臭氧(O3)氧化降解2,4,6-三氯酚的动力学研究
冯冬梅,高乃云,王希诚,马艳,周超
(同济大学 污染控制与资源化研究国家重点实验室,上海,200092)
摘要:采用臭氧氧化技术处理2,4,6-三氯酚(2,4,6-TCP),研究其反应动力学,同时研究2,4,6-TCP的初始质量浓度、臭氧发生量、pH、水中常见阴离子对降解速率的影响。研究结果表明:臭氧氧化能有效降解2,4,6-TCP,且降解规律符合准一级动力学模型;2,4,6-TCP初始质量浓度由3 mg/L增加到10 mg/L,降解速率k由0.134 1 min-1降低到0.058 1 min-1;固定初始质量浓度5 mg/L,当臭氧发生量由0.68 mg/min增加到2.34 mg/min时,降解速率常数由0.087 7 min-1提高到0.328 4 min-1;当pH由2.96逐步增加至11.00时,2,4,6-TCP的降解速率常数由0.050 3 min-1提高到0.168 1 min-1,且对于相同的pH增幅对降解反应速率的影响,酸性条件较碱性条件更大;SO42-对降解速率的影响不明显,HCO3-,CO32-,NO3-和Cl-均有利于2,4,6-TCP的降解,反应速率常数由大至小依次为:HCO3-,CO32-,NO3-,Cl-和SO42-。
关键词:2,4,6-三氯酚;臭氧;动力学;阴离子;pH
中图分类号:X592 文献标志码:A 文章编号:1672-7207(2013)05-2130-06
Kinetics of 2,4,6-TCP degradation by ozonation
FENG Dongmei, GAO Naiyun, WANG Xicheng, MA Yan, ZHOU Chao
(State Key Laboratory of Pollution Control and Resource Reuse, Tongji University, Shanghai 200092, China)
Abstract: Ozone was used to degrade 2,4,6-TCP and its kinetics was studied. The effects of initial mass concentration of 2,4,6-TCP, ozone dose, pH, common anions in water on degradation rate were discussed. The results show that 2,4,6-TCP can be effectively degraded by ozone and that reactions fits well with the first-order kinetics. When the initial mass concentration of 2,4,6-TCP changes from 3 mg/L to 10 mg/L, the rate constant decreases from 0.134 1 min-1 to 0.058 1 min-1. The rate constant increases from 0.087 7 min-1 to 0.328 4 min-1, as ozone production varies from 0.68 mg/min to 2.34 mg/min. With the change of pH from 2.96 to 11.00, the rate constant increases from 0.050 3 min-1 to 0.168 1 min-1. The rate of increase on degradation of 2,4,6-TCP is bigger under acidic condition than that under alkaline condition. HCO3-, CO32-, NO3- and Cl- have different positive effects on the degradation of 2,4,6-TCP while the effects of SO42- are not obvious. The degradation rate, the degradation rate from large to small of the five anions can be arranged as: HCO3- , CO32-, NO3-, Cl- and SO42-.
Key words: 2,4,6-TCP; ozone; kinetics; anions; pH
2,4,6-三氯酚(2,4,6-TCP)已广泛应用于许多工厂生产中,包括制造业的防腐剂、细菌和真菌杀剂、木材防腐剂和纺织业中的防霉剂[1-2]。过量的污水排放以及污水泄漏或事故,导致大量三氯酚进入附近水体[2-3]。并且在饮用水加氯处理过程中,天然有机物(NOM)尤其是水体中存在广泛的酚类也可经氯化形成三氯酚[4]。其毒性大且难生物降解,具有遗传毒性和三致效应,人体长期暴露在三氯酚环境中可制发癌症,世界卫生组织已经规定了三氯酚在饮用水中的最大可允许质量浓度为200 μg/L,并且美国、欧盟各国和中国等均将其列为优先控制污染物[2-3, 5]。我国城市供水质标准CJ/T 206—2005规定2,4,6-TCP质量浓度不得超过0.01 mg/L。臭氧消毒及预处理已在水处理工艺中得到广泛应用。一般有机物的臭氧直接降解具有选择性,臭氧容易降解含有C=C,C=N和N=N等双键、芳香族和氨基等的有机物[6-7]。而苯酚类物质的氧化降解特别是臭氧降解,反应速率一般较高,因为其亲核中心能与氧化剂快速反应[8]。此外,臭氧还能生成具有极强氧化性的多种活性自由基对物质进行间接氧化,其中羟基自由基(·OH)是一种具有更少选择性且氧化性比臭氧分子更强的氧化剂,直接氧化与间接氧化共同作用,从而提高水的可生化性和矿化率[6]。本文作者研究臭氧氧化降解2,4,6-TCP的效果及主要影响因素,并探讨该过程的动力学模型,以便为采用臭氧降解有机污染物提供参考。
1 材料与方法
1.1 试验流程
试验流程如图1所示。臭氧发生仪型号为DHX-IX,试验为连续式反应,在反应瓶中装入所需质量浓度的2,4,6-TCP溶液,通过磁力搅拌器混合均匀。反应中臭氧尾气由质量分数为2%的碘化钾溶液吸收。臭氧质量浓度测定采用碘量法[9]。在2个吸收瓶中均通入质量分数为20% KI,通入O3 40 min后所得臭氧量与时间的比值即为臭氧发生量。反应瓶中每隔一定时间取样,滴加过量亚硫酸钠(质量分数为10%)终止氧化,后待测定。
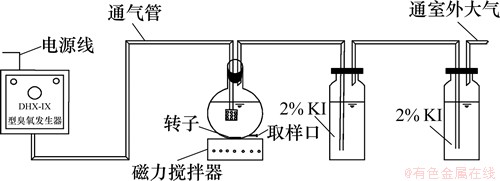
图1 试验工艺流程
Fig.1 Schematic flow of experiment system
1.2 试验药剂
药剂为:2,4,6-TCP(Sigma公司生产,纯度>98%)储备液,质量浓度为100 mg/L,用去离子水配制,使用时根据需要进行稀释;流动相甲醇、冰醋酸,为色谱级(Sigma公司生产);pH使用H2SO4和 NaOH溶液调节。试验期间水温为(12±2) ℃。调节离子浓度所用Na2SO4,NaCl,NaHCO3,Na2CO3和NaNO3皆为分析纯,购于国药集团,溶液浓度为10 mmol/L。
1.3 分析方法
2,4,6-TCP的质量浓度采用高效液相色谱仪(岛津LC-2010AHT)测定;使用Shim-pack VODS色谱柱(250.0 mm×4.6 mm);流动相为V(甲醇):V(水(含质量分数为1%的乙酸))=80:20;流动相流速为1.0 mL/min;分析时间为10 min;柱温为40 ℃;检测波长为289 nm。pH采用雷磁PHS-3C精密pH计测定。
2 结果与讨论
2.1 臭氧氧化2,4,6-TCP反应动力学分析
通过臭氧氧化动力学研究,可了解臭氧氧化工艺各主要影响因素在氧化过程中的作用。当水温稳定且反应液均匀混合时,可得到稳定的动力学常数。根据文献[6],有机污染物的降解过程中主要涉及分子态臭氧(O3)对污染物的直接氧化和臭氧转化产生的羟基自由基(·OH) 对有机污染物产生的间接氧化,即
O3+OH-→HO2-+O2 (1)
O3+HO2-→ ·OH + O2·-+O2 (2)
O3+O2·-→O3·-+O2 (3)
O3+2,4,6-TCP→中间产物 (4)
·OH+2,4,6-TCP→中间产物+H2O (5)
式(4)和(5)中,中间产物为甲酸和乙二酸等酸性物质,已由皮运正等[10]通过试验证实。由于羟基自由基(·OH)存在时间极短, 现有的技术手段还较难直接定量计算。目前,较可行的方法是采用拟一级动力学进行表征,即式(6)可转化为式(8)。实验采用式(6)和式(7)具体阐述pH、污染物初始质量浓度及臭氧发生量对降解过程的影响。
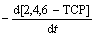 =kO3[2,4,6-TCP][O3]+ k·OH[2,4,6-TCP][·OH] (6)
=kO3[2,4,6-TCP][O3]+ k·OH[2,4,6-TCP][·OH] (6)
式中:kO3和 k·OH分别为臭氧直接氧化和间接氧化的速率常数,(mol·L-1)-1·s-1;[2,4,6-TCP]为2,4,6-TCP的浓度,mol/L;[O3]为O3浓度,mol/L;[·OH]为·OH浓度;t为反应时间,s。用Rc表示[·OH]与[O3]的比,即
 (7)
(7)
将式(7)代入式(6)可得:
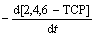 =[(kO3+k·OH Rc)[2,4,6-TCP][O3] (8)
=[(kO3+k·OH Rc)[2,4,6-TCP][O3] (8)
对于间歇式或者连续式反应器,
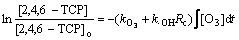 (9)
(9)
式中: 为实验过程中2,4,6-TCP的初始浓度,mol/L。实验所用反应器为连续式,臭氧流量为常量且磁力搅拌器将臭氧快速混合均匀,因此,可认为臭氧在水中溶解浓度恒定。通过式(9),可认为臭氧与2,4,6-TCP 的反应符合一级动力学,据式(9),准一级动力学反应常数k为
为实验过程中2,4,6-TCP的初始浓度,mol/L。实验所用反应器为连续式,臭氧流量为常量且磁力搅拌器将臭氧快速混合均匀,因此,可认为臭氧在水中溶解浓度恒定。通过式(9),可认为臭氧与2,4,6-TCP 的反应符合一级动力学,据式(9),准一级动力学反应常数k为
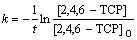 (10)
(10)
2.2 不同臭氧投加量对2,4,6-TCP的去除效果的影响
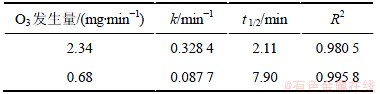
固定2,4,6-TCP初始质量浓度为5 mg/L,研究不同臭氧发生量( 0.68和2.34 mg/min)对2,4,6-TCP的降解效果的影响,并利用式(10)拟合ln([2,4,6-TCP]t/ [2,4,6-TCP]o)与反应时间t的变换规律,如图2所示。表1列出了相应的速率常数k、半衰期t1/2以及相关系数R2。由图2可以看出:随臭氧发生量的增加,拟合曲线的斜率明显增大,这说明2,4,6-TCP的降解速率随着臭氧发生量的增大而明显提高。这是因为:随着臭氧发生量的增加,水中存在的臭氧质量浓度相应升高,从而增加了臭氧分子攻击2,4,6-TCP的概率,同时也生成更多的羟基自由基(·OH),增强了氧化分解2,4,6-TCP的能力,提高了降解速率;如表1所示,当臭氧发生量增加到2.34 mg/min时,其对2,4,6-TCP的降解速率非常快,达到0.328 4 min-1,是臭氧发生量为0.68 mg/min时0.0877 min-1的近4倍。因此,适当增加臭氧发生量可明显提高降解效率。
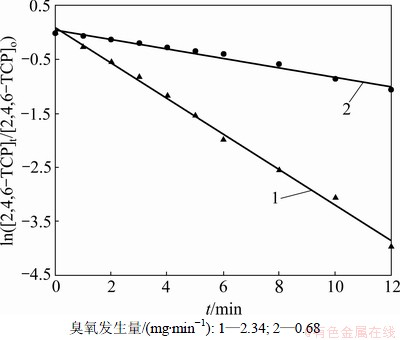
图2 不同臭氧发生量下对降解2,4,6-TCP的影响
Fig.2 Effect of different output mass fraction of O3 on degradation of 2,4,6-TCP
表1 不同臭氧发生量的臭氧降解2,4,6-TCP的准一级动力学模型的拟合参数
Table 1 Fitting parameters of kinetics models on degradation of 2,4,6-TCP under different output ozone concentrations
2.3 2,4,6-TCP初始质量浓度对降解效果的影响
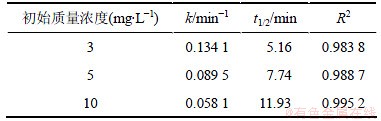
在臭氧发生量均为0.68 mg/min的条件下,对不同初始质量浓度(10, 5和3 mg/L)的2,4,6-TCP进行研究,并利用式(10)拟合ln([2,4,6-TCP]t/[2,4,6-TCP]o)与反应时间t的变化规律,如图3所示,相应的速率常数k、半衰期t1/2以及相关系数R2见表2。从图3与表2可看出:随着2,4,6-TCP初始质量浓度增加,ln([2,4,6-TCP]t/[2,4,6-TCP]o)对反应时间t的拟合曲线的斜率明显减小,这说明2,4,6-TCP的降解速率随着其初始质量浓度的增大而降低。因为臭氧发生量不变时,反应物的初始质量浓度增加,单位反应物所受到O3及羟基自由基(·OH)氧化的概率降低,因此,反应物的降解速率明显降低。当2,4,6-TCP初始质量浓度由3 mg/L增加到10 mg/L时,O3对2,4,6-TCP的降解速率也由0.134 1 min-1降低到0.058 1 min-1。因此,可在实际水处理生产中根据反应池内2,4,6-TCP的质量浓度确定臭氧投加量,保证处理效果与处理成本的最优化。
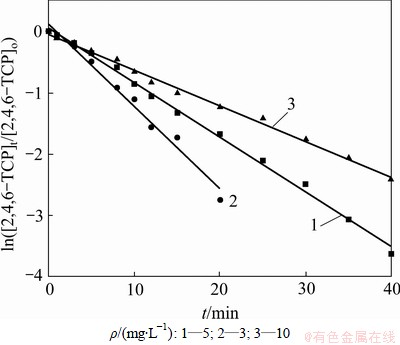
图3 不同2,4,6-TCP初始质量浓度的臭氧降解效果
Fig.3 Effect of different initial mass concentration on degradation of 2,4,6-TCP
表2 不同初始质量浓度下降解2,4,6-TCP的准一级动力学模型的拟合参数
Table 2 Fitting parameters of kinetics models on degradation of 2,4,6-TCP under different initial concentrations
2.4 pH对2,4,6-TCP降解速率的影响
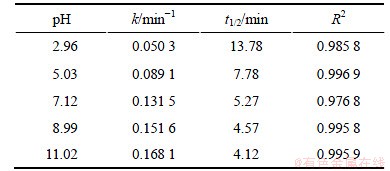
当2,4,6-TCP的初始质量浓度为5 mg/L,臭氧发生量约为0.68 mg/min时,考察反应液pH对降解速率的影响。在各个工况下,pH实测值分别为2.96,5.03,7.12,8.99和11.02,利用式(10)拟合ln([2,4,6-TCP]t/ [2,4,6-TCP]o)与反应时间t的变化规律如图4所示,相应速率常数k、半衰期t1/2以及相关系数R2见表3。从图4和表3可见:随着pH降低,ln([2,4,6-TCP]t/ [2,4,6-TCP]o)对反应时间t的拟合曲线的斜率减小,这说明2,4,6-TCP的降解速度随着pH的降低而降低;在酸性条件下,pH变化对降解速率的影响很大,当pH从2.96增至7.12时,降解速率k由0.050 3 min-1至0.131 5 min-1;而在碱性条件下,当pH由7.12到11.02时,降解速率由0.131 5 min-1至提高0.168 1 min-1,pH对降解速率的影响较小;当pH=11.02时,反应速率达到最大,这说明碱性条件有利于2,4,6-TCP臭氧氧化降解,文献[12]也证明了这一点。Graham等[11]使用O3降解2,4,6-TCP,测定出在pH=7.5的反应速率常数几乎是pH=2.0时的10倍。
在强酸性时,主要为臭氧分子直接参加反应,反应速率较慢;随水中pH逐渐增大,水中OH-浓度逐渐增大,臭氧与OH-反应生成·OH。由于·OH具有强氧化能力,使反应速率加快,表现为:在酸性条件下,提高pH时反应速率提高很快;而在碱性条件下,氧化反应以间接反应为主,当OH-足够充足时,易发生如下反应:
O3+OH-→HO2-+O2 (11)
O3+HO2-→·OH+O2·-+O2 (12)
所以,在碱性条件下,提高pH对间接反应的影响降低,表现为当pH从8.99增大至11.02时,2,4,6-TCP的降解速率增加幅度较小。可见:在强酸性条件下,2,4,6-TCP的降解以臭氧分子直接氧化去除为主,随pH增大,·OH间接氧化所占比例逐渐增大;而在碱性条件尤其当pH>9时,2,4,6-TCP的降解以·OH的间接氧化为主。

图4 pH对臭氧降解2,4,6-TCP的影响
Fig.4 Effect of pH on degradation of 2,4,6-TCP by ozonation
表3 不同pH条件下臭氧降解2,4,6-TCP的准一级动力学模型的拟合参数
Table 3 Fitting parameters of kinetics models on degradation of 2,4,6-TCP under different pH
2.5 水中常见阴离子对2,4,6-TCP降解速率的影响
2,4,6-TCP的初始质量浓度为5 mg/L,臭氧的发生量控制在0.68 mg/min,研究CO32-,SO42-,Cl-,HCO3-和NO3-分别存在时降解速率的变化规律,并利用式(10)拟合ln([2,4,6-TCP]t/[2,4,6-TCP]o)与反应时间t的变化规律,如图5所示;相应的速率常数k、半衰期t1/2以及相关系数R2见表4。从图5和表4可见:与未添加阴离子的水样相比,SO42-对降解反应的影响程度不太明显,除SO42-外所选择添加的阴离子均有利于2,4,6-TCP的降解,但是影响程度不相同;添加10 mmol/L阴离子的水样后,阴离子影响反应速率常数k从大到小依次为HCO3-,CO32-,NO3-,Cl-和SO42-。
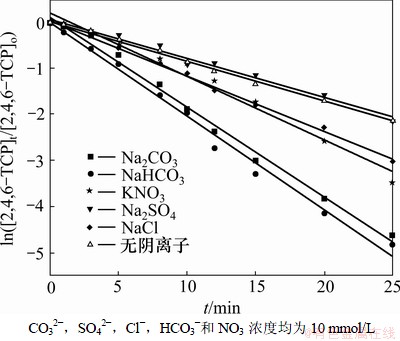
图5 阴离子对臭氧降解2,4,6-TCP的影响
Fig.5 Effect of anions on degradation of 2,4,6-TCP by ozonation
表4 不同阴离子条件下臭氧降解2,4,6-TCP的准一级动力学模型的拟合参数
Table 4 Fitting parameters of kinetics models on degradation of 2,4,6-TCP under different anions

根据Yapsakli等[12]的研究可知:HCO3-和CO32-对臭氧降解有一定的抑制作用,能消耗臭氧分解所产生的羟基自由基(·OH),生成惰性的CO3·-,中断自由基链式反应,影响氧化能力。
·OH+CO32-→OH-+CO3·- (13)
HCO3-和CO32-都是强碱弱酸盐,检测反应40 min前后pH变化,发现含CO32-的溶液pH从11.42变化至11.22,含HCO3-的溶液pH由8.99变化至8.68,说明分别含有HCO3-和CO32-反应液,降解反应始终在碱性条件下进行。碱性条件有利于羟基自由基(·OH)的生成,有利于2,4,6-TCP的去除。因而,当HCO3-和CO32-的浓度均为10 mmol/L时,其加快生成羟基自由基(·OH)的效果比生成惰性CO3·-的效果大。
NO3-对2,4,6-TCP的臭氧降解有协同促进作用,因为NO3-本身就是强氧化剂,且硝酸盐是天然水体中产生·OH的主要来源[7]。实验表明:在SO42-浓度为10 mmol/L时,其对臭氧降解2,4,6-TCP的降解速率的作用不明显。
根据Klaening等[13-14]的研究,Cl-可与·OH进行可逆反应,反应式为:
·OH+Cl-  ClOH (14)
ClOH (14)
k+=4.3×109 (mol·L-1)-1·s-1
k=6×109 s-1
ClOH+H+  Cl· +H2O (15)
Cl· +H2O (15)
k+=2×1010 (mol·L-1)-1·s-1
k=1.6×105 s-1
反应(14)是一个反应速度非常快的逆反应,它使得ClOH的稳定浓度很小,且ClOH的进一步反应生成Cl·和 H2O与pH有关;当pH=7.0时,通过·OH将Cl-氧化生成Cl原子的反应速率常数大约为103 (mol·L-1)-1·s-1。
本实验所用2,4,6-TCP反应液,使用去离子水(pH=5左右)配制,且2,4,6-TCP的臭氧降解会生成酸类物质,使pH逐渐降低,并已由实验证实。可见:Cl-对降解反应的影响是在酸性条件下进行,Cl-可生成Cl·并通过逆反应快速转化为·OH,并对2,4,6-TCP进行氧化。对于Cl-促进2,4,6-TCP的降解,有如下推测:(1) 由于降解反应所用反应器为连续式,臭氧不可避免地有一部分随气流逃逸,而由于Cl-大量存在,使得臭氧的利用率升高,从而提高反应的降解速率;(2) 由于存在大量Cl-,使臭氧在酸性条件下转化为羟基自由基(·OH)的能力增强,2,4,6-TCP的降解速率增大。
3 结论
(1) 臭氧氧化技术能有效降解2,4,6-TCP,且降解规律符合准一级动力学模型,相关系数均在0.97以上。
(2) 臭氧降解2,4,6-TCP的速率受2,4,6-TCP初始质量浓度、臭氧发生量、pH和水中常见阴离子的影响。降解速率随臭氧发生量的增加而提高,当臭氧投加量由0.68 mg/min增加至2.34 mg/min时,2,4,6-TCP的降解速率由0.087 7 min提高到0.328 4 min;降解速率随pH的升高而增大,当pH由2.96增大至11.02时,2,4,6-TCP的降解速率由0.050 3 min-1提高到0.168 1 min-1。对于相同的pH增幅对降解反应速率增幅的影响,酸性条件下比碱性条件下更大;当2,4,6-TCP的初始质量浓度由3 mg/L增大到10 mg/L时,降解速率由0.134 1 min-1降低至0.058 1 min-1,因此,可由反应物的量确定最佳的臭氧的投加量,保证处理效果与处理成本的最优化;SO42-对降解反应的影响程度不太明显,HCO3-,CO32-,NO3-和Cl-不同程度地有利于2,4,6-TCP的臭氧降解,反应速率常数从大至小为:HCO3-,CO32-,NO3-,Cl-和SO42-。
参考文献:
[1] Abe K, Tanaka K. Fe3+ and UV-enhanced ozonation of chlorophenolic compounds in aqueous medium[J]. Chemosphere, 1997, 35(12): 2837-2847.
[2] Chu W, Wong C C. A disappearance model for the prediction of trichlorophenol ozonation[J]. Chemosphere, 2003, 51(4): 289-294.
[3] Díaz-Díaz G, Celis-García M, Blanco-López M C, et al. Heterogeneous catalytic 2,4,6-trichlorophenol degradation at hemin–acrylic copolymer[J]. Applied Catalysis B: Environmental, 2010, 96(1/2): 51-56.
[4] Md A. Adsorption of phenolic compounds on low-cost adsorbents: A review[J]. Advances in Colloid and Interface Science, 2008, 143(1/2): 48-67.
[5] 马艳, 高乃云, 刘欣然, 等. 粉末活性炭吸附2,4,6-三氯酚的性能研究[J]. 同济大学学报: 自然科学版, 2011, 39(12): 1821-1826.
MA Yan, GAO Naiyun, LIU Xinran, et al. Performance of 2,4,6-trichlorophenol adsorption by powdered activated carbon[J]. Journal of Tongji University: Natural Science, 2011, 39(12): 1821-1826.
[6] von Gunten U. Ozonation of drinking water: Part Ⅰ. Oxidation kinetics and product formation[J]. Water Research, 2003, 37(7): 1443-1467.
[7] 张可佳, 高乃云, 殷娣娣, 等. 臭氧氧化降解微囊藻毒素-LR的动力学研究[J]. 同济大学学报: 自然科学版, 2009, 37(7): 919-924.
ZHANG Kejia, GAO Naiyun, YIN Didi, et al. Study on kinetics of microcystin-LR degradation by ozonation[J]. Journal of Tongji University: Natural Science, 2009, 37(7): 919-924.
[8] Beltrán F J, Rodríguez E M, Romero M T. Kinetics of the ozonation of muconic acid in water[J]. Journal of Hazardous Materials, 2006, 138(3): 534-538.
[9] 周云, 戴婕, 梁小虎. 自来水厂中臭氧浓度的监控与测定[J]. 给水排水, 2002, 28(10): 9-12.
ZHOU Yun, DAI Jie, LIANG Xiaohu. Ozone concentration measurement and monitoring in waterworks[J]. Water & Wastewater Engineering, 2002, 28(10): 9-12.
[10] 皮运正, 王建龙. 臭氧氧化水中2,4,6-三氯酚的反应机理研究[J]. 环境科学学报, 2005, 25(12): 1619-1623.
PI Yunzheng, WANG Jianglong. The pathway of the ozonation of 2,4,6-trichlorophenol in aqueous solution[J]. Acta Scientiae Circumstantiae, 2005, 25(12): 1619-1623.
[11] Graham N, Wei Chu, Lau C. Observations of 2,4,6-trichlorophenol degradation by ozone[J]. Chemosphere, 2003, 51(4): 237-243.
[12] Yapsakli K, Can Z S. Interaction of ozone with formic acid: A system which suppresses the scavenging effect of HCO3-/CO32-[J]. Water Quality Research Journal of Canada, 2004, 39(2): 140-148.
[13] Klaening U K. Laser flash photolysis of HClO, ClO-, HBrO, and BrO-, in aqueous solution[J]. Reaction of Cl- and Br- Atoms, 1985, 89: 243-245.
[14] von Gunten U. Ozonation of drinking water: Part Ⅱ. Disinfection and by-product formation in presence of bromide, iodide or chlorine[J]. Water Research, 2003, 37(7): 1469-1487.
(编辑 陈灿华)
收稿日期:2012-05-10;修回日期:2012-07-22
基金项目:国家科技重大专项项目(2012ZX07403-001);住房和城乡建设部研究项目(2009-K7-4)
通信作者:高乃云(1950-),女,陕西府谷人,博士,教授,博士生导师,从事饮用水处理技术和建筑给水排水技术研究;电话:13816869935;E-mail: gaonaiyun1@126.com