
J. Cent. South Univ. (2018) 25: 1849-1861
DOI: https://doi.org/10.1007/s11771-018-3874-9

Effect of postweld heat treatment on interface microstructure and metallurgical properties of explosively welded bronze–carbon steel
Khanzadeh GharahShiran Mohammad Reza1, Khoshakhlagh Ali2,Khalaj Gholamreza3, Bakhtiari Hamid4, Banihashemi Ali Reza5
1. Center for Advanced Engineering Research, Majlesi Branch, Islamic Azad University, Isfahan, Iran;
2. Young Researchers and Elites Club, Science and Research Branch, Islamic Azad University, Tehran, Iran;
3. Young Researchers and Elites Club, Saveh Branch, Islamic Azad University, Saveh, Iran;
4. Department of Materials Engineering, Najafabad Branch, Islamic Azad University, Najafabad, Iran;
5. Department of Materials Engineering, Shahreza Branch, Islamic Azad University, Shahreza, Iran
 Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Central South University Press and Springer-Verlag GmbH Germany, part of Springer Nature 2018
Abstract: The effects of postweld heat treatment on the microstructure and metallurgical properties of a bronze–carbon steel (st37) explosively bonded interface were studied. Explosive welding was done under 1.5- and 2-mm standoff distances and different conditions of explosive charge. Samples were postweld heat treated for 4 and 16 h in the furnace at 250 °C and 500 °C and then air cooled. Laboratory studies using optical microscopy, scanning electron microscopy, and microhardness testing were used to evaluate the welded samples. Microstructural examinations showed that by increasing the standoff distance and the explosive charge, the interface of bronze to steel became wavier. The microhardness test result showed that the hardness of the samples was higher near the joint interface compared with other areas because of the intensive plastic deformation, which was caused by the explosion force. The results show that increasing the heat treatment temperature and time caused the intermetallic compounds’layer thickness to increase, and, because of the higher diffusion of copper and tin, the iron amount in the intermetallic compounds decreased. Also, because of the increase in heat treatment temperature and time, internal stresses were released, and the interface hardness decreased.
Key words: heat treatment; explosive welding; intermetallic compound; standoff distance; diffusion layer
Cite this article as: Khanzadeh GharahShiran Mohammad Reza, Khoshakhlagh Ali, Khalaj Gholamreza, Bakhtiari Hamid, Banihashemi Ali Reza. Effect of postweld heat treatment on the interface microstructure and metallurgical properties of explosively welded bronze–carbon steel [J]. Journal of Central South University, 2018, 25(8): 1849–1861. DOI: https://doi.org/10.1007/s11771-018-3874-9.
1 Introduction
Explosion welding utilizes the explosion energy to form a metallic bond by electron sharing between two components that are kept at a specified standoff distance. Two welding surfaces approach each other with very high localized pressure when the collision occurs. Because of the collision between surfaces, a local pasty area forms at the interface, which causes the joining [1].
Explosion welding is an advanced solid-state welding process that is used for similar and dissimilar materials and alloys that could not be welded by fusion welding methods due to differences in their liquid points and the formation of harmful intermetallic compounds. The main metallurgical limitation of this process relates to forming joints with good flexibility and enough fracture resistance to endure fast plastic deformation during welding [2–4].
Cladding via explosion welding is making inroads into such industries as shipbuilding, vacuum equipment, aircraft and aerospace industries, automatic industries, and other machinery. The joining of bronze to steel is difficult to perform by fusion welding because of the difference in the component’s temperature, chemical, and physical properties [5, 6].
In the studies performed by SONG et al [7] for cladding of pure titanium on low-carbon steel, KACAR et al [8] for joining 316 L stainless steel to a steel tank (DIN- P355GH), ETTAQI et al [9] for deposition of cobalt-base superalloy on X38CrMoV5 steel, ZHAO et al [10] for joining of Inconel alloy-800 to 304 stainless steel, and AKBARI MOUSAVI [11] for joining of pure titanium to 304 stainless steel, the effects of standoff distance, explosive load, and other parameters of explosion welding have been investigated. They reported a waveform of the formed interface with alternation and fixed wave amplitude, also they also observed stretching of grains around the joint interface, increasing of wavelengths because of the explosive load, and grain refining caused by intensive plastic deformation at the joint interface. Microhardness results showed that the hardness of areas near the interface has increased at the flyer and base plates.
FINDIK et al [12] have studied the effects of temperature and time on the explosion welding of low-carbon steel and austenitic steel 304. Also, these effects have been investigated by ACARER et al [13] for explosion welding of steel on steel, AKBARI MOUSAVI et al [14] for explosion joining of 304 steel and titanium, and SAMARDZIC et al [15] for the threefold joining of AlMg5–Al–St 52-3. Their results indicated that increasing temperature causes more formed intermetallic compounds in the microstructure and that heat treatment after welding causes grain sizes, strength, and ductility to be significantly affected.
PENGSAKUL et al [16] researched the influence of temperature (300 °C) on intermetallic compounds of triple explosion joints of Al 5083–Al 1050–St 52-3N for 5 and 15 min. Their research shows that the amount of intermetallic compounds in Al 5083 increases considerably after the critical condition at 300 °C, but it did not have a significant effect on the amount of intermetallic compounds of Al 1050 and St 52-3N.
TRICARICO et al [17] evaluated the effect of standoff distance and heat treatment on the interface of aluminum and steel intermetallic layers. LOKAJ et al [18] joined aluminum to an austenitic steel by explosion welding and evaluated the heat treatment process. Also, DANESH MANESH et al [19] investigated the effect of annealing heat treatment on mechanical properties of aluminum cladding on steel. BANKER et al [20] also studied the influence of temperature and time variations on mechanical properties of an aluminum–steel joint. They concluded that the intermetallic compounds form or develop gradually with increasing temperature because of diffusion mechanism activation. Intermetallic compounds between aluminum and iron have formed more quickly than aluminum–titanium and aluminum chrome intermetallic compounds. Because of fast intermetallic compounds forming, the tensile strength of the joint decreased.
In the present study, the effects of heat treatment (temperature and time) on the microstructure of intermetallic compounds at the interface and mechanical properties of an explosion-welded joint of bronze and ST37 steel are investigated.
2 Experimental
2.1 Materials
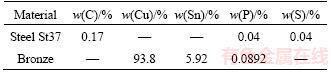
In this study, bronze was used as the flyer plate and ST37 steel as the base plate with the following dimensions: 1) steel base plate: 150 mm×150 mm with a thickness of 4 mm; 2) bronze flyer plate: 150 mm×150 mm with a thickness of 2 mm. The chemical compositions of the utilized alloys are given in Table 1.
Table 1 Chemical compound of bronze and steel
2.2 Selection of welding method
In this investigation, the dissimilar joint of ST37 carbon steel and bronze was explosion welded. Figure 1 shows the primary arrangement of the plates for explosion welding. Flyer and base plates were prepared with dimensions of 150 mm× 150 mm. The explosive material was amatol with a composition of 5 wt% TNT and 95% ammonium nitrate. The detonator was M8 with the detonation velocity of 2507 m/s.
The velocity of the explosive material was measured by a fiberoptic system. The explosion- welding parameters are presented in Table 2. The standoff distance was 1.5 and 2 mm, and the thickness of the explosive material was adjusted to form enough collision kinetic energy.

Figure 1 Initial configuration of plates for explosive welding
Table 2 Characteristics of explosive welding and heat treatment after welding tests

2.3 Postwelding heat treatment
To determine the influence of the heat treatment parameters (temperature and time) on intermetallic compounds and their properties, the welding samples were heat treated at 250 °C and 500 °C for 4 and 16 h according to Table 2. The effects of the standoff distance, thickness of the explosive material, heat treatment temperature, and time on diffusion at the interface were studied.
2.4 Microstructure and metallurgical evaluation of joint interface
2.4.1 Joint interface evaluation by optical microscopy (OM)
Some samples were prepared with dimensions of 10 mm×10 mm×8 mm. For more-accurate dimensions and prevention of warming up during cutting, a wire cut device (Model: GT600 DIN) was used. To study the microstructure of the samples, the prepared samples were sanded by papers of 60 to 3000 grades, and the final polish was performed by alumina solution. After polishing of the samples, the steel sections were etched using nital (2%) solution for 8 s and then washed with alcohol and, finally, dried. The microstructures of the interface and intermetallic compounds were analyzed by the OM (Model: Olympus). Afterward, the bronze sections were polished again and etched using the nitric acid solution (100 mL) and distilled water (100 mL) for 20 s and then washed with alcohol and dried.
2.4.2 Joint microstructure evaluation by scanning electron microscopy (SEM)
In this study, the shape, amount, and kind of intermetallic compounds and cracks around the interfaces were analyzed by SEM (SEM-model: VEGA\\ TESCAN-LMU), which was equipped with an energy dispersive X-ray spectrometry (EDS) analysis system.
2.4.3 Microhardness test
Microhardness tests (Vickers) were performed by a microhardness measurement device (model: KOOPA) with an applied load of 200 g for 20 s. The device measured 8 microhardness traces from different areas of weld and base metal with distances of 50, 100 and 200 μm from the joint interface.
3 Results and discussion
3.1 Joint interface evaluation by OM
3.1.1 Microstructure evaluation of BS1 samples
Figure 2 presents the OM images of BS1 samples taken at the longitudinal direction of the joint in the form of waves. As seen, the joint interface has a wavelike form in this condition. Because of the higher explosive load of this sample compared with BS2, BS3 and BS4 samples, it had a thicker melted layer before heat treatment and thicker diffusion layer after heat treatment (Table 3).

Figure 2 OM images of joint interface for BS1 samples:
Table 3 Thickness changes of local melted layer at different temperatures and time of heat treatment

The studies also showed that the increase of temperature caused an enhancement of the diffusion layer thickness [1, 4, 21]. The flyer plate’s minimum velocity also had the minimum amount of collision kinetic energy, which is essential for joining. As a result of the flyer plate’s collision, the consumable kinetic energy changes to potential energy that finally causes the plastic deformation of collision surface. If the plastic deformation amount is not enough, the short waves form, and the local melted area does not appear. Increasing of collision kinetic energy causes an intensive plastic deformation at beneath and the peak of the wave.
Because of higher collision pressures, vortices can form at the joint interface, and they could create local melted areas in the vicinity of the interface. These areas could be formed by internal heating created by higher pressure from the explosion shock waves and intensive plastic deformation; also, the production of adiabatic heat could result from the sticking vortices ahead of some wave fronts, which causes conversion of kinetic energy to thermal energy during the collision. Also, the adiabatic heat could be produced by trapped gasses between plates. These areas are surrounded by cold metal, and they would be under a high solidification velocity of 105–107 K/s [11].
3.1.2 Microstructure evaluation of BS2 samples
Figure 3 shows the waves formed in the perpendicular direction of the explosion axis. The maximum thickness of the melted layer was 38.28 μm for this sample, which decreased compared with the BS1 samples (4.26 μm). Also, the maximum thickness of the diffusion layer was 51.36 μm, which was less than that of BS1 samples (13.66 μm). The main reason is the decrease of the explosive load amount that causes a reduction of the collision velocity. Other studies also indicated that increasing temperature and time of heat treatment caused the width of the intermetallic compound area to develop [7, 9, 22].
3.1.3 Microstructure evaluation of BS3 samples
According to Figure 4, the thickness of the local melted layer and diffusion layer of the joint interface decreased compared with BS2 samples with constant standoff distance (2 mm) before and after heat treatment. Because of the lower thickness of the explosive charge compared with the BS2 samples, the velocity of the flyer plate decreased and lower plastic deformation occurred at the joint interface [23]. Before heat treatment, the collision kinetic energy also decreased, and the shape of the interface was not greatly similar to the vortex compared with BS2 samples [8].
3.1.4 Microstructure evaluation of BS4 samples
The joint interface of the BS4 samples is shown in Figure 5. The results demonstrated that the decrease of the explosive load and velocity of the flyer plate caused a reduction of the collision kinetic energy, so the required energy for diffusion at the interface decreased, leading to a reduction of the local melted layer thickness before heat treatment and for the diffusion layer after heat treatment.
Table 3 shows the thickness changes of the local melted layer before heat treatment and the diffusion layer after heat treatment. The increase in the local melted layer and diffusion layer thickness resulted from the temperature and time of the heat treatment, which simplified the diffusion condition, and the increase of explosive material thickness and standoff distance caused by the enhancement of collision velocity and transferred kinetic energy to the interface, which plays the role of the diffusion motivation energy during heat treatment and causes the increase of diffusion layer thickness at interface. These results were obtained before in other investigations [7, 10].

Figure 3 OM images of joint interface for BS2 samples:

Figure 4 OM images of joint interface for BS3 samples:

Figure 5 OM images of joint interface for BS4 samples:
3.2 Microstructure evaluation of samples by SEM
3.2.1 Microstructure evaluation of BS4 samples
Figure 6 shows the SEM images of the interface and the melted layer of the BS4 samples with a standoff distance of 1.5 mm and an explosive charge of 20 mm (thickness); EDS analysis of the local melted layers and diffusion layers of the interface are presented in Figure 7. Due to the lower standoff distance and the explosive load of this sample, the thickness of the melted layer decreased. The reason for the higher copper amount (at%) was related to the high diffusion coefficient of copper. Because of the changes in collision angle during explosion welding and collision velocity variations, the shape of the waves changed along the joint interface; the interface energy differed for different vicinities. Because of these changes, the energies of alloying elements varied after postweld heat treatment, which acted as the diffusion motivation energy. Increasing the temperature and time of the heat treatment enhanced the amounts of copper or iron; this depended on the amount of collision energy change along the interface.
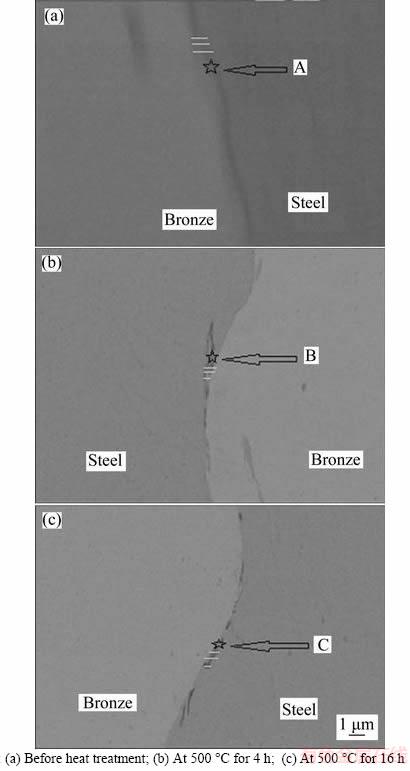
Figure 6 SEM images of local melted layer variations and specified diffusion layer at interfaces of BS4 samples:

Figure 7 EDS analysis of specified local melted layer areas and diffusion layer of BS4 sample:
3.2.2 Microstructure evaluation of BS3 samples
The thickness of the melted layer before heat treatment and the diffusion layer after heat treatment for BS3 samples increased compared with the BS4 samples. As shown in Figure 8(a) for the BS3 samples before heat treatment, the thickness of the local melted layer decreased compared with the diffusion layer after heat treatment at 500 °C for 16 h (point A, Figure 8(c)). The reason for this was the effect of heat treatment and diffusion of the alloying elements compared with the pre-heat treatment, which also increased the thickness of the diffusive layers. An EDS analysis of the local melted layers and diffusion layers of the interface is presented in Figure 9. The EDS results show that the melted layer chemical composition at point A before heat treatment (Figure 9(a)) included 64.44 at% of Fe, 34.42 at% of Cu, and 1.14 at% of Sn; the main reason for this composition was related to forming the local melted layer area and combining the base and flyer plates because of the rotation of the trapped jet at the joint interface. As the heat treatment time increased due to the reduced heat conduction of steel (ST37), the percentage of the iron element slightly decreased, and the percentage of copper increased considerably. The available copper at the joint copper part penetrated to the interface. The diffusion activation energy increased because of the temperature rise; therefore, the width of the reactant was enlarged. Diffusion layer variations depend on several major elements—diffusion, residual stresses of welding that cause strain at the diffusion area, the formation of strains due to the physical incompatibility of material during heat treatment, and the softening of the base metal matrix caused by the complete destruction of structural defects [24].

Figure 8 SEM images of local melted layer variations and specified diffusion layer at interfaces of BS3 samples:
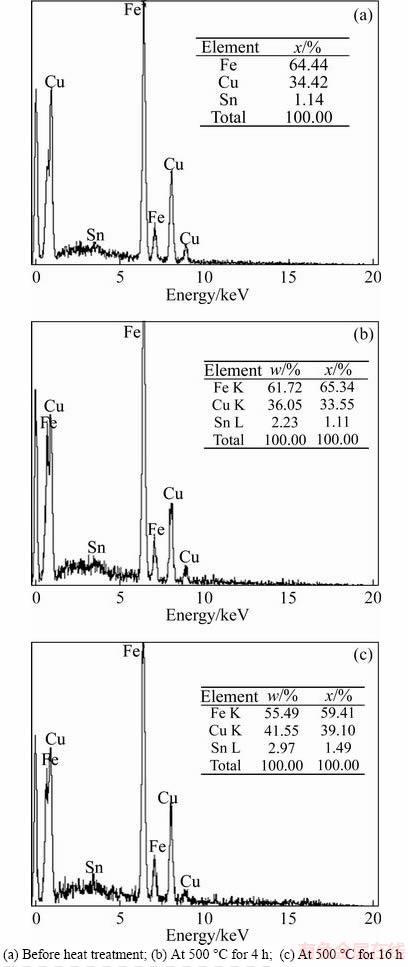
Figure 9 EDS analysis of specified local melted layer areas and diffusion layer of BS3 sample:
3.2.3 Microstructure evaluation of BS2 samples
Figure 10 shows the SEM images of the local melted layer and diffusion layer of BS2 samples with a standoff distance of 2 mm and an explosive charge of 30 mm (thickness). The reason for the thickness enhancement was related to increasing the explosive load and the collision kinetic energy at the interface. EDS analysis of point A is given in Figure 11, and the SEM image of the specified area is shown in Figure 10. According to results, the composition of the local melted layer at point A before heat treatment included 31.25 at% of Fe, 66.88 at% of Cu, and 1.87 at% of Sn (Figure 11(a)). For the chemical composition of the diffusion layer after heat treatment at 500 °C for 4 h at point A (shown in Figure 11(b)), the atomic percentages of Fe, Cu, and Sn were 27.56 at%, 71.19 at% and 1.25 at%, respectively. For the chemical composition of the diffusion layer after heat treatment at 500 °C for 16 h at point A (shown in Figure 11(c)), the atomic percentages of Fe, Cu, and Sn were 53.42 at%, 45.87 at% and 0.7 at%, respectively. Comparing the local melted layer compositions before heat treatment and after heat treatment at 500 °C for 4 h (Figures 11(b) and (c)) indicates that the Sn amount decreased from 1.87 at% to 1.25 at%, the Cu amount increased from 66.88 at% to 71.19 at%, and the Fe amount decreased from 31.25 at% to 27.56 at%. Comparing the compositions before heat treatment and after that for 16 h demonstrated that the amount of Sn decreased from 1.87 at% to 0.7 at%, the Cu amount decreased from 66.88 at% to 45.87 at%,and the Fe amount increased from 31.25 at% to 53.42 at%.

Figure 10 SEM images of local melted layer variations and specified diffusion layer at interfaces of BS2 samples:
3.2.4 Microstructure evaluation of BS1 samples
Figure 12 shows the SEM images of the local melted layer and diffusion layer of the BS1 samples. The reason for the thickness enhancement was the increase of the explosive load and more collision kinetic energy at the interface, as shown in Figure 12. Because the copper diffusion coefficient is higher than that of steel and there is more thermal conductivity, higher amount of copper is present at the diffusion layer. The diffusion layer composition of the samples heat treated for 16 h at the interface and point A (shown in Figure 13) showed that the atomic percentages of Fe, Cu, and Sn were 60.86 at%, 38.25 at% and 0.89 at% respectively; the amount of Fe increased, and the Sn and Cu atomic percentages decreased.

Figure 11 EDS analysis of specified local melted layer areas and diffusion layer of BS2 sample:
3.3 Mechanical properties evaluation
3.3.1 Microhardness test
In explosion welding, the flyer and base plates are exposed to an intense stress wave because of the material explosion. These intense stresses cause some changes in metallurgical properties and lead to increasing microhardness. Microhardness is a function of chemical compositions, percentage of alloying elements, intermetallic compounds, heat changes, explosive load, and standoff distance [13, 18].

Figure 12 SEM images of local melted layer variations and specified diffusion layer at interfaces of BS1 samples:
According to the results, the shock hardening would happen because of the explosion waves and microhardness increases when approaching the joint interface. The reason for this increase is the explosive load increase, which causes an enhancement of the flyer plate velocity and dynamic angle of collision for the samples with higher standoff distance. Because of the increasing standoff distance, the kinetic energy grows, an intense plastic deformation occurs at the joint interface, and hardening happens due to explosion waves. Increasing interface microhardness caused by raising the standoff distance has been reported [4, 10].

Figure 13 EDS analysis of specified local melted layer areas and diffusion layer of BS1 sample:
Tables 4 and 5 present the bronze and steel microhardness variations before and after heat treatment at a distance of 50 μm from the joint interface.
In the areas close to the joint interface, the amount of microhardness was high. By increasing the distance from the interface, the hardness was reduced. Because of the explosive force at the collision area, the flyer plate (bronze) and base metal (ST37 steel) collided intensely, causing plastic deformation of the flyer and base metal at the areas close to the interface. Plastic deformation causes work-hardening, and the amount of hardness increases at these areas; the increase of the microhardness of steel was more than that for bronze. The cause of the hardness reduction for bronze was related to the stress relief formed due to collision stresses after heat treatment; further increases of temperature and time caused further reduction of microhardness. However, for the heat-treated sample at 250 °C for 4 h, the hardness amount increased from two sides compared with those before heat treatment, which were HV 237 for the bronze part and HV 181 at the steel area. The reason for this increase was dislocation movement formed during the passing of the explosion shock at the material; because of the temperature increase, the possibility of forming new dislocation locks is enhanced, and the microhardness amount also increases.
Table 4 Microhardness (Vickers) variations of steel before and after heat treatment

Table 5 Microhardness (Vickers) variations of bronze before and after heat treatment

4 Conclusions
In this study, the influence of heat treatment on the microstructure and microhardness of explosion- welded ST37 steel sheets and bronze was studied, and the following results were obtained.
1) The increase of standoff distance and explosive material thickness caused the formation of a vortex-shaped joint interface, and local melted areas were created due to the enhancement of the collision pressure at the vicinity of the interface vortex waves. The composition of these areas was a mix of the alloying elements of the flyer and base plates, which combined due to the rotation of the jet.
2) Because of internal stress relief, the hardness of bronze decreased after heat treatment; the highest reduction occurred at a distance of 50 μm from the interface (from HV180 to HV86), which was related to the sample that was heat treated at 500 °C for 16 h and had a standoff distance of 2 mm and explosive charge of 30 mm.
3) For the samples heat treated at high temperatures for longer time, significant diffusion happened at the interface, and the thickness of the intermetallic compounds increased subsequently.
4) Because of the dynamic nature of the collision and collision kinetic energy variations, the compositions of the diffusion layers differed along the interface.
5) When the heat treatment time was increased from 4 to 16 h at 500 °C, the intermetallic compound layer thickness reached 30 to 65 μm because of the higher diffusion of Cu and Sn and the reduction of the Fe amount in the intermetallic compounds.
6) The increase of the temperature and time of the heat treatment caused less hardness near the interface area.
7) Heat treatment of the samples caused the enhancement of the intermetallic compound layer thickness at a distance of 50 μm from two sides of the interface, and the hardness amount decreased.
References
[1] Temizel G. Intermetallic phase formation at Fe-Al film interfaces [J]. Environmental Science, 2007, 31: 71–78.
[2] Zlobin B S. Explosion welding of steel with aluminum [J]. Materials and Design, 2003, 24: 617–622.
[3] Tricarico L, Spina R, Sorgente D, Brandizzi M. Effects of heat treatments on mechanical properties of Fe/Al explosion welded [J]. Materials and Design, 2009, 30: 693–700.
[4] Nobili A. Explosion bonding process [M]. France: Risvsaltes Plant, 1999.
[5] Cowan G R, Holtzman A H. Flow configuration in colliding plate [J]. Explosive Bonding Journal of Applied Physics, 1963, 34: 928–939.
[6] Shtertser A A, Zlobin B S. Flows, strains, and the formation of joints in oblique collision of metal plates [J]. Journal of Applied Mechanics and Technical Physics, 2015, 56: 927–935.
[7] Song J, Kostka A, Veehmayer M, Raabe D. Hierarchical microstructure of explosive joints: Example of titanium to steel cladding [J]. Materials Science and Engineering A, 2011, 528: 2641–2647.
[8] Kacar R, Acarer M. An investigation on the explosive cladding of 316L stainless steel-din-P355GH steel [J]. Materials Processing Technology, 2009, 153: 91–96.
[9] Ettaqi S, Langlois L, Bigot R. Cobalt-based super alloy layers deposited on X38CrMoV5 steel base metal by explosion cladding process [J]. Surface Coatings and Technology, 2008, 202: 3306–3315.
[10] Zhao H, LI P, Zhou Y, Huang Z, Wang H. Study on the technology of explosive welding Incoloy800- SS304 [J]. Materials Engineering and Performance, 2010, 20: 911–917.
[11] Akbari Mousavi S A A, Farhadi Sartangi P. Experimental investigation of explosive welding of cp-titanium/AISI 304 stainless steel [J]. Materials and Design, 2009, 30: 459–468.
[12] Findik F, Yilmaz R, Somyurek T. The effects of heat treatment on the microstructure and microhardness of explosive welding [J]. Scientific Research and Essays, 2011, 6: 4141–4151.
[13] Acarer M, Gulenc B, Findik F. Investigation of explosive welding parameters and their effects on microhardness and shear strength [J]. Materials and Design, 2003, 24: 659–664.
[14] Akbari Mousavi S A A, farhadi sartangi P. Effect of post-weld heat treatment on the interface microstructure of explosively welded titanium-stainless steel composite [J]. Materials Science and Engineering A, 2008, 494: 329–336.
[15] Samardzic I, Matesa B, Kladaric I. The influence of heat treatment on properties of three-metal explosion joint: AlMg-Al-steel [J]. Metalurgija, 2011, 50: 159–162.
[16] Phengsakul S, Rodchanarowan A. Effect of thermal treatment on intermetallic phases of Fe/Al structural transition joints [J]. Energy and Materials Science and Engineering, 2013, 34: 782–790.
[17] Tricarico L, Spina R. Mechanical strength of Fe/Al structural transition joints subject to thermal loading [J]. Archives of Materials Science and Engineering, 2009, 37: 85–93.
[18] Lokaj J, Benak m. X ray microanalysis of Al-austenitic steel boundary formed by explosion welding [J]. Metal, 2010, 5: 18–20.
[19] Danesh Manesh H, Karimi Taheri A. The effect of annealing treatment on mechanical properties of aluminum clad steel sheet [J]. Materials and Design, 2003, 24: 617–622.
[20] Banker J, NOBILI A. Aluminum-steel electric transition joints, effects of temperature and time upon mechanical properties [C]// Light Metals: Proceedings of Sessions, TMS 131st Annual Meeting. Warrendale, Pennsylvania, 2002: 439–445.
[21] Acarer M, Gulenc B, Findik F. Study of some welding parameters of explosively joined steel parts [C]// Proceedings of the 8th Denizli Materials Symposium. Denizli, Turkey, 2000.
[22] Shiran M K G, Bakhtiari H, Akbari Mousavi S A, Khalaj G, Mirhashemi S M. Effect of stand-off distance on the mechanical and metallurgical properties of explosively bonded 321 austenitic stainless steel-1230 aluminum alloy tubes [J]. Materials Research, 2017, 20(2): 291–302.
[23] Shiran M K G, Khalaj G, Pouraliakbar H, Jandaghi M, Bakhtiari H, Shirazi M. The effect of heat treatment on the intermetallic compounds and mechanical properties of the explosive welding interface of stainless steel 321 to aluminum 1230 [J]. International Journal of Minerals, Metallurgy, and Materials, 2017, 24(11): 1267–1277.
[24] Yan Y B, Zhang Z W, Shen W, Wang J H, Zhang L K, Chin B A. Microstructure and properties of magnesium AZ31B–aluminum 7075 explosively welded composite plate [J]. Materials Science and Engineering A, 2010, 527: 2241– 2245.
(Edited by YANG Hua)
中文导读
焊后热处理对爆炸焊接青铜–碳钢界面组织和冶金性能的影响
摘要:研究了焊后热处理对青铜–碳钢爆炸结合界面组织和冶金性能的影响。在不同装药条件下,分别在1.5 mm和2 mm距离下进行了爆炸焊接。在250 °C和500 °C的炉中进行4和16 h的焊后热处理,再进行风冷处理。采用光学显微镜、扫描电子显微镜和显微硬度测试对焊接试样进行了实验研究。显微组织测试表明,随着距离的增加和装药量的增加,青铜与钢的界面逐渐变薄。显微硬度测试结果表明,由于爆炸力引起的强烈塑性变形,接头界面附近的硬度较高。结果表明,随着热处理温度的升高和热处理时间的延长,金属间化合物的层厚增加,金属间化合物中的铁含量降低,铜和锡的扩散越快,金属间化合物中的铁含量越低。同时,由于热处理温度的升高和热处理时间的延长,内部应力释放,界面硬度降低。
关键词:热处理;爆炸焊接;金属间化合物;凝固距离;扩散层
Received date: 2017-03-20; Accepted date: 2017-10-01
Corresponding author: KHALAJ Gholamreza, PhD, Assistant Professor; Tel: +98-8642433342; E-mail: gh.khalaj@ziau.ac.ir; ORCID: 0000-0001-8510-4981