands at 798 and 450 cm–1 ascribed to Si—O—Si stretching vibrations were observed, indicating that SiO2 coating layers were formed on the latex surfaces after hydrolysis of sodium silicate. Meanwhile, a weak band at 956 cm–1 ascribed to CTAB was also detected, revealing that CTAB was combined with SiO2 coating layers. After calcination, only three bands at 1093, 794, and 443 cm–1 ascribed to the Si—O—Si vibrations were observed. It can be concluded that calcination caused further polycondensation between the silica gels and the latex template was completely removed.
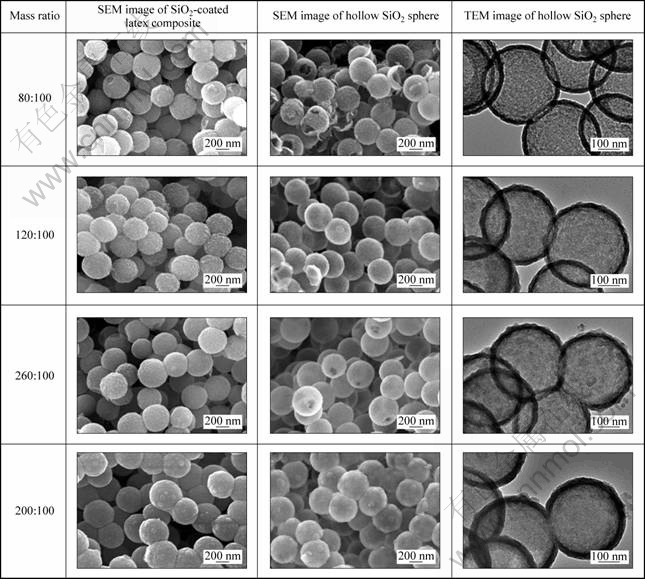
Fig. 3 SEM images of SiO2-coated latex composites, and SEM and TEM images of hollow SiO2 spheres with different mass ratios of SiO2 to latex template

Fig. 4 FT-IR spectra of polystyrene latex (a), polystyrene- methyl acrylic acid latex (b), CTAB (c), SiO2-coated polystyrene-methyl acrylic acid latex composite (d), and hollow SiO2 sphere (e)
3.3 Adsorption/desorption of nitrogen
The specific surface area, average pore diameter, and average pore volume of the hollow SiO2 spheres measured by nitrogen adsorption/desorption are listed in Table 3. When SiO2 was coated on the polystyrene latex template (T1), the resultant hollow SiO2 spheres had larger specific surface area, but smaller average pore diameter and lower pore volume than those prepared by using polystyrene-methyl acrylic acid latexes (T2-T5) as the templates had the same SiO2 load. When SiO2 was coated on the polystyrene-methyl acrylic acid latex templates (T2-T5) with a fixed mass ratio of SiO2 to latex template of 80:100, the specific surface area and pore volume of the resultant hollow SiO2 spheres increased with the decrease in the diameter of latex templates. When the latex template (T4) was coated with different SiO2 loads, the specific surface area and pore volume of the resultant hollow SiO2 spheres decreased with increasing the SiO2 load. For all the hollow SiO2 samples, the average pore diameter was more than 3 nm, indicating that mesoporous SiO2 shells were formed.
Under the present experimental conditions, different-sized hollow SiO2 spheres with mesoporous shells were constructed. The pore volume and specific surface area were affected by both latex template diameter and SiO2 load. The evolution process of the hollow SiO2 spheres was explained as follows. Polystyrene-methyl acrylic acid latex templates with negatively charged surfaces favorably adsorbed positively charged CTAB. Then the adsorbed positively charged CTAB, as a structure-directing agent, adsorbed negatively charged silica gels to form SiO2 coating layers on the latex template surfaces. After removing the latex templates and CTAB by calcination, hollow SiO2 spheres with mesoporous shells were formed.
3.4 Glyphosate release
The hollow SiO2 spheres of different shell thicknesses were used as glyphosate release vehicles. The release curves are shown in Fig. 5. When the glyphosate-loaded hollow SiO2 spheres with shell thicknesses of 22, 24, 27, and 33 nm were stirred for 120 min in water, the release rates of glyphosate were 75%, 60%, 47%, and 30%, respectively. The initial release rates within the first 10 min in terms of unit hollow sphere were 2.23×10–3, 1.73×10–3 and 1.34×10–3 and 9.7×10–4 g/(g·min), respectively, for the hollow SiO2 spheres with shell thicknesses of 22, 24, 27, and 33 nm. The results showed that glyphosate release rate decreased with the increase in the shell thicknesses.
Table 3 Specific surface areas, average pore diameters, and average pore volumes of hollow SiO2 spheres prepared under different experimental conditions
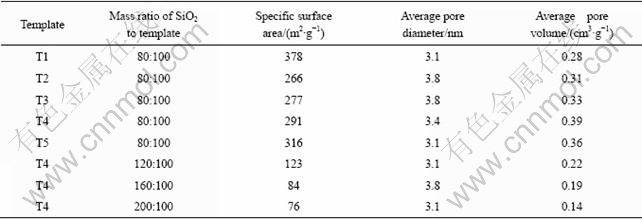

Fig. 5 Release curves of glyphosate from dipped hollow SiO2 spheres prepared with different mass ratios of SiO2 to latex template (T4)
4 Conclusions
1) Hollow SiO2 spheres were prepared from Na2SiO3 using different-sized polystyrene and polystyrene-methyl acrylic acid latex templates. When the diameter of the latex templates ranges from 1231 to 224 nm and the mass ratio of SiO2 to latex template is 80:100, the diameter and shell thickness of the as-prepared hollow SiO2 spheres decrease from 1348 to 249 nm and from 81 to 15 nm, respectively, with the decrease in the diameter of latex templates.
2) When polystyrene-methyl acrylic acid latex with the average diameter of 362 nm is used as the template, the average diameter and average shell thickness of the resultant hollow SiO2 spheres increase from 368 to 407 nm and from 22 to 33 nm, respectively, with increasing the mass ratios of SiO2 to latex template from 80:100 to 200:100.
3) The diameter and shell thickness of the hollow SiO2 spheres are tuned by varying both latex template diameter and SiO2 load. The hollow SiO2 spheres are constructed by mesoporous shells with large specific surface area. When glyphosate is used as a release model chemical, the glyphosate release rate is controlled by the shell thickness.
Acknowledgements
The authors thank Professor CHEN Kang-min (Analysis Center, Jiangsu University) very much for kindly supporting SEM and TEM measurement of the samples.
References
[1] LI J H, XU Y, WU D, SUN Y H. Hollow mesoporous silica sphere supported cobalt catalysts for F–T synthesis [J]. Catal Today, 2009, 148: 148-152.
[2] IKEDA S, KOBAYASHI H, IKOMA Y, HARADA T, YAMAZAKI S, MATSUMURA M. Structural effects of titanium (IV) oxide encapsulated in a hollow silica shell on photocatalytic activity for gas-phase decomposition of organics [J]. Appl Catal A, 2009, 369: 113-118.
[3] BAO J C, LIANG Y Y, XU Z, SI L. Facile synthesis of hollow nickel submicrometer sphere [J]. Adv Mater, 2003, 15: 1832-1835.
[4] LI Z B, SHEN B, DENG Y D, LIU L, HU W B. Preparation and microwave absorption properties of electroless Co–P-coated nickel hollow spheres [J]. Appl Surf Sci, 2009, 255: 4542-4546.
[5] MA H, JI W Q, ZHAO J Z, LIANG J, CHEN J. Preparation, characterization and catalytic NaBH4 hydrolysis of Co-B hollow spheres [J]. J Alloys Compd, 2009, 474: 584-589.
[6] AO Y H, XU J J, ZHANG S H, FU D G. A one-pot method to prepare N-doped titania hollow spheres with high photocatalytic activity under visible light [J]. Appl Surf Sci, 2010, 256: 2754-2758.
[7] WANG J P, BAI Y, MU M Y, YIN J, ZHANG W F. Preparation and electrochemical properties of TiO2 hollow spheres as an anode material for lithium-ion batteries [J]. J Power Sources, 2009, 191: 614-618.
[8] YU J G, FAN J J, ZHAO L. Dye-sensitized solar cells based on hollow anatase TiO2 spheres prepared by self-transformation method [J]. Electrochimica Acta, 2010, 55: 597-602.
[9] ZHAO Y C, CAI Y, TIAN J N, LAN H X. Facile preparation and excellent catalytic performance of PtRuPd hollow spheres nanoelectrocatalysts [J]. Mater Chem Phys, 2009, 115: 831-834.
[10] TAO F F, GAO C L, WEN Z H, WANG Q, LI J H, XU Z, Cobalt oxide hollow microspheres with micro-and nano-scale composite structure: Fabrication and electrochemical performance [J]. J Solid State Chem, 2009, 182: 1055-1060.
[11] AN Z G, ZHANG J J, PAN S L. Low-density core–shell composite hollow microspheres with tunable magnetic properties [J]. J Phys Chem Solids, 2009, 70: 1083-1088.
[12] TADA M, KANEMARU T, HARA T, NAKAGAWA T, HANDA H, ABE M. Synthesis of hollow ferrite nanospheres for biomedical applications [J]. J Magn Magn Mater, 2009, 321: 1414-1416.
[13] JIANG J H, GAO Q M, ZHENG Z J, XIA K S, HU J. Enhanced room temperature hydrogen storage capacity of hollow nitrogen-containing carbon spheres [J]. Int J Hydrogen Energ, 2010, 35: 210-216.
[14] XU J Q, WANG D, QIN L P, YU W J, PAN Q Y. SnO2 nanorods and hollow spheres: Controlled synthesis and gas sensing properties [J]. Sensor Actuat B, Chem, 2009, 137: 490-495.
[15] ZHANG M L, CHANG J. Surfactant-assisted sonochemical synthesis of hollow calcium silicate hydrate (CSH) microspheres for drug delivery [J]. Ultrason Sonochem, 2010, 17: 789-792.
[16] YANG J, LEE J, KANG J, LEE K, SUH J, YOON H. Hollow silica nanocontainers as drug delivery vehicles [J]. Langmuir, 2008, 24: 3417-3421.
[17] ZHU Y F, SHI J L, CHEN H R, SHEN W H, DONG X P. A facile method to synthesize novel hollow mesoporous silica spheres and advanced storage property [J]. Microporous and Mesoporous Materials, 2005, 84: 218-222.
[18] CARUSO F, CARUSO R A, M?HWALD H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating [J]. Science, 1998, 282: 1111-1114.
[19] TSAI M S, LI M J. A novel process to prepare a hollow silica sphere via chitosan-polyacrylic acid (CS-PAA) template [J]. J Non-Cryst Solids, 2006, 352: 2829-2833.
[20] LE Y, CHEN J F, WANG W C. Study on the silica hollow spheres by experiment and molecular simulation [J]. Appl Surf Sci, 2004, 230: 319-326.
[21] YANG J, LIND J U, TROGLER W C. Synthesis of hollow silica and titania nanospheres [J]. Chem Mater, 2008, 20: 2875-2877.
[22] LIU C, GE C, WANG A L, YIN H B, REN M, ZHANG Y S, YU L B, JIANG T S. Synthesis of porous hollow silica spheres using functionalized polystyrene latex spheres as templates [J]. Korean J Chem Eng, 2011, 28: 1458-1463.
[23] GE C, ZHANG D Z, WANG A L, YIN H B, REN M, LIU Y M, JIANG T S, YU L B. Synthesis of porous hollow silica spheres using polystyrene-methyl acrylic acid latex template at different temperatures [J]. J Phys Chem Solids, 2009, 70: 1432-1437.
中空二氧化硅微球的尺寸控制制备及草甘膦释放
刘 纯1,2,殷恒波1,王爱丽1,吴占敖3,吴 刚3,姜 涛3,沈玉堂1,姜廷顺1
1. 江苏大学 化学化工学院,镇江 212013;
2. 北华大学 化学与生物学院,吉林 132013;
3. 中国人民解放军 第359医院,镇江 212001
摘 要:以硅酸钠为硅源,聚苯乙烯与苯乙烯-甲基丙烯酸共聚乳液为模板,制备直径为249~1348 nm的不同尺寸的中空二氧化硅微球。在给定二氧化硅与乳液模板质量比的前提下,中空二氧化硅微球直径与壳厚度随着乳液模板直径的增加而增加。中空二氧化硅微球直径与壳厚度也随着二氧化硅与乳液模板质量比的增加而增加。苯乙烯-甲基丙烯酸共聚乳液模板表面存在羧基有利于形成致密、均匀的二氧化硅微球球壳。中空二氧化硅球壳具有介孔结构和大的比表面积。将草甘膦用作释放模型化合物时,其释放速率可通过改变球壳厚度而进行调节。
关键词:中空二氧化硅球;聚苯乙烯;苯乙烯-甲基丙烯酸共聚物;草甘膦释放
(Edited by LI Xiang-qun)
Foundation item: Projects (11KJB530002, CX10B-259Z) supported by Research Funds from Jiangsu Provincial Department of Education, China; Project (10zxfk35) supported by Sichuan Province Nonmetallic Composites and Functional Materials Key Laboratory Project, China
Corresponding author: YIN Heng-bo; Tel: +86-511-88787591; Fax: +86-511-88791800; E-mail: yin@ujs.edu.cn; WU Zhan-ao; E-mail: waz_007@qq.com
DOI: 10.1016/S1003-6326(11)61300-9