Sol-gel derived HA/TiO2 double coatings on Ti scaffolds for orthopaedic applications
来源期刊:中国有色金属学报(英文版)2006年第z1期
论文作者:XU W HU W Y LI M H MA Q Q P. D. HODGSON WEN C E
文章页码:209 - 216
Key words:Ti scaffolds; sol-gel method; Hydroxyapatite; Titania; HA/TiO2 coatings; simulated body fluid
Abstract: Hydroxyapatite/titania (HA/TiO2) double layers were coated onto Ti scaffolds throughout for orthopaedic applications by sol-gel method. Differential scanning calorimetry (DSC), thermogravimetric analysis (TG) and X-ray diffractometry (XRD) were used for the characterisation of the phase transformations of the dried gels and coated surface structures. Scanning electron microscope (SEM) equipped with energy dispersive spectrometry (EDS) was used for the observation and evaluation of the morphology and phases of the surface layers and for the assessment of the in vitro tests. The in vitro assessments were performed by soaking the HA/TiO2 double coated samples into the simulated body fluid (SBF) for various periods. The TiO2 layer was coated by a dipping-coating method at a speed of 12 cm/min, followed by a heat treatment at 600 ℃ for 20 min. The HA layer was subsequently dipping-coated on the outer surface at the same speed and then heat-treated at difference temperatures. The results indicat that the HA phase begins to crystallize after a heat treatment at 560 ℃. The crystallinity increases obviously at 760 ℃. SEM observations find no delamination or crack at the interfaces of HA/TiO2 and TiO2/Ti. The HA/TiO2 coated Ti scaffolds displays excellent bone-like apatite forming ability when it is soaked into SBF. Ti scaffolds after HA/TiO2 double coatings can be anticipated as promising implant materials for orthopaedic applications

XU W1, HU W Y1, LI M H1, MA Q Q1, P. D. HODGSON2,WEN C E2
1. Department of Applied Physics, Hunan University, Changsha 410082, China;
2. Centre for Material and Fibre Innovation, Deakin University, Geelong Vic 3217, Australia
Received 10 April 2006; accepted 25 April 2006
Abstract: Hydroxyapatite/titania (HA/TiO2) double layers were coated onto Ti scaffolds throughout for orthopaedic applications by sol-gel method. Differential scanning calorimetry (DSC), thermogravimetric analysis (TG) and X-ray diffractometry (XRD) were used for the characterisation of the phase transformations of the dried gels and coated surface structures. Scanning electron microscope (SEM) equipped with energy dispersive spectrometry (EDS) was used for the observation and evaluation of the morphology and phases of the surface layers and for the assessment of the in vitro tests. The in vitro assessments were performed by soaking the HA/TiO2 double coated samples into the simulated body fluid (SBF) for various periods. The TiO2 layer was coated by a dipping-coating method at a speed of 12 cm/min, followed by a heat treatment at 600 ℃ for 20 min. The HA layer was subsequently dipping-coated on the outer surface at the same speed and then heat-treated at difference temperatures. The results indicat that the HA phase begins to crystallize after a heat treatment at 560 ℃. The crystallinity increases obviously at 760 ℃. SEM observations find no delamination or crack at the interfaces of HA/TiO2 and TiO2/Ti. The HA/TiO2 coated Ti scaffolds displays excellent bone-like apatite forming ability when it is soaked into SBF. Ti scaffolds after HA/TiO2 double coatings can be anticipated as promising implant materials for orthopaedic applications
Key words: Ti scaffolds; sol-gel method; Hydroxyapatite; Titania; HA/TiO2 coatings; simulated body fluid
1 Introduction
Bone injuries and failures often require the inception of implant biomaterials. Researches in this area are receiving increasing attention worldwide. A variety of artificial bone materials, such as metals, polymeric materials, composites and ceramics are explored to replace diseased bones [1-3]. In particular, metallic implant materials, e.g. SUS316L stainless steel, Co-Cr-Mo type alloys, Ti and Ti alloys (e.g. Ti-6Al-4V) are widely used as orthopaedic and dental implant materials. Among these, Ti and some of Ti alloys are preferred load-bearing implant materials due to their relatively low modulus, excellent strength-to-weight ratio, good fracture toughness, and superior biocompatibility and corrosion resistance [4]. It has been demonstrated that Ti and some of Ti alloys are well accepted by human tissues compared to other metal materials [5]. Moreover, Ti scaffolds demonstrated desirable biomechanical properties since their elastic moduli can be tailored to be very close to those of cancellous bones [6]. Such kind of commensurate elastic moduli can be expected to eliminate the stress shielding effect, which may lead to the failure of the implant material. It is thus possible, that Ti alloy and its scaffolds have a high biomedical potential due to its unique property combination of biocompatibility and biomechanical property.
Nevertheless, in metallic biomaterials there is a lack of direct chemical bonding between the implant material and the host bone tissue after implantation due to the encapsulation phenomena by the fibrous tissues. The presence of this fibrous capsule isolates the implant materials from the surrounding bone and has been a crucial problem. Since 1970 s, this issue has been extensively investigated and bioactive ceramics, e.g. hydroxyapatite coatings on the implant biomaterials has been developed because they can bond to and integrate with bone in living body spontaneously. There is a general rule that an essential requirement for an artificial implant material to bond to a living bone is by the ormation of a hydroxyapatite layer on its surface in the living body. Hydroxyapatite (HA) has many biological benefits, such as direct bonding to bone and enhancement of new bone formation around it due to its chemical similarity with hard tissues [7-14]. Many methods including plasma spraying, pulsed laser deposition and electrophoretic deposition have been studied to produce HA coatings over the last 15 years [15-22]. Among these techniques, only plasma-spraying has achieved commercial success [15]. But the coated layer by this method was easily separated from the surfaces or resorbed in the body environment because of the unstable characteristics through its rapid solidification, inhomogeneous composition, melted and decomposed phases, etc. Furthermore, methods such as plasma spraying and electrophoresis may produce highly crystalline coatings, which are difficult to resorb in the body [23]. Lastly, the plasma spraying process requires an extremely high temperature, which may be as high as 12 000 ℃ and therefore causes high equipment require-
ments.
In recent years, sol-gel technique has been developed to synthesise HA for the coating of the implant materials [7-10, 24, 25]. Compared to conventional thin film forming process, this process offers several advantages, e.g. allowing a better control of the chemical composition of the coating, the preparation of homogenous films, the control of the film microstructure and a reduction of the densification temperature of the ceramic layer, and finally, requiring less equipment and potentially less expensive. The precursors used in the sol-gel process are solutions. It is thus especially easy to purify them by distillation or crystallisation. Moreover, the precursors are mixed at the molecular level in the solution. This intimate mixture of the coating components allows lower sintering temperature. It has been reported that HA can even be synthesised at a temperature as low as 400 ℃ [18]. On the other hand, TiO2 coatings, place between the HA and Ti, have been used to improve the bonding strength of the HA layer and the Ti substrate, as well as the corrosion resistance of Ti. The corrosion resistance is known to increase with the increase of the thickness of the TiO2 coating [22]. Therefore, HA/TiO2 coatings can be expected to combine the advantages of TiO2 with those of HA.
In the present study, Ti scaffolds were subjected to a sol-gel HA/TiO2 coating process. The characteristics of the HA/TiO2 coatings of the titanium scaffolds before and after the soaking into a simulated body fluid (SBF) were investigated using X-ray diffractometry (XRD), differential scanning calorimetry (DSC), thermogravime-
tric (TG) analysis, and scanning electron microscope (SEM) equipped with energy dispersive spectrometer (EDS). The purpose of this study is to evaluate the effect of sol-gel process on formation of HA/TiO2 coatings on the Ti scaffolds throughout and the bone-like apatite forming ability, i.e. the bioactivity of the Ti scaffolds, when the coated scaffolds was soaked into a SBF.
2 Experimental2.1 Preparation of Ti scaffold samples
For the preparation of the Ti scaffolds, commercially available Ti powders (purity ≥99.9%, powder size ≤10 μm) and ammonium bicarbonate particles were used as starting materials in the present study. The fabrication process consisted of the mixing, pressing and heat-treating steps as described in our previous study [6]. At first, Ti powders and spacer holder particles were mixed thoroughly in an agate mortar. After the ingredients homogeneous mixed, powders of the mixture were uniaxially pressed into green compacts in steel dies with a pressure of 80 MPa. The green compacts were then heat-treated to burn out the spacer holder particles, and to sinter into porous Ti scaffolds. The heat-treatment process is consisted of two steps: at 180 ℃ holding for 30 min and 1 200 ℃ holding for 2 h. Cylindrical scaffold specimens with a porosity of 35% and an average pore size of 250 μm were fabricated for the examining of the macro- and micro-structural characteristics. Scaffold disc samples with a diameter of 10 mm were machined from for the sintered scaffold samples for the HA/TiO2 coatings and the in vitro assessments.
The Ti scaffold disc samples were polished progressively using 240, 600, and 800-grit silicate-carbon papers to remove macro-level surface defects and contaminations. After polishing, all the discs were ultrasonically cleaned in distilled water and then washed in acetone for 10 min and in 40% ethyl alcohol solution for 15 min, and rinsed in distilled water for 20 min. The samples were dried at 80 ℃ under vacuum in an electric oven for 24 h finally. The surface morphology of Ti scaffold disc samples before and after coatings, and after further soaking into simulated body fluid for various periods were characterised.
2.2 HA/TiO2 coatings
The sol-gel process started with the preparation of the TiO2 sol. A tetrabutylorthotitanate (C16H36TiO4) was first diluted with absolute ethanol, and then a small amount of distilled water mixed with diethanolamine (NH(CH2CH2OH)2) which was used as catalyzer was added for hydrolysis, followed by vigorous stirring for 24 h. Subsequently, the mixed sol was aged for 24 h. For the preparation of the HA sol, triethyl phosphite (C2H5O3PO)) diluted with anhydrous ethanol was first hydrolysed for 24 h with distilled water under vigorous stirring. A stoichiometric amount of 2 mol/L calcium nitrate (Ca(NO3)2?4H2O)) dissolved in anhydrous ethanol was added into the hydrolysed phosphite sol which had been aged for 24 h (n(Ca) ∶n(P)= 1.67∶1). Vigorous stirring was continued for 4 h after the titration, and then aged for 24, 48 and 72 h for characterisation. It is found that ageing for 48 h for the HA sol is necessary for obtaining a stable gel condition and HA composition.
The TiO2 layer was coated by a dipping-coating method at a speed of 12 cm/min, followed by a heat treatment at various temperatures, i.e. 300, 340, 560 and 800 ℃ for 20 min for the evaluation of the effect of temperature. The HA film was subsequently dipping-coated on the outer surface at the same speed and then heat-treated at various temperatures, i.e. 300, 400, 560, 680, 760 ℃ for 20 min using the 48 h aged HA sol for characterisation of the effect of the temperature. The process was repeated several times to get a thicker later of coating. DSC analysis and TG (STA449C DIL402PC), XRD (Simens D500), and SEM equipped with energy dispersive spectrometry EDS (Oxford) were used for the characterisation of the dried gels and coated samples.
2.3 Soaking in SBF for in vitro assessments
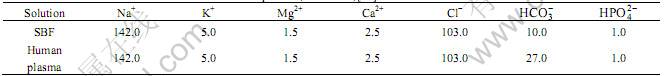
In vitro experiments were carried out through soaking the HA/TiO2 coated Ti scaffold disc samples into SBF [26] and incubated at 37 ℃ in a humidified atmosphere of 95% air and 5% CO2. The SBF with ion concentrations nearly equal to the human blood plasma was prepared by dissolving reagent-grade chemicals: NaCl, NaHCO3, Na2CO3, KCl, K2HPO4?3H2O, MgCl2?H2O, CaCl2 and Na2SO4 in distilled water, and buffered at 36.5 ℃ and pH value 7.25 with trishydroxymethylamminomethane ((CH2OH)3CNH3) and HCl. The ion concentrations are listed in Table 1. The SBF solution was refreshed every 24 h. After various incubation periods (up to 14 d), the Ti scaffold samples were taken out of SBF, rinsed with distilled water, and then dried in a vacuum oven at 50 ℃ for 24 h for the following characterisation.
3 Results and discussion3.1 Thermal analysis of TiO2 and HA gels
DSC and TG analyses were performed in the temperature range from 20 to 800 ℃ with a heating rate of 10 K/min for TiO2 gel samples. The DSC/TG curves of the TiO2 gel heating in flowing N2 gas and air respectively are shown in Fig.1. The TG curve of TiO2 gel sample heating in air can be divided into four stages. The first stage is from room temperature to 260 ℃. The rate of mass loss is 12%, which can be attributed to the evaporation of the absorbed water in the gel. The second stage is from 260 to 480 ℃. The rate of mass loss is 33%, which is attributed to the combustion and carbonization of organic substance. The third stage is from 480 to 600 ℃. The rate of mass loss is 15%, which is caused by the decomposition of residual organic materials and the transformation of anatase phase to rutile phase of TiO2. No further mass loss can be observed in the last stage from 600 to 800 ℃. In this stage, the anatase TiO2 has transformed into rutile phase. This is corresponding to the broad exothermic peaks in DSC traces in the temperature range from 600 to 800 ℃, which is presented below.
The DSC trace of the TiO2 gel sample heating in flowing air exhibited four exothermic peaks also. There are two small exothermic peaks at 276 and 327 ℃, as corresponding to the first and second stages shown in the TG curves. The strong exothermic peak at 538 ℃ is representative of the exothermic reaction indicating the formation of TiO2. In contrast to the small exothermic peak at the temperature near 538 ℃ heating in N2, there is a strong peak at approximately 538 ℃ while heating in air. It can be concluded that there is more organic materials produced and burned in air around 538 ℃, resulting in the release of large quantity of heat as indicated by the obvious exothermic peak at 538 ℃,
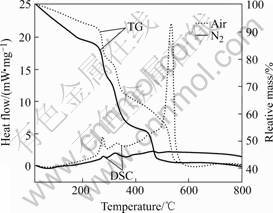
Fig.1 DSC/TG curves of TiO2 gel samples
Table 1 Ion concentration of SBF and human blood plasma (10-3 mol/L)[26]
compared to that when it is heated in N2.
Fig.2 shows the TG curves of HA dried gel samples heating in flowing air. The samples were aged for 24, 48 and 72 h, respectively. It can be seen that the three curves exhibit very similar declining tendency during the heating process. However, the mass loss decreases with the increase of the aging time of the sample. The sample aged for 24 h show the largest mass loss, while the mass loss for the sample aged for 72 h is just slightly smaller than that of the sample aged for 48 h.
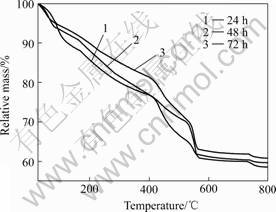
Fig.2 TG curves of HA gel samples aged for different time
Carefully observation of the three TG curves reveales that there are three stages from room temperature to 800 ℃. In the first stage(25-100 ℃), the three curves are very close; especially, the curve for the 48 h aged sample is almost overlapped by the curve for the 72 h aged sample. The mass loss for the three samples is approximately the same in this stage. For the second stage from 100 to 550 ℃, the mass loss of 72 h aged sample and 48 h aged sample are less than that of the 24 h aged sample. Subsequently, the three curves exhibited a steep drop at 550 ℃. This rapid drop can be contributed to the formation of the hydroxyapatite, which is in consistent with the DSC results as elaborated below and is further confirmed by the following XRD analysis. From 550 to 800 ℃, the three curves show a relative plateau stage, which indicate there are little mass loss in this period.
Fig.3 shows the DSC curves of three HA dried gel samples heated in air. The three samples were aged for 24, 48 and 72 h, respectively. It can be seen that the tendency of the occurring of the endothermic peaks and the exothermic peaks with the increase of the temperature is very similar for the three samples. During the entire heating process, the curves for 48 h aged sample and 72 h aged sample are relatively close. The curve of the 24 h aged sample is obviously separated from the other two curves for the 48 h and 72 h aged samples. Inspections reveal that in the temperature range
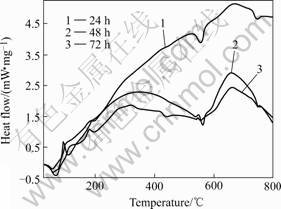
Fig.3 DSC curves of HA gel samples aged for different time
from 25 to 100 ℃, the three curves show very similar thermal behaviours. The first endothermic peak occurr at 54 ℃ for the three samples. This peak can be attributed to the evaporation of the absorbed water in the samples. From 100 to 800℃, three curves separate obviously, but the positions of the endothermic peaks and the exothermic peaks at the temperature axis are very close. The DSC curves show two exothermic peaks at 108 and 185 ℃, respectively, which can be attributed to the decomposition of the nitrates and organic compounds in the HA gel samples. The endothermic peak at 555 ℃ on the three DSC curves is representative of the endothermic reaction indicating the crystallization of HA. The last endothermic peak is observed at 749 ℃, which is caused by the removal of the residuals in the samples. Compared to the 48 h and 72 h aged HA samples, the 24 h aged sample shows lager exothermic heat. This is because there are more organic materials in the gel sample burned or decomposed. It can be concluded that the HA gel should be aged for a time of 48 h or longer to achieve a stable gel condition and HA composition.
3.2 XRD analysis
The crystal structure of TiO2 gels and films was characterized using XRD analysis. The XRD patterns of TiO2 film samples coated on the Ti scaffold and annealed at various temperatures are shown in Fig.4. The annealing temperatures were 300, 340, 560, and 800 ℃, respectively. The TiO2 film coated on the Ti scaffold and heat treated at 560 ℃ shows sharp peaks corresponding to the anatase phase, indicating that the film annealed at this temperature is mainly consisted of anatase phase (Fig.4(c)). It can be deduced that the TiO2 phase with an anatase structure can be obtained in the film by annealing at a temperature of 560 ℃. Fig.4(d) shows the sharp peaks corresponding to the rutile phase, indicating the phase transformation of the TiO2 from anatase to rutile by annealing at 800 ℃. The relatively sharp peaks can be explained by the high crystallinity of the rutile
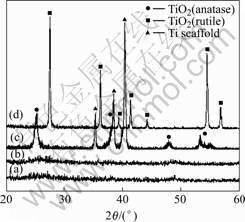
Fig.4 XRD patterns of TiO2 gel samples after heat treatment at various temperatures: (a) 300℃; (b) 340 ℃; (c) 560 ℃; (d) 800 ℃
TiO2 phase. The samples annealed at 300 and 340 ℃ are found to be amorphous phase, as shown in the curves in Figs.4(a) and (b).
It has been reported that the efficacy of apatite nucleation through the functional groups of Ti—OH on the metal surface is determined not only by their composition alone, but also by their concentration and structural arrangements [26]. The TiO2 gel possesses three kinds of structures as amorphous, anatase and rutile after annealing at 340, 560 and 800 ℃, respectively (Fig.4). Among these, the anatase phase forms bone-like apatite most effectively, followed by the rutile phase; the amorphous phase forms no apatite. Therefore, it can be concluded that the temperature of 560 ℃ is the most suitable temperature of annealing, which ensures the attaining of the anatase phase TiO2 and therefore the efficiency of bone-like apatite nucleation.
Fig.5 shows the XRD patterns of HA/TiO2 coatings on the Ti scaffold after heat treatments at 300, 400, 560, 680 and 760 ℃, respectively. It can be seen that there is no HA phase peaks occurring after the heat treatment at 300 ℃ and only very weak peaks belonging to HA phase appeared at 400 and 500 ℃, as shown in Fig.5. However, there are peaks attributable to HA phase emerged on the pattern of the sample after heat treatment at 560 ℃, as curve (c) shown in Fig.5 and the intensity of these peaks increases after heat treatments at 680 and 760 ℃ as curves (d) and (e) shown in Fig.5. For the samples after heat treatment at higher temperatures of 760 ℃, the crystallinity of HA phase increases obviously.
Fig.6 shows the XRD patterns of the HA/TiO2 coatings on the Ti scaffold before and after the soaking into the SBF for various periods. The main phases before soaking into SBF are HA, TiO2 and tricalcium phosphate (Ca3(PO4)2, TCP) (Fig.6 (a)). It can be seen that there are
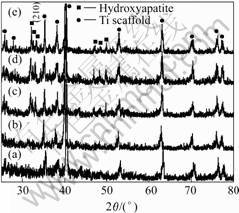
Fig.5 XRD patterns of HA/TiO2 coatings on Ti scaffold after heat treatments at various temperatures: (a) 300℃; (b) 400 ℃; (c) 560 ℃; (d) 680 ℃; (e) 760 ℃
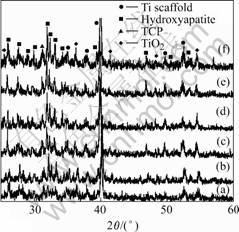
Fig.6 XRD patterns of HA/TiO2 coated Ti scaffold samples soaked in SBF for various days: (a) 0; (b) 1 d; (c) 4 d; (d) 7 d; (e) 10 d; (f) 14 d
already weak diffraction peaks belonging to HA phase detectable after soaking in SBF for 1 d as shown in Fig.6 (b). The diffraction peaks attributable to bone-like apatite phase become obviously after 4 d soaking in SBF (Fig.6 (c)). The intensity of the diffraction peaks increases with the increase of the soaking time as shown in Figs.6 (d)-(f).
3.3 SEM observations of HA/TiO2 coatings on Ti scaffolds before and after soaking into SBF
The surface structure of the TiO2 coated Ti scaffold sample, the HA/TiO2 coated samples before and after soaking into SBF were characterised using SEM. The SEM image of the Ti scaffold sample after TiO2 coating and heat treated at 600 ℃ is shown in Fig.7. It can be seen that the TiO2 film exhibites a cracked surface. There are a number of cracks distributed on the TiO2 layer. These cracks were caused by the mechanical constraints occurred during the heat treatment process. Such cracked surface is beneficial to the adhesive strength between the TiO2 and HA layers because the subsequently coated HA gel can fill into the cracks and cover the surface of TiO2 film completely. Therefore the high surface roughness of the TiO2 film can be expected to improve the quality of the HA layer deposition in the following HA coating process.
The SEM images of the surface microstructures for the HA/TiO2 coated Ti scaffold sample after soaking into SBF for various days are shown in Fig.8. It can be seen that after soaking into SBF for 1 d, there is a very thin layer of the HA phase formed throughout the porous structure of the Ti scaffold; for the sample after soaking for 4 d, granules of HA phase emerge on the wall surface of the scaffold (Figs.8 (a) and (b)). The HA granules grow gradually with the increase of the soaking time from 7 to 14 d in SBF as shown in Figs.8 (c)-(e).
Fig.8 (f) shows the local enlarged image of the sample soaked for 14 d. It can be seen that a porous structure of HA phase has formed. EDX analysis was performed on the SBF soaked samples. Fig.9 shows the EDX analysis
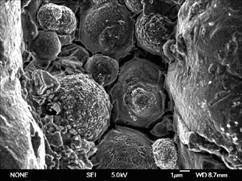
Fig.7 SEM image of TiO2 film prepared by annealing at 600 ℃
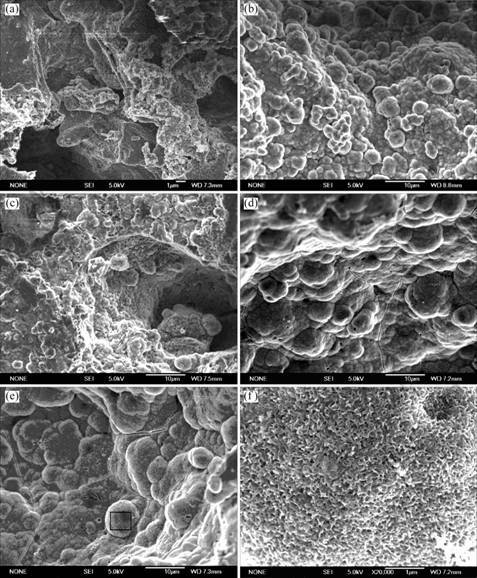
Fig.8 SEM images of surface structures of HA/TiO2 films after soaking into SBF for different time: (a) 1 d; (b) 4 d; (c) 7 d; (d) 10 d; (e) 14 d; (f) Local enlarged image for samples soaked for 14 d
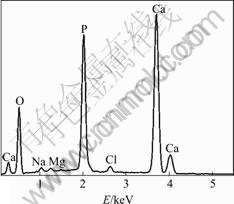
Fig.9 EDX analysis of HA/TiO2 coated Ti scaffold sample after soaking into SBF for 14 d
of the HA/TiO2 coated sample soaked into SBF for 14 d. It can be seen that a bone-like apatite layer with elements traces for Ca, P, O, Na, Mg and Cl have been formed on the Ti scaffold surface.
Fig.10 shows the Ca/P mole ratio of the coatings on Ti scaffold sample after soaking in SBF for various days. Before soaking into SBF, the Ca/P atomic ratio is 1.688, which is the ratio for HA coating layer. After 1 d soaking, the ratio decreases rapidly to 1.46, and then it gradually decreases to 1.43 within the next 7 d, and continuing to decrease to 1.41 for 10 d and then maintained this level for longer time SBF soaking. In this process, an amorphous calcium phosphate phase forms on the coated surfaces at first. This amorphous calcium phosphate phase grows gradually with the increase of soaking time and appeared granule morphology. After soaking into SBF for 14 d, the Ca/P mole ratio maintained 1.41. SEM observations reveal that the amorphous calcium phosphate granules transform into bone-like apatite. These are caused by the OH- and PO43- ions on coating surfaces, which can combine with the Ca2+ ions in the SBF to induce the formation of the bone-like apatite. It can be concluded that the HA/TiO2 films coated on the Ti
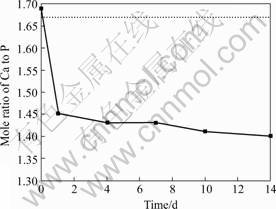
Fig.10 Mole ratio of Ca to P of HA/TiO2 coated Ti scaffold after soaking into SBF for various days
scaffold display good bone-like apatite forming ability in SBF. Therefore, good bioactivity can be imparted to the Ti scaffold via the HA/TiO2 coatings.
HA-only coatings are bioactive but the adhesive strength between the coating and the substrate might be unsatisfactory. TiO2-only coatings strongly adhere to the substrate but their bioactivity is limited. Therefore, the ideal coating is to combine the advantages of both HA and TiO2: the adhesive strength of TiO2 and the bioactivity of HA. In the present study, HA/TiO2 double coatings are successfully achieved through the sol-gel process. Detailed characterisation indicates that the bone-like apatite layer consisted of nano-crystals of apatite phase that exhibits a defective structure and low crystallinity. These features are very similar to those of the mineral phase of bone. Therefore, osteoblasts can preferentially proliferate on the apatite and differentiate to form an extracellular matrix composed of biological apatite and collagen. The surrounding bone tissues can bond to the bone-like apatite layer directly. Therefore, it can be concluded that the HA/TiO2 coated Ti scaffold exhibited good bioactivity after HA/TiO2 coatings. The mechanism of the bone-like apatite formation can be described as follows. The HA coating layer releases Ca2+, Na+ or K+ ions from its surface via the exchange with the H3O+ ions in the SBF to form the functional Ti-OH groups on their surface. Water molecules in the SBF then react with the Ti-OH groups. The Ti-OH groups induce apatite nucleation, and the released Ca2+, Na+, or K+ ions accelerate apatite nucleation by increasing the ionic activity product of apatite in the SBF. Once the apatite nuclei are formed, these nuclei can grow spontaneously by consuming the calcium and phosphate ions in the surrounding SBF. In the present study, the results indicate that the functional groups of Ti-OH are effective for the bone-like apatite formation.
4 Conclusions
1) Sol-gel derived TiO2 coating is successfully dipping-coated on Ti scaffold samples at a speed of 12 cm/min, followed by a heat treatment at 600 ℃. It is found that the temperature of 600 ℃ for annealing is effective for the TiO2 crystallization into an anatase structure. The HA film is subsequently dipping-coated at the same speed and by a heat treatment at 560 ℃. The results indicate that the HA phase begins to crystallise at 560 ℃ and the crystallinity increases with the increase of the temperature.
2) SEM observations find no delamination or crack at the interfaces of HA/TiO2 and TiO2/Ti. The in vitro assessments are performed by soaking the HA/TiO2 double coated samples into the SBF for various periods. The HA/TiO2 coated Ti scaffolds displayed excellent bone-like apatite forming ability when it is soaked into SBF. Ti scaffolds after HA/TiO2 double coatings can be anticipated as promising implant materials for orthopaedic applications.
References
[1] HENCH L L. Bioceramics: from concept to clinic[J]. J Am Ceram Soc, 1991, 74: 1485-1510.
[2] HUTMACHER D W. Polymeric scaffolds in tissue engineering bone and cartilage[J]. Biomaterials, 2000, 21: 2529-2543.
[3] NIINOMI M. Fatigue performance and cyto-toxicity of low rigidity titanium alloy, Ti-29Nb-13Ta-4.6Zr[J]. Biomaterials, 2003, 24: 2673-2683.
[4] LONG M, RACK H J. Titanium alloys in total joint replacement-a materials science perspective[J]. Biomaterials, 1998, 19: 1621-1639.
[5] BRUNETTEe D M, TENGVALL P, TEXTOR M, THOMSON P. Titanium in Medicine [M]. Heidelberg: Springer-Verlag, 2001.
[6] WEN C E, YAMADA Y, SHIMOJIMA K, CHINO Y, ASAHINA T, MABUCHI M. Processing and mechanical properties of autogenous titanium implant materials[J]. J Mater Sci Mater Med, 2002, 13: 397-401.
[7] SAMUNEVA B, KOZHUKHAROV V, TRAPALIS C H, KRANOLD R. Sol-gel processing of titanium-containing thin coatings[J]. J Mater Sci, 1993, 28: 2353-2360.
[8] KIM H W, KOH Y H, LI L H, LEE S, EE H. Hydroxyapatite coating on titanium substrate with titania buffer layer processed by sol-gel method[J]. Biomaterials, 2004, 25: 2533-2538.
[9] NIINOMI M, AKAHORI T, TAKEUCHI T, KATSURA S. Dental precision casting of Ti-29Nb-13Ta-4.6Zr using calcia mold[J]. Mater Sci Forum, 2005, 475-479: 2303-2308.
[10] YU J G, ZHAO X J, ZHAO Q N. Effect of surface structure on photocatalytic activity of TiO2 thin films prepared by sol-gel method[J]. Thin Solid Films, 2000, 379: 7-14.
[11] HARIZANOV O, HARIZANOVA A. Development and investigation of sol-gel solutions for the formation of TiO2 coatings[J]. Solar Energy Materials & Solar Cells, 2000, 63: 185-195.
[12] MONTENERO A, GNAPPI G, FERRARI F, CESARI M, SALVIOLI E, MATTOGNO L, KACIULIS S, FINI M. Sol-gel derived hydroxyapatite coatings on titanium substrate[J]. J Mater Sci, 2000, 35: 2791-2797.
[13] HSIEH M F, PERNG L H, CHIN T S. Hydroxyapatite coating on Ti6Al4V alloy using a sol-gel derived precursor[J]. Mater Chem Phys, 2002, 74: 245-250.
[14] ISHIZAWA H, OGINO M. Hydrothermal precipitation of hydroxyapatite on anodic titanium oxide films containing Ca and P[J]. J Mater Sci, 1999, 34: 5893-5898.
[15] BERNDT C C, HADDAD G N, FARME A J D, GROSS K A. Spraying for bioceramic applications—a review[J]. Mater Forum, 1990, 14: 161-173.
[16] COTELL C M, CHRISEY D B, GRABOWSKI K S, SPRAGUE J A. Pulsed laser deposition of hydroxyapatite thin films on Ti-6AL-4V[J]. J Appl Biomater, 1992, 3: 87-93.
[17] ZHITOMIRSKY I, OR L G. Electrophoretic deposition of hydroxyapatite[J]. J Mater Sci Mater Med, 1997, 8: 213-219.
[18] LIU D M, YANG Q, TROCZYNSKI T. Sol-gel hydroxyapatite coating on stainless steel substrates[J]. Biomaterials, 2002, 23: 691-698.
[19] WEN C E, YAMADA Y, SHIMOJIMA K, CHINO Y, HOSOKAWA H, MABUCHI M. Novel titanium foam for bone tissue engineering[J]. J Mater Res, 2002,17: 2633-2639.
[20] GU Y W, KHOR K A, CHEANG P. In vitro studies of plasma-sprayed hydroxyapatite / Ti-6Al-4V composite coatings in simulated body fluid (SBF)[J]. Biomaterials, 2003, 24: 1603-1611.
[21] CLERIES L, FERNANDEZ-PRADAS J M, MORENZA J L. Behavior in simulated body fluid of calcium phosphate coatings obtained by laser ablation[J]. Biomaterials, 2000, 21: 1861-1865.
[22] KOIKE M, FUJII H. The corrosion resistance of pure titanium in organic acids[J]. Biomaterials, 2001, 22: 2931-2936.
[23] GROSS K A, BERNDT C C. In vitro testing of plasma-sprayed hydroxyapatite coatings[J]. J Mater Sci Mater Med, 1994, 5: 219-224.
[24] KIM H W, KONG Y M, BAE C J, NO Y J, KIM H E. Sol-gel derived fluor-hydroxyapatite biocoatings on zirconia substrate[J]. Biomaterials, 2004, 25: 2919-2926.
[25] PIVETEAU L D, GASSER B, SCHLAPBACH L. Evaluating mechanical adhesion of sol-gel titanium dioxide coatings containing calcium phosphate for metal implant application[J]. Biomaterials, 2000, 21: 2193-2201.
[26] KOKUBO T, KUSHITANI H, SAKKA S, KITSUGI T, YAMAMURO T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W[J]. J Biomed Mater Res, 1990, 24: 721-734.
Corresponding author: WEN C E; Tel: +6-3-52273354; Fax: +6-3-52273354; E-mail: cwen@deakin.edu.au

