机械活化黄铜矿的团聚-集聚及浸出性能
来源期刊:中国有色金属学报(英文版)2021年第5期
论文作者:赵素兴 王改荣 杨洪英 陈国宝 丘学民
文章页码:1465 - 1474
关键词:黄铜矿;机械活化;团聚;集聚;浸出
Key words:chalcopyrite; mechanical activation; agglomeration; aggregation; leaching
摘 要:研究机械活化对黄铜矿粒度参数、显微组织和浸出性能的影响,并探讨活化过程中团聚和集聚的发生和转变。结果表明,研磨8 h前黄铜矿的团聚与显微组织变化互不影响;然而,研磨8 h后晶粒尺寸的降低达到极限,显微组织变化停滞,使得团聚被集聚取代。浸出实验结果表明,机械活化能显著提高黄铜矿的浸出性能,而集聚对浸出的阻碍作用远强于团聚。经4 h酸浸实验后,活化8 h的矿样获得最高铜浸出率,其值为80.13%。
Abstract: The effect of mechanical activation on the granulometric parameters, microstructure, and leaching efficiency of chalcopyrite was evaluated, and the occurrence/transition of agglomeration and aggregation was discussed. The results showed that in 8 h of milling treatment, the agglomeration and the microstructure did not affect each other. However, with prolonging milling time, the crystallite size tended to reach a saturation value, and the stagnating microstructural changes led to the replacement of agglomeration by aggregation. The leaching results indicated that the mechanical activation can strongly enhance the reactivity of chalcopyrite and the hindering effect of aggregation on leaching was considerably greater than that of agglomeration. Consequently, after 8 h of milling, the maximum Cu leaching rate of 80.13% was achieved after 4 h of acid leaching.
Trans. Nonferrous Met. Soc. China 31(2021) 1465-1474
Su-xing ZHAO, Gai-rong WANG, Hong-ying YANG, Guo-bao CHEN, Xue-min QIU
School of Metallurgy, Northeastern University, Shenyang 110819, China
Received 28 May 2020; accepted 24 December 2020
Abstract: The effect of mechanical activation on the granulometric parameters, microstructure, and leaching efficiency of chalcopyrite was evaluated, and the occurrence/transition of agglomeration and aggregation was discussed. The results showed that in 8 h of milling treatment, the agglomeration and the microstructure did not affect each other. However, with prolonging milling time, the crystallite size tended to reach a saturation value, and the stagnating microstructural changes led to the replacement of agglomeration by aggregation. The leaching results indicated that the mechanical activation can strongly enhance the reactivity of chalcopyrite and the hindering effect of aggregation on leaching was considerably greater than that of agglomeration. Consequently, after 8 h of milling, the maximum Cu leaching rate of 80.13% was achieved after 4 h of acid leaching.
Key words: chalcopyrite; mechanical activation; agglomeration; aggregation; leaching
1 Introduction
Chalcopyrite is the most abundant copper resources, which is mainly treated by pyro-metallurgical methods. With the reduction of high-grade copper resources and increased environmental regulation, the hydrometallurgical approaches become more important due to their economic and environmental advantages, such as the low-cost of processing, and the absence of harmful gas emissions [1]. However, the chalcopyrite leaching performance is negatively affected by the formation of surface passive layers [2] containing compounds such as polysulfides, elemental sulfur allotropes and jarosite [3], limiting the application of hydro- metallurgical treatments. Many attempts have been made to solve this problem including the use of non-sulfate oxidants [4], bioleaching [5] and catalysts [6]. However, these methods still have the drawbacks of long leaching time, high-cost and/or low-leaching rates.
The fine milling is an effective method to promote leaching processes [7]. Finely milled chalcopyrite particles (<20 μm), however, are known to become passivated shortly after the initiation of the leaching process [8]. It should also be mentioned that the mechanical activation (MA) caused by the high-energy ball milling process often changes the microstructure and surface properties of the ball-milled materials [9]. MA can promote the chalcopyrite leaching performance [7,10], thus providing an efficient and low-cost alternative for traditional pressure leaching methods [11].
It is worth mentioning that the effect of MA on chalcopyrite leaching can be explained based on the increase of the specific surface area [12], distortion of the crystal structure [13], the surface oxidation [14], and the increase of amorphization degree [15]. On the other hand, the leaching performance is known to decrease for prolonged-milled samples, which is attributed to the occurrence of agglomeration in the milled samples [16].
JUHASZ and OPOCZKY [17] suggested distinguished three stages of interaction between the particles in fine milling, namely, adherence, aggregation, and agglomeration. They also reported that the MA and microstructural changes only occur at the last agglomeration stage. It should be considered that with the development of milling technologies, MA could take place within a few minutes of milling. In this case, the aggregation and agglomeration are frequently interchanged, and therefore, the three-stage mechanism mentioned above is not clearly distinguishable.
Agglomeration can be defined as the clustering of ground particles, which are held together by physical interactions in order to reduce their surface free energy. The bonding of agglomerates is weak and can usually be readily broken [18,19], which makes it difficult for agglomeration to dramatically affect the reactivity of materials [20]. In contrast, the aggregation is defined as the clustering of particles interconnected by chemical bonds. This interparticle bonding is strong, and provides a barrier for the accessibility of the internal surfaces for the leaching agents [18], which may seriously counteract the positive influence of MA on leaching.
In this study, the MA of chalcopyrite concentrate using a planetary ball mill, and its effects on the subsequent leaching process were reported. Particular emphasis was put on the distinguishment of agglomeration and aggregation by the analysis of granulometric parameters and microstructure. Meanwhile, the effects of agglomeration and aggregation on the reactivity of chalcopyrite were also investigated, and a considerable leaching rate was obtained.
2 Experimental
2.1 Materials
The chalcopyrite concentrate used in this study was obtained from Shenyang, China. The chemical composition of the sample was measured by XRF (ZSX100e, Japan) to be 28.4 wt.% Cu, 30.0 wt.% Fe, 35.7 wt.% S, and 4.3 wt.% Si.
2.2 Experimental methods
A laboratory high energy ball milling equipment (QM-3SP2 planetary mill, China) was used in this study for MA. Four 100 mL jars of ZrO2, charged with 4 and 12 ZrO2 balls with diameters of 20 and 5 mm, respectively, were used as the milling medium [21,22]. The ball-to-powder mass ratio was constant at 20:1 and the milling speed was kept at 400 r/min. The rotation direction was reversed every 15 min for different milling periods from 10 min to 10 h. The milling was operated at ambient temperature. After milling, the received samples were washed, filtered, dried, sealed in plastic bags, and stored at -37 °C.
2.3 Analytical methods
The particle size distribution (PSD) of non- activated and activated samples was measured by a laser particle size analyzer (Malvern Instruments Mastersizer 3000). The uniformity (U), span (S), and specific granulometric surface area (SG) were calculated according to the following formulas [23]:
 (1)
(1)
 (2)
(2)
 (3)
(3)
where xi and Di are the volume fraction and mean diameter in class i, respectively, D10, D50, and D90 are related to the particle sizes below which 10%, 50%, and 90% of particles exist, ρ is the density of particles, and D3,2 is the surface area moment mean diameter.
The specific surface areas of the initial and milled minerals were measured by the BET method (N2 adsorption) using a surface area and porosity analyzer (ASAP 2460, Micromeritics, USA). The micromorphology was evaluated by SEM (SIGMA 300, ZEISS, Germany). The mineral phases were analyzed by X-ray diffractometer (XRD) (XRD-7000, Shimadzu, Japan) with Cu Kα radiation (λ=0.154 nm). The XRD patterns of the samples were recorded using a step size of 0.0200° and a counting time of 30 s/step at 40 mV and 30 mA. The XPowder 12 software was used for qualitative and quantitative characterization such as peak search and phase identification. The amorphization degree of the samples during MA was calculated according to the following equation [24]:
 (4)
(4)
where A is the amorphization degree and X is the degree of crystallinity, U0 and UX are the background intensities of non-activated and activated samples, while I0 and IX are the integral intensities of non-activated and activated samples, respectively.
2.4 Acid leaching
The leaching experiments were conducted at 80 °C in a 250 mL baker containing 200 mL of leaching solution (0.5 mol/L H2SO4 + 0.5 mol/L NaCl) and 1 g of the chalcopyrite sample. The stirring speed was set at 200 r/min [25]. The Cu2+ concentration in solutions was measured by the atomic absorption spectroscopy (AAS, Varian 220Z, Australia). The leaching rate of copper  was obtained using the following equation:
was obtained using the following equation:
 (5)
(5)
where ρ1 is the Cu2+ concentration in leaching solution (g/L), V is the volume of leaching solution (L), x is the copper content in the chalcopyrite concentrate (wt.%), and m is the mass of concentrate used in each leaching experiment (g).
3 Results and discussion
3.1 Particle size and surface area
Figure 1 shows the PSD of non-activated and activated samples as a function of the milling time, and the corresponding changes of granulometric parameters such as D10, D50, D90, D3,2, D4,3 (volume moment mean diameter), U, and S are shown in Table 1.
It can be found from Fig. 1 and Table 1 that, the particle sizes of the activated samples decrease rapidly after 10 min of MA. Also, the reductions in the uniformity (U) and span (S) observed in Table 1 suggest that an overall comminution has occurred in the first 10 min. In this period, the particle size distribution is characterized as strong unimodal with gentle left slope and sharp right slope, showing that the fracture mechanism is shattering [26].

Fig. 1 Particle size distributions of chalcopyrite samples milled for different periods
Table 1 Particle size distribution and surface area of non-activated and activated chalcopyrite samples

After 15 min of MA, the left half of the PSD curve and D10 change slightly, while the right half of the PSD curve moves toward left and the other parameters decrease, indicating the continuous comminution of the sample. However, a little tail appears in the rightmost curve, and the maximum particle size increases from 58.90 to 76.00 μm, indicating the occurrence of agglomera- tion and the first apparent grinding limit [27]. With increasing the MA time to 60 min (1 h), the little tail is replaced by a new peak, leading to the transformation of unimodal distribution into a bimodal one. However, the D values in Table 1 keep decreasing, especially for the D90. It should be noticed that the effect of dry milling on PSD includes two elementary counteracting processes, namely breakage and agglomeration [28], and the relative proportions change with milling time. With 240 min (4 h) of milling, the bimodal distribution becomes more obvious. The main peak representing fine particles shifts towards left and the tail peak representing agglomerated particles keeps increasing, indicating that the proportions of fine and agglomerated particles increase simultaneously with prolonging time [29]. During 240-480 min (4-8 h) of milling, the main peak stops shifting and its height declines, while the peak of agglomerated particles heightens slowly. Meanwhile, the stable D values indicate that the milling enters a state of dynamic equilibrium [18].
After 10 h of milling, the existence of breakage is still manifested by the decreased D10. Meanwhile, the PSD curve extends towards the right and the bimodal distribution transforms into a trimodal one. By this time, D50, D90, D4,3 (Table 1), and the maximum particle size (Figs. 1(g, h)) increase from 3.60, 31.60, 10.80, and 86.40 μm (8 h) to 5.60, 52.40, 17.30, and 144.10 μm, respectively. The dramatic increase in PSD is drastically different from that of the dynamic equilibrium in the agglomeration state, which may be due to the occurrence of aggregation.
For a better distinction of the agglomeration and aggregation, the specific surface areas at different milling time were measured, and results are shown in Fig. 2. As can be observed, both SG and BET surface area (SBET) dramatically increase after milling, because the comminution intrinsically increases the surface area. When comparing the results obtained up to 8 h, it is obvious that the SBET slowly increases while SG reaches the maximum at milling time of 2 h and then levels off. Additionally, the SBET becomes much larger than SG and their changing trends are different, which is the typical phenomenon of particle agglomeration [30]. We need to emphasize that the agglomerates act as individual particles in the dynamic light scattering, while in the BET method, the nitrogen could access most of the inner surfaces of agglomerates, thus providing a higher value of surface area [31].

Fig. 2 Variation of specific surface area of non-activated and activated chalcopyrite samples at various milling time
The SBET increases slowly to the maximum value in the first 8 h. This changing trend is in close agreement with previous studies on chalcopyrite [20]. However, both SG and SBET decrease at prolonged milling time of 10 h, which is consistent with the change of PSD. When the aggregation happens, an increase in D3,2 (see Table 1) can cause a decrease in SG, according to Eq. (3). Meanwhile, the particles are tightly compressed after aggregation, leading to a decrease of porosity. Thus, the nitrogen could not penetrate to the inner surfaces, resulting in a decrease of SBET [18].
3.2 SEM images
Figure 3 presents the SEM images of non- activated and activated chalcopyrite concentrates. As seen in Fig. 3(a), the non-activated sample has the characters of unequal size, irregular shape, and smooth surface. Figure 3(b) indicates that after milling for 4 h, large flaky particles become rough with small particles sticking on the surface, which display the agglomeration. Meanwhile, in large particles, slightly layered structures could be found, transforming from the bulk structure. This can be explained by the brittle-ductile transition in MA process. The chalcopyrite particles approaching the grinding limit (proved by PSD analysis) undergo plastic deformation instead of fracture, and the brittle-ductile transition occurs. In this way, the input energy can be stored [32], and then the ductile chalcopyrite particles repeat flattening, cold welding, fracturing, and rewelding [33], leading to the formation of the layered structure. When the milling time increases to 8 h, the surfaces of particles remain rough, while the layered structure becomes much more obvious (Fig. 3(c)). Figure 3(d) illustrates that the surface is slightly smooth after 10 h of milling and the joints between layers are inconspicuous, which forms a sharp contrast with different stacking directions displayed in Fig. 3(c). Consequently, the layer-packed particles are tightly compressed, indicating the occurrence of aggregation instead of agglomeration after long time milling, which agrees with previous observations [15]. At a higher magnification, fine particles are necked together (Fig. 3(e)), showing obvious sintering effect and local heating in the planetary ball mill.

Fig. 3 SEM images of chalcopyrite samples milled for different periods
3.3 XRD patterns
XRD analysis was carried out to study the microstructural changes of chalcopyrite during agglomeration and aggregation. Figure 4 shows the XRD patterns of samples milled up to 10 h. As can be seen, the main phases of the non-activated sample are chalcopyrite, pyrite, and quartz. The comparison of non-activated and activated samples shows that as the milling goes on, the chalcopyrite peaks broaden and the intensities of peaks gradually decline. Furthermore, some adjacent peaks broaden to overlap, such as peaks (220) with (204) and (312) with (116). This may be due to the microstructural changes such as the lattice strain, and the decrease in crystallite size [34]. Also, some weak peaks with low intensities become invisible, such as (400) and (008), indicating the selective rupture of the lattice through these planes, and the amorphization of the well-crystallized chalco- pyrite [35]. However, there is neither a new peak nor a shifted peak, implying no phase transformation or new phase formation. Moreover, the changes of quartz and pyrite peaks are much less than that of chalcopyrite, which is due to the lower hardness of chalcopyrite (3.5-4 on Mohs scale), whilst the hardness of pyrite (6) and quartz (7) is much higher, and the microstructural change in softer phase is preferred during milling [18].

Fig. 4 XRD patterns of non-activated and activated chalcopyrite samples at various milling time
The rising of the background intensity with prolonging milling time manifests the reduction of the degree of crystallinity. Usually, the amorphization degree (A) and the full width at half maximum (FWHM) were used to measure the effect of MA. In this work, the A (Eq. (4)) and FWHM were investigated using the main peak (112), and their variations versus milling time are shown in Fig. 5. The results illustrate that the increase of A is substantial in the first 3 h, while the increasing rate diminishes gradually to 8 h and then levels off till 10 h. Besides, the FWHM increases with prolonging milling time, corresponding to the induced lattice strain and the decrease of crystallite size. The FWHM changes little after 8 h, indicating the stability of crystallite size and lattice strain.
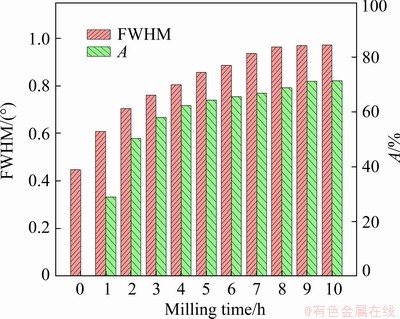
Fig. 5 Amorphization degree (A) and FWHM of non-activated and activated chalcopyrite samples at various milling time
Combined with the PSD and SEM analysis, it can be seen that in 8 h of milling treatment, the agglomeration and the microstructure do not affect each other. Because the agglomeration does not involve the micro-level, it is only the result of particles gathering together to reduce their surface free energy. While after milling for 8 h the crystallite size reaches a saturation value and the change of microstructure is stagnating [36]. To store the input energy, the chalcopyrite particles are sintered and tightly compressed [37], and then the aggregation occurs.
4 Leaching results
The effect of MA on the leaching rate was evaluated, and the results are shown in Fig. 6. In this figure, the leaching rate is plotted against the milling time for the non-activated and activated samples. It is observed that in the first 0.5 h, the similar leaching speed of activated samples is much faster than that of the non-activated sample, which can mainly be explained due to the dissolution of surface oxidation products [35]. Both the leaching speed and the Cu leaching rate increase with the increase of milling time, indicating that the reactivity of chalcopyrite is enhanced by MA. After milling for 8 h, the highest Cu leaching rate of 80.13% is achieved, which decreases to 58.42% with 10 h of milling, due to the occurrence of aggregation.
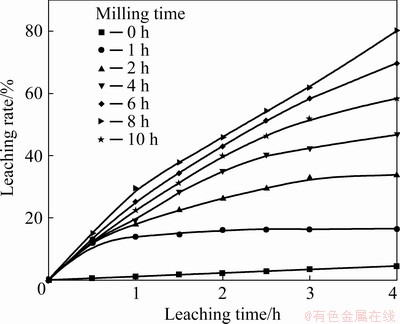
Fig. 6 Leaching rate of Cu as function of milling time
To examine the kinetics of the Cu leaching, a special case of the Erofejev–Kolmogorov equation was selected to fit the leaching data [38]. The equation is expressed as
 (6)
(6)
where t is the leaching time, and k is an empirical rate constant. This equation is a purely empirical equation without assumptions about the mechanism of the leaching reaction, which is often used for the description of solid-liquid reactions.
The comprehensive evaluation of MA properties on the leaching reaction rate constant is shown in Fig. 7. The relationship between leaching rate constant and the milling time and the violation degree of the structure (SBET/X) [39] is shown in Figs. 7(a) and (b), respectively. Besides, the rate constant is divided by the surface area, and plotted against the milling time (Fig. 7(c)) [40]. As can be seen from Fig. 7(a), although the agglomeration occurs in the first 8 h of milling, the leaching rate constant still increases with the extension of milling time, indicating that the hindering effect of agglomeration on leaching is not important. While after milling for 8 h, the leaching rate constant decreases significantly due to the occurrence of aggregation. From Fig. 7(b), it can be concluded that the promoting effect brought about by the increased amorphization degree on k is equal to that of the BET surface area, indicating the structural sensitivity of the reaction [41].

Fig. 7 Effect of MA on leaching of chalcopyrite samples
However, the specific surface area decreases dramatically after the occurrence of aggregation after 10 h milling, and then the Cu leaching rate decreases with the slight increase of the amorphization degree. The value of k/SBET increases in the first 8 h of milling, as shown in Fig. 7(c), indicating the overlapping effect of the structural imperfection as a result of MA [40]. Also, the value of k/SBET decreases with prolonging milling time to 10 h, indicating that the effective surface area in leaching is less than the BET surface area, and the leaching is limited seriously. The reasons for this observation include three aspects: (1) the structure of aggregated particles is compact, therefore, the inaccessibility of the internal surface of the aggregates decreases the solid-liquid contact surface in the leaching process; (2) the microtopography-enhanced dissolution decreases due to the flat surface of large aggregate particles [42]; (3) the aggregated particles are much more stable than the agglomerated ones, and these particles could not be dispersed during the leaching, sharply decreasing the reactivity. It can be concluded that the hindering effect of aggregation on the leaching process is considerably greater than that of agglomeration.
5 Conclusions
(1) The PSD transformation of chalcopyrite in MA can be distinguished as three stages: quick comminution (0 to 15 min), dynamic process of comminution and agglomeration (15 min to 8 h), and the transition from agglomeration to aggregation (after 8 h).
(2) The SBET was much larger than SG and their changing trends were different, while the aggregation occurred in the later stage of MA, then both SG and SBET decreased.
(3) Plastic deformation, reduction in crystallite size, and the increase in lattice strain and amorphization degree occurred after MA. In 8 h of milling treatment, the agglomeration and the microstructure did not affect each other, after that the crystallite size reached a saturation value, the chalcopyrite particles were sintered and tightly compressed, and aggregation took place.
(4) The promoting effect of amorphization and surface area on leaching was important, while, the hindering effect of aggregation on leaching was severer than that of agglomeration. After milling for 8 h (before aggregation), the Cu leaching rate reached its maximum value of 80.13%.
Acknowledgments
This work was supported by the Special Fund for the National Natural Science Foundation of China (U1608254), and the National Key R&D Program of China (2018YFC1902002).
References
[1] WATLING H R. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate- chloride and sulfate-nitrate process options [J]. Hydrometallurgy, 2013, 140: 163-180.
[2] PAN Hao-dan, YANG Hong-ying, TONG Lin-lin, ZHONG Cong-bin, ZHAO Yu-shan. Control method of chalcopyrite passivation in bioleaching [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(9): 2255-2260.
[3] KLAUBER C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution [J]. International Journal of Mineral Processing, 2008, 86(1-4): 1-17.
[4] SHIERS D W, COLLINSON D M, KELLY N J, WATLING H R. Copper extraction from chalcopyrite: Comparison of three non-sulfate oxidants, hypochlorous acid, sodium chlorate and potassium nitrate, with ferric sulfate [J]. Minerals Engineering, 2016, 85: 55-65.
[5] WU Shi-fa, YANG Cong-ren, QIN Wen-qing, JIAO Fen, WANG Jun, ZHANG Yan-sheng. Sulfur composition on surface of chalcopyrite during its bioleaching at 50 °C [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(12): 4110-4118.
[6] WANG M H, ZHANG Y, DENG T L, WANG K. Kinetic modeling for the bacterial leaching of chalcopyrite catalyzed by silver ions [J]. Minerals Engineering, 2004, 17(7-8): 943-947.
[7] KAMALI A R, VAHDATI KHAKI J. Copper leaching from nanoparticles of chalcopyrite concentrate [J]. Russian Journal of Non-ferrous Metals, 2008, 49: 138-143.
[8] DUTRIZAC J E. Elemental sulphur formation during the ferric chloride leaching of chalcopyrite [J]. Hydrometallurgy, 1990, 23(2-3): 153-176.
[9] FARHANG M R, KAMALI A R, NAZARIAN-SAMANI M. Effects of mechanical alloying on the characteristics of a nanocrystalline Ti-50at.%Al during hot pressing consolidation [J]. Materials Science and Engineering B, 2010, 168(1): 136-141.
[10] MAURICE D, HAWK J A. Ferric chloride leaching of mechanically activated chalcopyrite [J]. Hydrometallurgy, 1998, 49(1-2): 103-123.
[11] MAURICE D, HAWK J A. Ferric chloride leaching of a mechanically activated pentlandite-chalcopyrite concentrate [J]. Hydrometallurgy, 1999, 52(3): 289-312.
[12] BAFGHI M S, EMAMI A H, ZAKERI A. Effect of specific surface area of a mechanically activated chalcopyrite on its rate of leaching in sulfuric acid-ferric sulfate media [J]. Metallurgical and Materials Transactions B: Process Metallurgy and Materials Processing Science, 2013, 44(5): 1166-1172.
[13] ACHIMOVICOVA M, BALAZ P, BRIANCIN J. The influence of mechanical activation of chalcopyrite on the selective leaching of copper by sulphuric acid [J]. Metalurgija, 2006, 45(1): 9-12.
[14] AGNEW C J, WELHAM N J. Oxidation of chalcopyrite by extended milling [J]. International Journal of Mineral Processing, 2005, 77(4): 208-216.
[15] MOHAMMADABAD F K, HEJAZI S, KHAKI J V, BABAKHANI A. Mechanochemical leaching of chalcopyrite concentrate by sulfuric acid [J]. International Journal of Minerals, Metallurgy and Materials, 2016, 23(4): 380-388.
[16] VAFAEIAN S, AHMADIAN M, REZAEI B. Sulphuric acid leaching of mechanically activated copper sulphidic concentrate [J]. Minerals Engineering, 2011, 24(15): 1713-1716.
[17] JUHASZ A Z, OPOCZKY L. Mechanical activation of minerals by grinding, pulverizing and morphology of particles [M]. Chichester: Ellis Horwood, 1990.
[18] BALAZ P. Extractive metallurgy of activated minerals [M]. Amstedam: Elsevier, 2000.
[19] POURGHAHRAMANI P, FORSSBERG E. Changes in the structure of hematite by extended dry grinding in relation to imposed stress energy [J]. Powder Technology, 2007, 178(1): 30-39.
[20] BAFGHI M S, EMAMI A H, ZAKERI A, KHAKI J V. Development and verification of a mathematical model for variations of the specific surface area of mineral powders during intensive milling [J]. Powder Technology, 2010, 197(1-2): 87-90.
[21] GHAYOUR H, ABDELLAHI M, BAHMANPOUR M. Optimization of the high energy ball-milling: Modeling and parametric study [J]. Powder Technology, 2016, 291: 7-13.
[22] TAKACS L, PARDAVI-HORVATH M. Nanocomposite formation in the Fe3O4-Zn system by reaction milling [J]. Journal of Applied Physics, 1994, 75(10): 5864-5866.
[23] JONES G C, CORIN K C, van HILLE R P, HARRISON S T L. The generation of toxic reactive oxygen species (ROS) from mechanically activated sulphide concentrates and its effect on thermophilic bioleaching [J]. Minerals Engineering, 2011, 24(11): 1198-1208.
[24] OHLBERG S M, STRICKLER D W. Determination of percent crystallinity of partly devitrified glass by X-ray diffraction [J]. Journal of the American Ceramic Society, 1962, 45(4): 170-171.
[25] YEVENES L V, MIKI H, NICOL M. The dissolution of chalcopyrite in chloride solutions: Part 2: Effect of various parameters on the rate [J]. Hydrometallurgy, 2010, 103(1-4): 80-85.
[26] KING R P. Modeling and simulation of mineral processing systems [M]. Oxford: Butterworth- Heinemann, 2001.
[27] ZHAO Q Q, YAMADA S, JIMBO G. The mechanism and grinding limit of planetary ball milling [J]. Journal of the Society of Powder Technology, 1989, 302(7): 297-302.
[28] OPOCZKY L, FARNADY F. Fine grinding and states of equilibrium [J]. Powder Technology, 1984, 39(1): 107-115.
[29] EBADI H, POURGHAHRAMANI P. Effects of mechanical activation modes on microstructural changes and reactivity of ilmenite concentrate [J]. Hydrometallurgy, 2019, 188: 38-46.
[30] LI J J, HITCH M. Characterization of the microstructure of mechanically-activated olivine using X-ray diffraction pattern analysis [J]. Minerals Engineering, 2016, 86: 24-33.
[31] AKBARI B, TAVANDASHTI M P, ZANDRAHIMI M. Particle size characterization of nanoparticles: A practical approach [J]. Iranian Journal of Materials Science and Engineering, 2011, 8(2): 48-56.
[32] TKACOVA K. Mechanical activation of minerals [M]. Amsterdam: Elsevier, 1989.
[33] ZHAO Q Q, SAITO F. A review on mechanochemical syntheses of functional materials [J]. Advanced Powder Technology, 2012, 23(5): 523-531.
[34] CULLITY D B. Elements of X-ray diffraction second edition [M]. Reading MA: Addison-Wesley, 1978.
[35] GRANATA G, TAKAHASHI K, KATO T, TOKORO C. Mechanochemical activation of chalcopyrite: Relationship between activation mechanism and leaching enhancement [J]. Minerals Engineering, 2019, 131: 280-285.
[36] LUCKS I, LAMPARTER P, MITTEMEIJER E J. Uptake of iron, oxygen and nitrogen in molybdenum during ball milling [J]. Acta Materialia, 2001, 49(13): 2419-2428.
[37] BOLDYREV V V, PAVLOV S V, GOLDBERG E L. Interrelation between fine grinding and mechanical activation [J]. International Journal of Mineral Processing, 1996, 44-45: 181-185.
[38] ZHAO Zhong-wei, ZHANG You-xin, CHEN Xing-yu, CHEN Ai-liang, HUO Guang-sheng. Effect of mechanical activation on the leaching kinetics of pyrrhotite [J]. Hydrometallurgy, 2009, 99(1-2): 105-108.
[39] TKACOVA K, BALAZ P. Structural and temperature sensitivity of leaching of chalcopyrite with iron(III) sulphate [J]. Hydrometallurgy, 1998, 21(1): 103-112.
[40] SENNA M. Determination of effective surface area for the chemical reaction of fine particulate materials [J]. Particle & Particle Systems Characterization, 1989, 6(1-4): 163-167.
[41] AMMOU-CHOKROUM M, SEN P K, FOUQUES F. Electro-oxidation of chalcopyrite in an acid chloride medium: I. Merit and kinetics of the reaction [J]. Les Memoires Scientifiques de la Revue de Metallurgie, 1979, 4: 271-283.
[42] TROMANS D, MEECH J A. Enhanced dissolution of minerals: Stored energy, amorphism and mechanical activation [J]. Minerals Engineering, 2001, 14(11): 1359-1377.
赵素兴,王改荣,杨洪英,陈国宝,丘学民
东北大学 冶金学院,沈阳 110819
摘 要:研究机械活化对黄铜矿粒度参数、显微组织和浸出性能的影响,并探讨活化过程中团聚和集聚的发生和转变。结果表明,研磨8 h前黄铜矿的团聚与显微组织变化互不影响;然而,研磨8 h后晶粒尺寸的降低达到极限,显微组织变化停滞,使得团聚被集聚取代。浸出实验结果表明,机械活化能显著提高黄铜矿的浸出性能,而集聚对浸出的阻碍作用远强于团聚。经4 h酸浸实验后,活化8 h的矿样获得最高铜浸出率,其值为80.13%。
关键词:黄铜矿;机械活化;团聚;集聚;浸出
(Edited by Wei-ping CHEN)
Corresponding author: Hong-ying YANG, Tel: +86-13889803669, E-mail: yanghy@smm.neu.edu.cn
DOI: 10.1016/S1003-6326(21)65590-5
1003-6326/ 2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press

