
Preparation and characterization of lithium hexafluorophosphate for lithium-ion battery electrolyte
LIU Jian-wen(刘建文), LI Xin-hai(李新海), WANG Zhi-xing(王志兴),
GUO Hua-jun(郭华军), PENG Wen-jie(彭文杰), ZHANG Yun-he(张云河), HU Qi-yang(胡启阳)
School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China
Received 12 December 2008; accepted 6 May 2009
Abstract: A promising preparation method for lithium hexafluorophosphate (LiPF6) was introduced. Phosphorus pentafluoride (PF5) was first prepared using CaF2 and P2O5 at 280 ℃ for 3 h. LiPF6 was synthesized in acetonitrile solvent by LiF and PF5 at room temperature (20-30 ℃) for 4 h. The synthesized LiPF6 was characterized by infrared spectrometry and X-ray diffraction(XRD). Atomic absorption and ion chromatography results show that the purity of synthesized LiPF6 reaches 99.98%. Thermal stability of self-synthesized LiPF6 was analyzed by differential thermal analysis and thermogravimetry. The results indicate that the self-synthesized LiPF6 has higher purity, lower impurity contents and better thermal stability than the commercial LiPF6.
Key words: lithium-ion batteries; lithium hexafluorophosphate; phosphorus pentafluoride; acetonitrile
1 Introduction
Lithium hexafluorophosphate (LiPF6) is a typical electrolyte salt for lithium-ion batteries. This salt has many advantages over conventional electrolyte salts such as LiAsF6, LiBF4 and LiClO4: 1) It can form suitable SEI membrane in electrodes, especially in cathode; 2) it can implement passivation for anode current collectors to prevent their dissolution; 3) it has wide windows of electrical stability; 4) it has excellent solubility and high conductivity in various solvents; and 5) it is environment-friendly[1-2].
Traditional preparation of LiPF6 continuously follows the HF solvent method. However, there are three major disadvantages for this method. First, high toxicity of HF brings great danger to preparation and rigorous demands for reaction devices; second, high energy consumption is essential because of deeply-cold reaction; third, large amount of impurity always remains in the product as a form of LiPF6·HF. In normal cases, it is very difficult to decrease the amount of HF to below 10 μg/mL. The remaining HF causes erosion to electrodes, which directly affects the capacity of batteries. Therefore, the factors mentioned above all lead to the restriction of HF method nowadays [1-4].
In this work, a promising preparation method of LiPF6 is introduced. In order to strictly control the influence of H2O, CaF2 and P2O5 are used to prepare PF5, which is defined as a “thoroughly-dry method”. Acetonitrile with low toxicity is used instead of HF as a solvent, which has no pollution to environment and production. Acetonitrile is extremely beneficial to separation and purification of LiPF6.
2 Experimental
2.1 Reagents
LiF(self-made[5]), anhydrous acetonitrile, anhydrous aether, CaF2, P2O5 were all supplied by Tianjin Kemiou Chemical Reagent Co., China. Argon gas was supplied by Changsha Gaoke Gas Plant of China.
2.2 Equipment
The equipment in the experiment was self- constructed. The whole equipment is schematically shown in Fig.1. Reactor Ⅰ(C) was mainly composed of a stainless steel cylinder vessel (C2) and crucible heater box (C1). Hermetical circles and liquid-sealing system were designed at the mouth of heater box to guarantee no leak of gas in the experiment. Reactor Ⅱ (D) comprised a two-neck round bottom flask equipped with a magnetic stirrer (D), circumfluence equipment (D1) and condenser (D2). Among them the circumfluence equipment was designed to increase the utility rate of materials, and the condenser was to prevent the volatilization of solvent in the flask. Argon gas of high purity in the carrier gas system (A) was used to build up protection environment for the whole experiment. Many drier towers (B, B′) were set in various places in the experiment to satisfy rigorous demand for water. Waste gas multiple- absorption equipment (E) was adopted in the experiment.
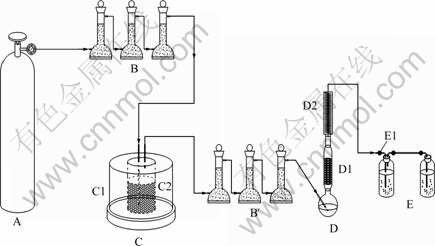
Fig.1 Schematic diagram of equipment: A Carrier gas system; B, B′ Drier towers; C Reactor Ⅰ; C1 Crucible heater box; C2 Stainless steel cylinder; D Reactor Ⅱ; D1 Circumfluence equipment; D2 Condenser; E Waste gas absorption equipment; E1 Desiccant
2.3 Preparation of PF5
The preparation process of PF5 in the experiment was monitored by Gas Chromatography-Mass Spectroscopy-Selected Ion Monitoring (GC-MS-SIM). Because only liquid sample can meet the request of determination in GC-MS-SIM, PF5 must be pretreated [6]. In the experiment, PF5 was dissolved in anhydrous aether. Firstly, anhydrous aether was enclosed in a two-neck round bottom flask equipped with a magnetic stirrer and a condenser. After the flask was placed in 0 ℃-water-bath and the air in it was removed by dry argon gas, PF5 was dissolved into anhydrous aether for 0.5-1 h. Finally, the sample was fetched out from the flask for determination.
2.4 Preparation of LiPF6
LiPF6 was synthesized by CaF2, P2O5 and LiF according to the following procedures. 1) 50.0 g CaF2 and 150.0 g P2O5 were sealed in a pottery ball-grinding machine to be mixed for 2 h. The mixture was then quickly transferred to the stainless steel cylinder vessel in crucible heater box. 2) 10.0 g LiF and 200 mL anhydrous acetonitrile were enclosed into the two-neck round bottom flask. They were stirred under magnetism to form suspending solution. 3) 8.0 g LiF was filled in the circumfluence tower by carrier of glass fibre. Two reactors were connected. 4) Air in whole system was removed by dry argon gas. 5) The temperature of reactor Ⅰ was controlled between 280 ℃ and 300 ℃. The temperature of reactor Ⅱwas controlled between 20 ℃ and 30 ℃. Reaction process was maintained for 3 h. 6) After the solution in the flask was heated at 50 ℃ for 4 h, it was filtrated quickly. 7) The received filtrate was cooled at -20 ℃ for crystallization. The product was dried in vacuum at 60 ℃ to obtain LiPF6.
2.5 Characterization and determination
All the operation in the experiment was carried out in a glove box under H2O and CO2 free atmosphere, which was provided by Scientific Instrument Plant of Nanjing University, China. Infrared spectrum was measured by AVATAR-360 FT-IR instrument provided by Nicolet Magna Co., USA. 1-2 mg LiPF6 was quickly transferred in agate mortar to be mixed with KBr for 5-6 min. It was then pressed into pieces for measurement. The structures of self-synthesized LiPF6 were characterized using Cu Kα radiation in the range of 10?-90? with a scanning rate of 2 (?)/min by D/max 2550 X-ray diffraction (XRD) instrument provided by Japan. Thermogravimetric (TG) analysis was done by SDT Q600 V8.0 instrument provided by USA. The quantity of Li+ was determined by atomic absorption spectrometry with the instrument provided by Beijing Rayleigh Analytical Instrument Co., China. The concentrations of Li+ standard solutions were 0.5, 1, 2, 4, 6, 8 mg/L, respectively. 1 mL 2.0 g/L KCl solution was used as ionization buffering agent. The testing solution was self-made LiPF6-aether after dilution. The quantity of PF6- was determined by Dionex 500 ion chromatography instrument from USA. The concentrations of standard solutions were 10.0 μg/mL F-, 5.0 μg/mL PO43- and 80 μg/mL PF6-, respectively. The preparation of PF5 was monitored by GC-MS Philigen series of Thermo Electron Corporation. The type of GC was Trace GC ultra. The GC column was db-5. The type of MS was Trace dsq. The adopted selected ions in the experiment were m/z=50, 69, 88, 104, 107.
3 Results and discussion
3.1 Monitoring to preparation of PF5
The monitoring figure of GC-MS-SIM to the preparation of PF5 at different temperatures is shown in Fig.2(a). It can be seen that the peak area at 200 ℃ is clearly small, which results from no reaction of materials at this temperature. The peak area reaches the maximum at temperatures between 280 ℃ and 300 ℃. However, many impurity peaks appear when the temperature is continuously increased over 300 ℃. Therefore, the monitoring results reveal that continuously increasing temperature brings disadvantages to the preparation of PF5. It is determined that the best heating temperature of CaF2 and P2O5 is between 280 ℃ and 300 ℃. The monitoring figure of GC-MS-SIM with different time at 280 ℃ is shown in Fig.2(b). It can be obviously found that the best reaction time is 3 h. Therefore, the optimal preparation condition of PF5 is that CaF2 and P2O5 are heated between 280 ℃ and 300 ℃ for 3 h. Meanwhile, it can be concluded from MS information that PF5 prepared at optimal conditions is in high purity.
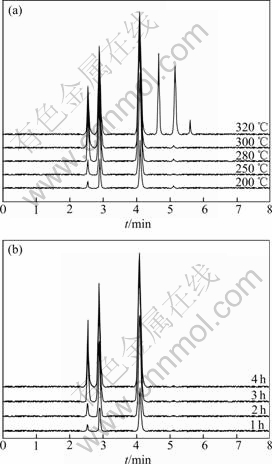
Fig.2 GC-MS-SIM graphs of preparation of PF5: (a) At different temperatures; (b) At 280 ℃ with different time
3.2 Infrared spectrum characterization
PF6- belongs to Oh point group. Six kinds of vibration modes are included in the molecule, whose basic frequency number is 15. The characteristic of Oh point group is listed in Table 1[7-8].
Table 1 Characteristic of Oh point group

It can be calculated from Table 1 that:
Г=A1g+Eg+2F1u+F2g+F2u
Then,
Гt=2F1u, Гv=A1g+Eg+F2g+F2u
And,
Гα= A1g+Eg+F2g
So, for LiPF6 molecule, two energy levels belonging to F1u have infrared activity, which results from the flexing vibration and bending vibration of F—P bond, respectively[9-13]. Therefore, two strong absorption peaks of LiPF6 molecule should appear at 820-860 cm-1 and 550-565 cm-1.
The infrared spectra of standard LiPF6 and self-synthesized LiPF6 are shown in Fig.3. It can be found that the position and intensity of peaks in the two spectra are the same. Strong absorption peaks appear at 560 cm-1 and 831 cm-1, respectively. Therefore, it can be confirmed that the received production is LiPF6.
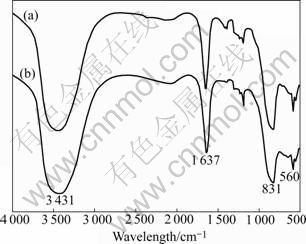
Fig.3 Infrared spectra of LiPF6 tested at differentiate rate of 4 cm-1, and scan degree of 32 times: (a) Standard LiPF6; (b) Self- synthesized LiPF6
3.3 XRD characterization
Strong lines of LiPF6 in standard PDF card appear at 21?-26?. Hypo-strong lines appear at 42?, 51?, 57?, etc [14-15].
The XRD pattern of self-synthesized LiPF6 is shown in Fig.4. It can be observed that the strong peaks of self-synthesized LiPF6 appear at 22.00? and 25.24?, and hypo-strong peaks appear at 42.28?, 52.72? and 57.72?, which is the same as the standard PDF card. Meanwhile, it can be confirmed from the calculation results of diffraction peaks that the crystal structure of LiPF6 is hexagonal series and space group belongs to Oh point group. It can be also observed that the diffraction peaks are smooth and clear, and the diffraction peak of I/I0=100 appears at 2θ=26.83?, which accounts for the integrated crystal structure of LiPF6.
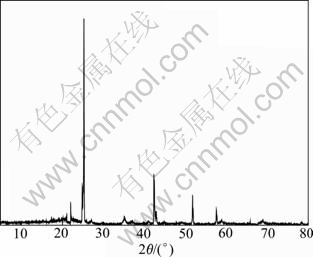
Fig.4 XRD pattern of self-synthesized LiPF6 tested by Cu Kα radiation in range of 10?-90? with scanning rate of 2(?)/min
3.4 Atomic absorption analysis
Fig.5 shows the standard curve of Li+ solution, whose correlative coefficient is 0.999 92. 5.0 g self- synthesized LiPF6 was firstly dissolved in 1 000 mL anhydrous aether. It was then diluted for analysis at the same conditions as standard Li+ solution. The analysis result is ρ(Li+)=0.291 mg/L. The concentration of self- synthesized LiPF6 through conversion is ρ(Li+)=0.291 g/L. This final result indicates that self-synthesized LiPF6 has low amount of impurity metal ions.
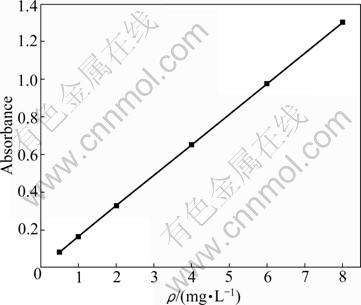
Fig.5 Standard curve of lithium ion
3.5 Ion chromatography(IC) analysis
The standard IC graphs of 10.0 μg/mL F-, 5.0 μg/mL PO43-, 80 μg/mL PF6- are shown in Fig.6. 5.0 g self-made LiPF6 was firstly dissolved in 1 000 mL anhydrous aether. It was then diluted for analysis at the same conditions as standard solutions. The analysis result is ρ(PF6-)=47.07 μg/mL. The concentration of self-synthesized LiPF6 through conversion is ρ(PF6-)= 4.707 g/L. The final result indicates that no other impurity ions (such as F-, PO43-) are testified, and the purity of self-synthesized LiPF6 reaches 99.98%.
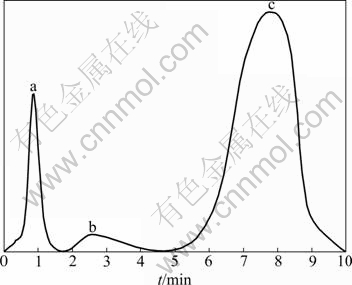
Fig.6 Standard IC graphs: (a) 10.0 μg/mL F-; (b) 5.0 μg/mL PO43-; (c) 80 μg/mL PF6-
3.6 TG-DTG analysis
The thermal decomposition curve of self- synthesized LiPF6 is shown in Fig.7. It can be seen that strong decomposition peaks of LiPF6 appear at 78.33 ℃ and 202.15 ℃, and the mass loss is 12.44% and 80.38%, respectively. However, it is reported in Refs.[1-2,16] that the initial decomposition temperature of LiPF6 is 60℃. Therefore, the thermal stability of self-synthesized LiPF6 is higher than the literature-reported LiPF6.
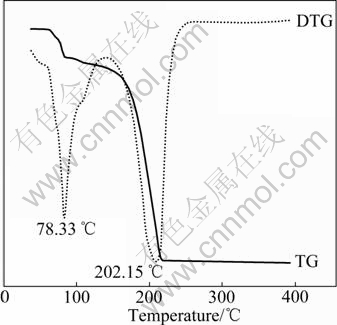
Fig.7 Thermal decomposition curve of LiPF6 tested with heating rate of 10 ℃/min under protection of N2 in corundum crucible
4 Conclusions
1) A promising preparation method of LiPF6 was introduced. Phosphorus pentafluoride (PF5) was first prepared using CaF2 and P2O5 at 280 ℃ for 3 h. LiPF6 was synthesized in acetonitrile solvent by LiF and PF5 at room temperature (20-30 ℃) for 4 h.
2) The synthesized LiPF6 was characterized by infrared spectrometry and X-ray diffractometry(XRD). Atomic absorption and ion chromatography results show that the purity of synthesized LiPF6 reaches 99.98%. The thermal stability of self-synthesized LiPF6 was analyzed by differential thermal analysis and thermogravimetry. The initial decomposition temperature of self- synthesized LiPF6 is 78.33 ℃, which is higher than the literature-reported decomposition temperature.
References
[1] GAVRITCHEY K S, SHAMATAVA G A, SMAGIN A. Calorimetric study of thermal decomposition of lithium hexafluorophosphate [J]. Journal of Thermal Analysis and Calorimetry, 2003, 73(1): 71-83.
[2] SMAGIN A, MATYUKHA V A, KOROBTSEV P. Application of thermo-gravimetric studies for optimization of lithium hexafluorophosphate production [J]. Journal of Power Sources, 1997, 68(8): 326-327.
[3] HOWLETT P C, MACFARLANE D R, HOLLENKAMP A F. A sealed optical cell for the study of lithium electrode and electrolyte interfaces [J]. Journal of Power Sources, 2003, 114(16): 277-284.
[4] CHANGE A, CARREB M, WILLMANN P. Modeling viscosity and conductivity of lithium salts in γ-2butyrolactone [J]. Journal of Power Sources, 2002, 109(9): 203-213.
[5] HU Qi-yang, LI Xin-hai, WANG Zhi-xing, PENG Wen-jie. Preparation of lithium fluoride in high purity: CN Patent 1962445 [P]. 2006.
[6] WANG Li, WANG Zheng-fan, MOU Shi-fen. Sample pretreatment of chromatography analysis [M]. Beijing: Chemical Industry Press, 2004: 151-154. (in Chinese)
[7] XIN Ming, HU Wen-hai. Group theory and chemistry [M]. Beijing: Higher Education Press, 1984: 222-225. (in Chinese)
[8] KE Yi-kan, DONG Hui-ru. Handbook of analytical chemistry [M]. Beijing: Chemical Industry Press, 1998: 51-59. (in Chinese)
[9] TENG Xiang-guo, DAI Ji-cui, MA Pei-hui. Preparation and characterization of LiPF6 for lithium ion secondary batteries [J]. Journal of Inorganic Chemistry, 2004, 20(9): 1109-1111. (in Chinese)
[10] AROCA R, NAZRI M, NAZRI G A. Vibration spectra and ion-pair properties of lithium hexafluorophosphate in ethylene carbonate based mixed-solvent systems for lithium batteries [J]. Journal of Solution, 2000, 29(10): 344-348.
[11] MOHAMED K S. Pyridine poly—A reagent for the preparation of hexafluorophosphate [J]. J Fluorine Chemistry, 1983, 23(15): 509-511.
[12] MOHAMED K S, PADMA D K. Spectral studies on pyridine hexafluorophosphate [J]. Spectrochim Acta, 1985, 41(5): 725-729.
[13] WILLMANN P. Solvate of lithium hexafluorophosphate and pyridine, its preparation and preparation process for lithium hexafluorophosphate using said solvate: US Patent 5993761 [P]. 1999.
[14] HUANG Ji-wu. Operation guide of X-ray diffraction experiment [M]. Changsha: Central South University Press, 2006: 102-108. (in Chinese)
[15] LI Wen. Research and practice of hexafluorophosphate for lithium ion battery electrolyte [D]. Beijing: Chinese Academy of Sciences, 2004: 55-60. (in Chinese)
[16] LI Li-yun, ZHANG Zhi-ye, CHEN Xin. Innovation of hexafluorophosphate for lithium ion battery electrolyte [J]. Chemistry Industry and Engineering, 2003, 22(11): 224-228. (in Chinese)
Foundation item: Project(2007CB613607) supported by the National Basic Research Program of China
Corresponding author: LIU Jian-wen; Tel/Fax: +86-731-88836633; E-mail:changdeljw@163.com
DOI: 10.1016/S1003-6326(09)60144-8
(Edited by YUAN Sai-qian)