Effect of Ti on microstructure, mechanical and corrosion properties of (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix bulk metallic glasses
Yan-xin ZHUANG, Shen-ci WANG, Chang-jiu WANG, Nai-peng WANG, Ji-cheng HE
Key Laboratory of Electromagnetic Processing of Materials, Ministry of Education,Northeastern University, Shenyang 110819, China
Received 29 January 2015; accepted 2 July 2015
Abstract: The (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix alloys were prepared using an in-situ suck-casting method in a copper mold. The effects of Ti addition on the microstructure, mechanical and corrosion properties of the (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix alloys were investigated by X-ray diffraction, scanning electron microscopy, compressive tests and corrosion tests. It has been found that the addition of Ti higher than 4% (mole fraction) causes the formation of many crystalline phases in the alloy. The alloys with 1%-3%Ti display an obvious yield stage on their compressive stress-strain curves. An appropriate addition of Ti can improve the strength and ductility of the alloys. All the alloys have high corrosion resistance in 1 mol/L NaOH solution, and are corroded in 1 mol/L HCl solution. However, the appropriate addition of Ti can significantly improve the corrosion resistance of the alloys in HCl solution.
Key words: Zr-based bulk metallic glass; Ti; mechanical properties; corrosion
1 Introduction
Zr-based bulk metallic glasses (BMGs) are promising candidates for the structural and functional applications due to their high strength, excellent glass forming ability, wide supercooled liquid region, and good corrosion resistance. In the last two decades, a large number of Zr-based BMGs with high glass forming ability have been developed [1,2]. Zr55Al10Ni5Cu30 BMG is one of the alloys having the largest glass forming ability [3]. However, the intrinsic brittleness of this alloy limits the range of its possible applications. To overcome the brittleness of many monolithic BMGs, different methods have been proposed. The in-situ or ex-situ introduction of crystalline phases at microscale or nanoscale into the glassy matrix could cause the generation of multiple shear bands in the entire sample, and thereby improve the ductility of the alloys [4-9]. The process has led to the invention of the bulk metallic glassy composites (BMGCs). It has been reported that the Zr55Al10Ni5Cu30 bulk metallic glassy composites reinforced by TiNb or ZrO2 have higher compressive fracture strength and better plasticity than the monolithic BMG [9].
Minor addition of alloying elements has great effects on the formation and properties of the BMGs and BMGCs [10]. In-situ formation of fine crystalline phases in the BMG matrix can be realized by an appropriate addition of the alloying elements. Proper addition of 0.6% Y (mole fraction) improves the room temperature ductility and the glass forming ability of Zr-based alloy [11]. The addition of element Ta into the Zr-Cu- Ni-Al bulk metallic glasses enhances the mechanical properties of the alloy, and the metallic glassy composites reinforced by Ta-solid solutions can be obtained when Ta is 5% (mole fraction) or higher [12]. Small addition of Ti improves the glass forming ability of the Zr62-xTixCu20Ni8Al10 [13] and Cu46Zr46Al8-xTix [14] bulk metallic glasses, and causes the formation of tiny CuTi phase in the MgCuY bulk metallic glass [15]. It has been well accepted that the introduction of nanocrystallines in amorphous matrix could improve the ductility of the alloys. However, how the introduced crystalline phases affect the corrosion properties of the alloys is still an open question. We investigated the effects of Nb on the formation, mechanical and corrosion properties of ZrAlNiCu bulk metallic glasses [16]. In this work, the minor addition of Ti into the Zr55Al10Ni5Cu30 alloy was performed. The effects of Ti addition on the formation of crystalline phases, mechanical properties, and corrosion properties of (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix alloys were investigated systematically.
2 Experimental
Ingots with the nominal composition of (Zr0.55Al0.1- Ni0.05Cu0.3)100-xTix (x=0, 1, 2, 3, 4, and 5, mole fraction, %) were prepared by arc-melting of 99.9% Zr, Al, Ni, Cu and Ti under a titanium-gettered argon atmosphere. The ingots were remelted at least four times to achieve chemical homogeneity. Then, a cylindrical rod with a diameter of 5 mm was prepared by suck-casting into a copper mold under argon atmosphere. The structure of the as-cast samples was characterized by X-ray diffraction (XRD) using Cu Kα radiation (X’Pert Pro diffractometer). Compressive tests were performed on a CMT5105 machine at a strain rate of 1×10-4 s-1, where the test samples had 5 mm in diameter and 10 mm in length. The fracture surfaces were characterized using a scanning electron microscope (SEM SSX-50) equipped with energy dispersive spectrometry (EDS). The corrosion tests were carried out using the samples with a diameter of 5 mm and a length of 5 mm. The surfaces of the samples were ground, polished and cleaned using a standard routine. The masses of the samples were weighed using an electrical balance. The samples were then put into a 1 mol/L HCl or 1 mol/L NaOH solution to test the corrosion properties of the alloys in the acidic and alkaline solutions. The samples were weighed at different time. The corrosion surfaces were characterized using SEM.
3 Results and discussion
3.1 Formation of alloys
Figure 1 gives the XRD patterns of as-cast (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix (x =0, 1, 2, 3, 4, and 5, mole fraction, %) alloys. A clear broad diffraction peak can be found on the XRD patterns of the alloys with Ti additions of 0, 1%, 2%, 3% and 4%, indicating that the main phase in these alloys is amorphous. It can also be observed that the position of the amorphous haloes is slightly changed by the addition of Ti, which can be attributed to different clusters with short-range-order existing in the alloys. No obvious sharp Bragg peaks corresponding to crystals are found on the XRD pattern of the Zr55Al10Ni5Cu30 alloy within the detection limitation of the XRD. When x is 1, a tiny Bragg peak is found at 2q=29°, which can be indexed to a NiZr phase. At x=3, tiny Bragg peaks corresponding to Zr2Cu and NiTi2 phase appear on the XRD pattern of the alloy. When x is 4, the NiZr, Zr2Cu, NiTi2 and unknown phases are found in the amorphous-based alloy. Finally, when x is 5, many crystalline phases are formed, and the amorphous phase is not the main phase in the alloy. It can be concluded that the appropriate addition of Ti (less than 4%) can enhance the formation of crystalline in the amorphous matrix, and thereby is beneficial to the formation of the BMGCs. However, an over addition of Ti element will completely destroy the structure of the amorphous phase.
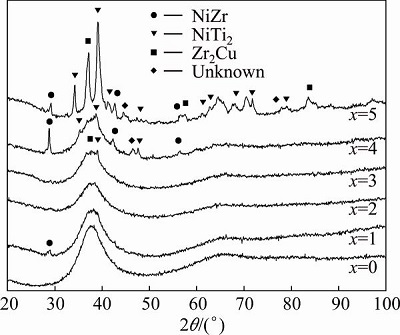
Fig. 1 XRD patterns of as-cast (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix alloys
3.2 Mechanical properties of alloys
Figure 2 shows the compressive stress-strain curves of the as-cast (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix alloys. The Zr55Al10Ni5Cu30 amorphous alloy displays a brittle fracture, and has a maximum compressive strength of 1426.5 MPa, which is similar to the values in other references [9,12]. When 1%-3% Ti was added into Zr55Al10Ni5Cu30 amorphous alloy, an obvious yield stage appears in the stress-strain curves of the three alloys. A few weak serrations are also found upon the onset of plastic yielding of (Zr0.55Al0.1Ni0.05Cu0.3)99Ti1 alloy. The serration events are associated with the shear band formation and propagation [17,18]. All the three alloys have large maximum compressive strength, which are 1662.4, 1657.6, and 1726.3 MPa when x is 1, 2 and 3, respectively. (Zr0.55Al0.1Ni0.05Cu0.3)96Ti4 and (Zr0.55Al0.1- Ni0.05Cu0.3)95Ti5 alloys display the same fracture mode as the Zr55Al10Ni5Cu30 alloy. An appropriate addition of Ti may enhance the formation of the fine crystalline phases, which hinder the development of shear bands, and thereby improve the mechanical properties and ductility of the alloys.
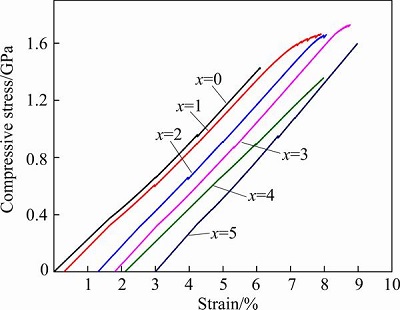
Fig. 2 Compressive stress-strain curves of as-cast (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix alloys
Figure 3 gives the SEM images of fracture surfaces of (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix alloys. Clear vein-like patterns can be observed on the fracture surfaces. It is also found that local melting within the shear bands and liquid droplets appear on the fracture surfaces. The density of the vein-like structure in the alloys with addition of 1% Ti is higher than that in the alloy without Ti addition, which can be attributed to the interaction between the second phase and the amorphous matrix. The alveolate patterns are also found on the fracture surfaces of the alloys with x values of 2 and 3, which could be caused by the brittle fracture of the second phase.
3.3 Corrosion behavior of alloys
Figure 4 gives the masses of (Zr0.55Al0.1Ni0.05- Cu0.3)100-xTix alloys immersed in 1 mol/L NaOH solution for different time. It can be found that all the alloys show no obvious mass change after being immersed in 1 mol/L NaOH solution for 144 h, and no corrosion pits are observed on the surfaces of the alloys. The results indicate that the amorphous and amorphous-based composites show excellent anti-corrosion properties in the alkaline solution. The nature of good corrosion resistance of the alloys in the alkaline solution can be attributed to the formation of metallic oxide on the surfaces.
Figure 5 shows the mass loss and corrosion rate of (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix alloys immersed in 1 mol/L HCl solution for different time. The mass loss and the average corrosion rate of the Zr55Al10Ni5Cu30 amorphous alloy immersed in 1 mol/L HCl solution for 168 h are 0.18% and 4.57 μg/(cm2·h), respectively. When 1%-4% Ti is added into the alloys, the mass loss and corrosion rate of the alloys decrease with the increase of Ti content. However, the mass loss and the average corrosion rate of (Zr0.55Al0.1Ni0.05Cu0.3)95Ti5 alloy in 1 mol/L HCl solution for 168 h are 0.27% and 6.01 μg/(cm2·h), respectively, which are much higher than those of Zr55Al10Ni5Cu30 amorphous alloy. The larger corrosion rate of (Zr0.55Al0.1Ni0.05Cu0.3)95Ti5 alloy could be related to the complex crystalline phases and the corresponding intergranular corrosion.
Figure 6 displays the SEM images of (Zr0.55Al0.1- Ni0.05Cu0.3)100-xTix alloys immersed in 1 mol/L HCl solution for 168 h. The bright areas in the images are the regions etched by 1 mol/L HCl solution, and the dark areas are not etched by 1 mol/L HCl solution. It can be found that the etched area in the Zr55Al10Ni5Cu30 alloy is the largest. With the addition of Ti, the etched area becomes smaller, suggesting that the addition of Ti can enhance the corrosion resistance of the alloys in the acidic solution.

Fig. 3 SEM images of fracture surfaces of (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix alloys
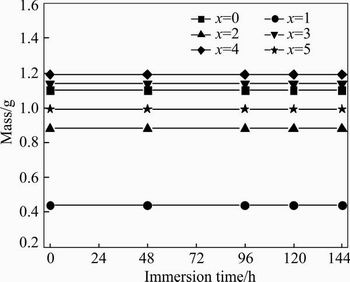
Fig. 4 Mass of (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix alloys as function of immersion time in 1 mol/L NaOH solution
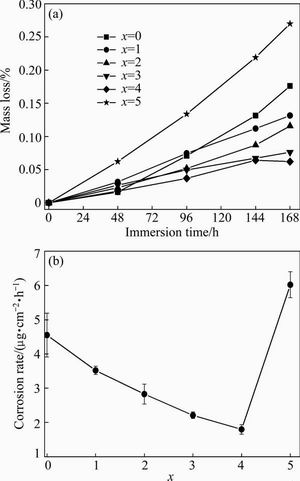
Fig. 5 Mass loss (a) and average corrosion rate (b) of (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix in 1 mol/L HCl solution
Figure 7 gives the enlarged SEM images of the corrosion areas of (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix alloys immersed in 1 mol/L HCl solution for 168 h. The continuous corrosion pits are found on the corrosion surfaces of Zr55Al10Ni5Cu30 and (Zr0.55Al0.1Ni0.05Cu0.3)99- Ti1 alloys. However, the corrosion pits in (Zr0.55Al0.1- Ni0.05Cu0.3)99Ti1 alloy are much smaller than those in the Zr55Al10Ni5Cu30 alloy. The discontinuous corrosion pits are found on the corrosion surfaces of the other four alloys. The corrosion pits in (Zr0.55Al0.1Ni0.05Cu0.3)95Ti5 are much larger and deeper than those in the (Zr0.55Al0.1Ni0.05Cu0.3)96Ti4 alloy. The SEM images suggest that (Zr0.55Al0.1Ni0.05Cu0.3)99Ti1 alloy has lower corrosion rate in 1 mol/L HCl solution than Zr55Al10Ni5Cu30 alloy, and (Zr0.55Al0.1Ni0.05Cu0.3)95Ti5 alloy has higher corrosion rate than (Zr0.55Al0.1Ni0.05- Cu0.3)96Ti4 alloys, which are in good agreement with the conclusions above.
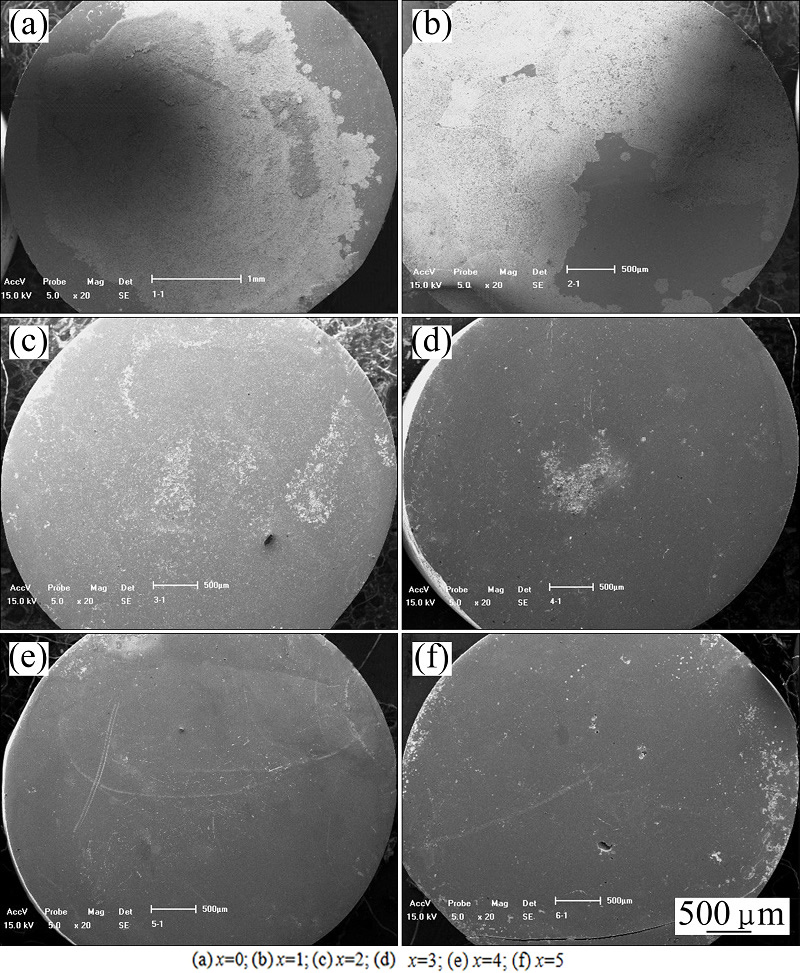
Fig. 6 SEM images of (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix alloys immersed in 1 mol/L HCl solution for 168 h
4 Conclusions
1) The effect of Ti addition on the formation and properties of (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix (x=0, 1, 2, 3, 4, and 5, mole fraction, %) alloys was investigated. The addition of Ti element improves the formation of metallic glassy composites. The addition of 1%-3% Ti increases the compressive strength and plasticity of the alloy, while the alloys with 4% Ti and 5% Ti display a brittle fracture.
2) All the alloys investigated have excellent anti-corrosion properties in 1 mol/L NaOH solution. An appropriate addition of Ti improves the corrosion resistance of the alloys in 1 mol/L HCl solution. When content of Ti in the alloys increases from 0 to 4%, the average corrosion rate decrease from 4.57 to 1.82 μg/(cm2·h), and (Zr0.55Al0.1Ni0.05Cu0.3)95Ti5 alloy has largest corrosion rate of 6.01 μg/(cm2·h).
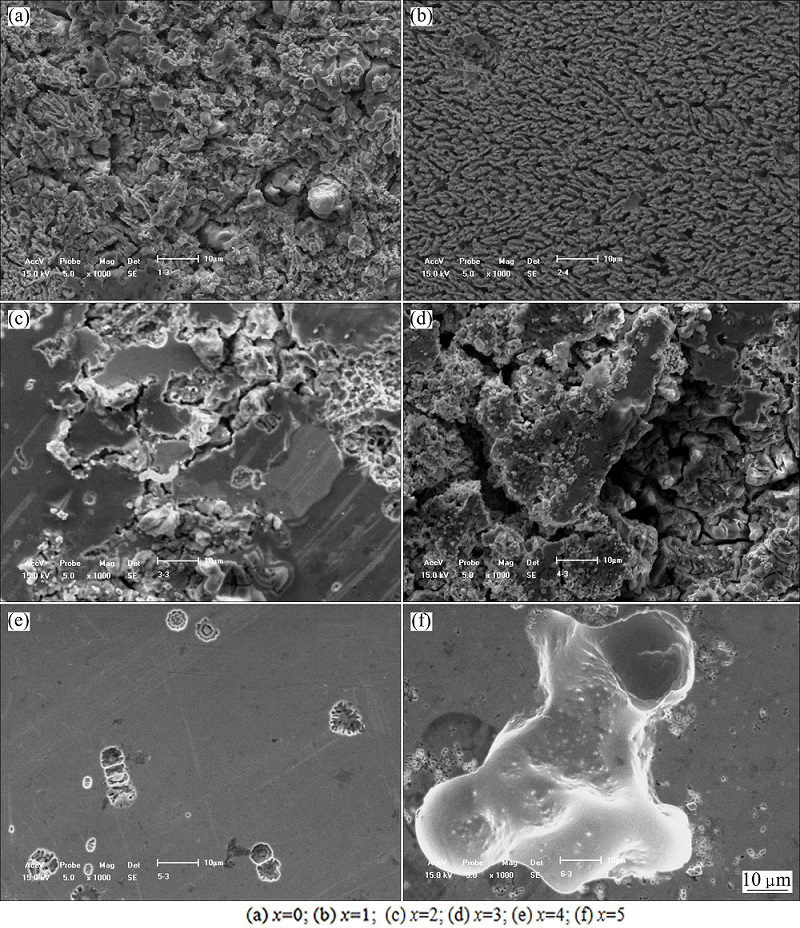
Fig. 7 Enlarged SEM images of (Zr0.55Al0.1Ni0.05Cu0.3)100-xTix alloys immersed in 1 mol/L HCl solution for 168 h
References
[1] INOUE A, TAKEUCHI A. Recent development and application products of bulk glassy alloys [J]. Acta Materialia, 2011, 59(6): 2243-2267.
[2] WANG W H, DONG C, SHEK C H. Bulk metallic glasses [J]. Materials Science and Engineering R, 2004, 44(2-3): 45-89.
[3] YOKOYAMA Y, MUND E, INOUE A, SCHULTZ L. Production of Zr55Cu30Ni5Al10 glassy alloy rod of 30 mm in diameter by a cap-cast technique [J]. Materials Transactions, 2007, 48(12): 3190-3192.
[4] HAYS C C, KIM C P, JOHNSON W L. Microstructure controlled shear band pattern formation and enhanced plasticity of bulk metallic glasses containing in situ formed ductile phase dendrite dispersions [J]. Physical Review Letters, 2000, 84(13): 2901-2904.
[5] FAN C, LOUZGUIZE D V, LI C, INOUE A. Nanocrystalline composites with high strength obtained in Zr-Ti-Ni-Cu-Al bulk amorphous alloys [J]. Applied Physics Letters, 1999, 75(3): 340-342.
[6] QIAO J W, WANG S, ZHANG Y, LIAW P K, CHEN G L. Large plasticity and tensile necking of Zr-based bulk-metallic-glass-matrix composites synthesized by the Bridgman solidification [J]. Applied Physics Letters, 2009, 94(15): 151905.
[7] HOFMANN D C, SUH J Y, WIEST A, DUAN G, LIND M L, DEMETRIOU M D, JOHNSON W L. Designing metallic glass matrix composites with high toughness and tensile ductility [J]. Nature, 2008, 451: 1085-1090.
[8] FAN C, QIAO D, WILSON T W, CHOO H, LIAW P K. As-cast Zr-Ni-Cu-Al-Nb bulk metallic glasses containing nanocrystalline particles with ductility [J]. Materials Science and Engineering A, 2006, 431(1-2): 158-165.
[9] CHU Z H, KATO H, XIE G Q, YUAN G Y, LU C, DING W J. Correlation between the enhanced plasticity of glassy matrix composites and intrinsic mechanical property of reinforcement [J]. Materials Science and Engineering A, 2013, 560: 40-46.
[10] WANG W H. Roles of minor additions in formation and properties of bulk metallic glasses [J]. Progress in Materials Science, 2007, 52(4): 540-596.
[11] PENG Wei-jie, ZHANG Yong. Micro-alloying of yttrium in Zr-based bulk metallic glasses [J]. Progress in Natural Science: Materials International, 2010, 20: 46-52.
[12] LIU L, CHAN K C, SUN M, CHEN Q. The effect of addition of Ta on the structure, crystallization and mechanical properties of Zr-Cu-Ni-Al-Ta bulk metallic glasses [J]. Materials Science and Engineering A , 2007, 445: 697-706.
[13] KUHN U, EYMANN K, MATTERN N, ECKERT J, GEBERT A, BARTUSCH B, SCHULTZ L. Limited quasicrystal formation in Zr-Ti-Cu-Ni-Al bulk metallic glasses [J]. Acta Materiala, 2006, 54(18): 4685-4692.
[14] MA G Z, SUN B A, PAULY S, SONG K K, KUHN U, CHEN D, ECKERT J. Effect of Ti substitution on glass-forming ability and mechanical properties of a brittle Cu-Zr-Al bulk metallic glass [J]. Materials Science and Engineering A, 2013, 563: 112-116.
[15] QIU Ke-qiang, WANG Lin, REN Ying-lei, LI Rong-de. Effect of Ti and Be addition on microstructure and mechanical properties of Mg58.5Cu30.5Y11 bulk metallic glass [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(2): 477-482.
[16] WANG C J, WANG S C, WANG W B, XING P F, ZHUANG Y X. Effect of Nb on formation, mechanical and corrosion properties of (Zr0.55Al0.1Ni0.05Cu0.3)100-xNbx bulk metallic glasses [J]. Materials Research Innovations, 2014, 18(S4): 761-765.
[17] GONZALEZ S, CHEN N, ZHANG Q S, LOUZGUINE-LUZGIN D V, PEREPEZKO J H, INOUE A. Effect of shear bands initiated in the pre-yield region on the deformation behaviour of Zr-based metallic glasses [J]. Scripta Materialia, 2011, 64(8): 713-716.
[18] LIU Y H, LIU C T, GALI A, INOUE A, CHEN M W. Evolution of shear bands and its correlation with mechanical response of a ductile Zr55Pd10Cu20Ni5Al10 bulk metallic glass [J]. Intermetallics, 2010, 18(8): 1455-1464.
Ti对(Zr0.55Al0.1Ni0.05Cu0.3)100-xTix块体金属玻璃显微组织、力学及腐蚀性能的影响
庄艳歆,王神赐,王长久,王乃鹏,赫冀成
东北大学 材料电磁过程教育部重点实验室,沈阳 110819
摘 要:采用铜模吸铸法制备(Zr0.55Al0.1Ni0.05Cu0.3)100-xTix合金,利用X射线衍射、扫描电镜、压缩实验及腐蚀测试等手段研究Ti对合金显微组织、力学和腐蚀性能的影响。结果表明,当Ti含量高于4% (摩尔分数) 时,合金中出现大量晶化相;而当Ti含量为1%~3%Ti时,合金的压缩应力-应变曲线上出现屈服现象。添加适量的Ti元素能提高合金的强度及韧性。在1 mol/L NaOH溶液中浸泡144 h后,(Zr0.55Al0.1Ni0.05Cu0.3)100-xTix合金质量无明显变化,表现出良好的耐腐蚀性能;而在1 mol/L HCl溶液中则会发生腐蚀,但适量的Ti元素添加可显著提高合金在盐酸溶液中的耐腐蚀性能。
关键词:Zr基块体金属玻璃;钛;力学性能;腐蚀
(Edited by Wei-ping CHEN)
Foundation item: Projects (51171041, 51104047) supported by the National Natural Science Foundation of China; Project (N100409001) supported by the Fundamental Research Funds for the Central Universities, China
Corresponding author: Yan-xin ZHUANG; Tel: +86-24-83680156; E-mail: yxzhuang@epm.neu.edu.cn
DOI: 10.1016/S1003-6326(16)64098-0