Dissolution kinetics of malachite in ammonia/ammonium sulphate solution
来源期刊:中南大学学报(英文版)2012年第4期
论文作者:刘志雄 尹周澜 陈启元
文章页码:903 - 910
Key words:copper; malachite; kinetics; ammonia; ammonium sulphate
Abstract: The dissolution kinetics of malachite was investigated in ammonia/ammonium sulphate solution. The effects of ammonia and ammonium sulphate concentration, pH, leaching time, reaction temperature, and particle size were determined. The results show that the optimum leaching conditions for malachite ore with a copper extraction more than 96.8% are ammonia/ammonium concentration 3.0 mol/L NH4OH + 1.5 mol/L (NH4)2SO4, liquid-to-solid ratio 25:1 mL/g, leaching time 120 min, stirring speed 500 r/min, reaction temperature 25 °C and particle size finer than 0.045 mm. The dissolution process of malachite with an activation energy of 26.75 kJ/mol is controlled by the interface transfer and diffusion across the product layer. A semi-empirical rate equation is obtained to describe the leaching process and the reaction orders with respect to concentration of ammonia and ammonium sulphate are 2.983 0 and 0.941 1, respectively.
J. Cent. South Univ. (2012) 19: 903-910
DOI: 10.1007/s11771-012-1091-5![]()
LIU Zhi-xiong(刘志雄)1, 2, YIN Zhou-lan(尹周澜)1, HU Hui-ping(胡慧萍)1, CHEN Qi-yuan(陈启元)1
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. College of Chemistry and Chemical Engineering, Jishou University, Jishou 416000, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract: The dissolution kinetics of malachite was investigated in ammonia/ammonium sulphate solution. The effects of ammonia and ammonium sulphate concentration, pH, leaching time, reaction temperature, and particle size were determined. The results show that the optimum leaching conditions for malachite ore with a copper extraction more than 96.8% are ammonia/ammonium concentration 3.0 mol/L NH4OH + 1.5 mol/L (NH4)2SO4, liquid-to-solid ratio 25:1 mL/g, leaching time 120 min, stirring speed 500 r/min, reaction temperature 25 °C and particle size finer than 0.045 mm. The dissolution process of malachite with an activation energy of 26.75 kJ/mol is controlled by the interface transfer and diffusion across the product layer. A semi-empirical rate equation is obtained to describe the leaching process and the reaction orders with respect to concentration of ammonia and ammonium sulphate are 2.983 0 and 0.941 1, respectively.
Key words: copper; malachite; kinetics; ammonia; ammonium sulphate
1 Introduction
Copper generally is found in nature in the form of oxidized and sulfide minerals, such as azurite [Cu3(OH)2(CO3)2], malachite [Cu2(OH)2CO3], chrysocolla (CuSiO3×2H2O), chalcopyrite (CuFeS2) covelline (CuS) and bornite (Cu5FeS4 ). In the past years, copper was mostly produced from sulfide ores by pyrometallurgical operations because sulfide ores are easily separated from gangue and concentrated by conventional flotation techniques. Recently, the depletion of high-grade copper sulfide ores has led to the focusing on the extraction of copper from oxidized copper ores in various carbonate and silicate minerals [1-6].
Unlike copper sulfides treated by pyrometallurgical operations, the processing of oxidized copper ore needs to follow different approaches. The oxidized copper ores are soluble either in acidic or alkaline medium at room temperature. Hydrometallurgical methods of processing ores or their concentrates have an increasing importance in the extraction of oxidized copper ore. Hydrometallurgical processes including leaching, solvent extraction and electrowinning (L-SX-EW) were applied [7]. Sulfuric acid is the most usual lixiviant for oxidized copper ore, and owing to the different nature of ores, acid consumption ranges from 0.4 to 0.7 t H2SO4 for 1 t copper recovered. Among oxidized copper minerals, malachite occurs as a secondary copper mineral in the upper oxidized zone of copper deposits, and it is soluble in acid medium. The leaching kinetics of malachite in sulfuric acid [8-9] and the extraction of copper from raffinate via solvent extraction (SX) have been studied intensively [10-11].
However, acid leaching of oxidized copper ores has some disadvantages. Not only azurite, malachite and copper silicates will consume acid, but also other gangue minerals, such as limestone and dolomite, usually consume acid [12-13]. The preponderance of carbonates in copper ores often causes excessive increase in the acid consumption. Moreover, many other metals, such as Fe, and Al, can be dissolved with sulfuric acid, which brings about the difficulty of purification. Therefore, a cost-effective leaching route needs to be developed and more selective reagents are necessary for the processing of oxidized copper ores. Alkaline leaching, which is more selective, less corrosive and has lower reagent consumption for calcareous or carbonated gangue, has attracted a lot of interests of researchers.
Sodium hydroxide, lime and ammonia are the most commonly used alkaline reagents. A large number of leaching systems in which complex ions are formed between the metal cation and complexing species in solutions are widely utilized. Ammonium hydroxidized is extensively used in nickel, cobalt, and copper industries due to the formation of metal-ammonia complexes which are highly soluble in ammonia and ammonium salt solutions. Ammonium hydroxidized or ammonia/ ammonium carbonate/chlorite leaching system is commonly applied in the leaching of malachite [14-18].
ARZUTUG et al [14] studied the dissolution of malachite in NH3-saturated water solution and examined the leaching kinetics by applying statistical method to the experimental data. They claimed that the leaching rate was fitted to a pseudo-second-order kinetic model with an activation energy of 85.16 kJ/mol.
BING?L et al [17] investigated the dissolution of malachite in ammonia/ammonium carbonate medium and found that the leaching process was controlled by the interface transfer and diffusion across the product layer and that the activation energy was 15 kJ/mol. YARTA?I and ?OPUR [18] studied the dissolution kinetics of malachite in ammonium chloride solution and a mathematical model was derived for the dissolution process which is controlled by diffusion through the product film.
K?NK?L et al [19] studied the leaching kinetics of malachite in ammonia solution and deduced a mathematical model for the leaching process with the activation energy of 22.338 kJ/mol. They declared that the leaching process was controlled by diffusion through the ash film. A kinetic study performed by Oudenne and Olson was reported that the leaching of malachite in ammonium carbonate solution took place in two stages [15]. The rate equations were defined as 1-(1-x)1/3=k1t for stage I and 1-(1-x)1/2=k2t for stage II (where x is the reaction fraction of the solid, k1 and k2 are rate constants, and t is the leaching time). The activation energies were 64 kJ/mol for stage I and 75 kJ/mol for stage II. EKMEKYAPAR and OYA [20] suggested that the dissolution rate of malachite in ammonium chloride solution was controlled by mixture kinetics and that the activation energy for the dissolution reaction was calculated as 71 kJ/mol. The semi-empirical model proposed to represent the reaction kinetics was 1-2(1-x)1/3+(1-x)2/3=kt.
Generally, these leaching kinetics investigations of malachite have been carried out in acid, ammonia, ammonia/ammonium carbonate and ammonia/ ammonium chloride media, but the leaching kinetic characteristics of malachite in ammonia/ammonium sulphate solutions have been rarely reported.
Tangdan oxidized copper ore, which contains high-grade calcium-magnesium carbonate gangues and has poor floatable characteristics, is one of the largest copper mine in China with a reserve of about 115×104 t. Its average content of copper is 0.75% (mass fraction) and the phase composition of copper is quite complex. The specimen mineral of malachite was from Tangdan. The leaching of the malachite ore in ammonia/ammonium sulphate solution is investigated to provide basal data for industry.
2 Experimental
2.1 Materials
The oxidized copper ore, which was a specimen mineral, was from Tangdan, Yunnan Province, China. The chemical composition of the sample ore is listed in Table 1. The element contents of copper, iron, magnesium, chromium, calcium and manganese were analyzed using an atomic absorption spectrophotometer (AAS). Zn content was determined by inductively coupled plasma atomic emission spectroscopy (ICP- AES, Thermo Fisher Scientific, IRIS Intrepid II XSP). Sulfur was determined by LECO-444SC analyzer. Quartz content was analyzed by classical digestion with HF method.
Table 1 Main chemical compositions of oxidized copper ore (mass fraction, %)

Mineralogical analysis was performed by X-ray diffractometer using a Philips RigakuTM DMAX 2250VB model. As shown in Fig. 1, it was indicated that the major mineral phases in the sample were malachite, dolomite and quartz.
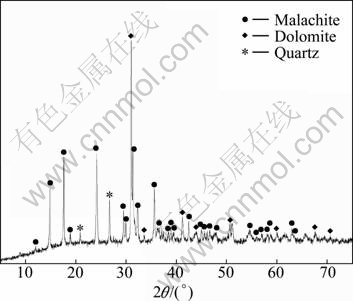
Fig. 1 X-ray diffraction pattern of copper ore
The copper ore was crushed and ground by using crusher, roller and mill, and sieved to gain the desired particle size fractions of 0-0.045, 0.045-0.075, 0.075- 0.150 and 0.150-0.212 mm for the leaching experiments. The experiments regarding the effects of ammonia/ ammonium sulphate concentration, liquid-to-solid ratio, temperature and stirring speed were performed with the ore sample of 0.100-0.150 mm. The experiments, in which the effects of particle size were studied, were carried out using ores with different sizes. pH measurements were carried out with LEICI PHS-3C. Leaching solutions were prepared using reagent grade chemicals (Ammonia, 25.7% NH3(aq); Ammonium sulphate, 99.5%) and distilled water.
2.2 Experimental procedure
A 500 mL round-bottomed split flask was used as a leaching reactor with four necked tops for taking samples from leaching solution. Mechanical stirrer, a mercury thermometer and a cooler were used to avoid evaporation loss of solution and ammonia. The desired temperature of the flask within the error of ±0.5 °C was adjusted by a thermo-statically controlled electric heating mantle. All experiments were controlled at temperatures between 15 and 55 °C. Agitation was provided by a mechanical stirrer. The 200 mL solution containing specific concentration of NH4OH/(NH4)2SO4 was added into the leaching reactor. After the desired stirring speed and reaction temperature were attained, solid sample was added to the solution in reactor. At selected time intervals, 2 mL of leaching solution sample was withdrawn from the reactor and filtered for analysis. The sample solution was analyzed for copper by an atomic absorbance spectrophotometer (AAS). At the same time, 2 mL fresh lixiviant was added into the reactor immediately to keep the volume of the solution constant. Filtration was made after each leaching experiment. The residue was weighed after being dried for 10 h at 110 °C.
3 Results and discussion
3.1 Dissolution principles
NH3 is a weak base and can form complexes with Cu2+ in aqueous solution. Within a specified range of pH, all of the complexes with Cu2+ in the solution are water-soluble, and they belong mainly to ![]() complex in the ammonia/ammonium salt aqueous solution whose pH value is between 9 and 12.
complex in the ammonia/ammonium salt aqueous solution whose pH value is between 9 and 12.
Malachite (Cu(OH)2×CuCO3) dissolves in NH4OH/ (NH4)2SO4 solutions by forming a stable ![]() complex. The ammonia leaching reactions for malachite take place as follows:
complex. The ammonia leaching reactions for malachite take place as follows:
![]() →
→
![]() (1)
(1)
![]() →
→
![]() (2)
(2)
The overall reaction for leaching can be written as
![]() →
→
![]() (3)
(3)
3.2 Effect of operation parameters on leaching of copper
3.2.1 Effect of agitation speed
The effect of agitation speed on the copper leaching was carried out in the range from 100 to 500 r/min. An adequate suspension of the solid was observed at 300 r/min and the copper dissolution was independent of the agitation above this speed. As a result, agitation speed was maintained at 500 r/min subsequently to eliminate the effect of agitation as a variable on the leaching rate. It is only indicated that hydrodynamic boundary layer around the particle has reached a limited value with the high stirring speed. Thus, diffusion across this minimum thickness boundary layer could still be the slow step. Therefore, additional experimental evidence such as the value of the activation energy and the application of kinetics model are needed to determine the rate controlling step.
3.2.2 Effect of leaching time
The effect of leaching time on the dissolution of malachite was investigated. The results are shown in Fig. 2. It is obvious that the extraction rate of copper increases with increasing the leaching time. At the beginning of leaching, the dissolution rate of malachite is very high. After 5 min, the copper extraction rate reaches 53.0%. After 25 min, the copper extraction rate increases slowly. This can be explained by the reason that the malachite is mainly dissolved and the content of malachite in the residue of leaching becomes lower. The copper extraction rate increases to 87.6% at the end of 180 min.
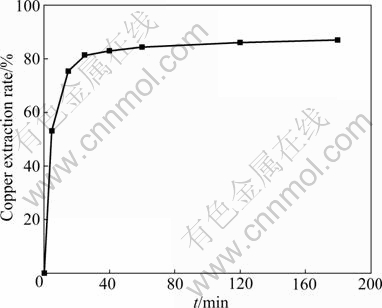
Fig. 2 Effect of leaching time on copper extraction of malachite (Ammoniacal concentration 3.0 mol/L NH4OH + 1.5 mol/L (NH4)2SO4; temperature 25 °C; particle size 0.100-0.150 mm; liquid-to-solid ratio 20 mL/g; stirring speed 500 r/min)
3.2.3 Effect of ammonia/ammonium concentration
The effects of ammonia concentration and pH of the leaching solution were investigated in ammonia solution. The results for the copper extraction and pH of the leaching solution are shown in Fig. 3. It is shown that the copper extraction rate increases with the increase of ammonia concentration. The extraction rate of copper increases greatly in the range of 0-5 mol/L NH4OH, and then it increases slowly. pH of the leaching solution increases with the increase of ammonia concentration. The copper extraction rate of malachite can reach 73.4% at 13.4 mol/L NH4OH, while it is only 7.5% at 1.0 mol/L NH4OH.
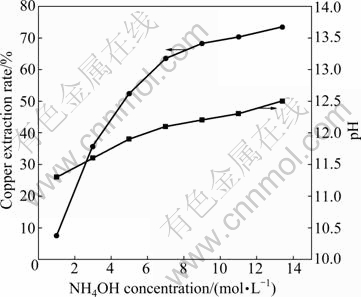
Fig. 3 Effect of NH4OH concentration on copper extraction of malachite and change of pH during leaching in ammonia solution (Temperature 25 oC; particle size 0.100-0.150 mm; liquid-to-solid ratio 20 mL/g; stirring speed 500 r/min; time 180 min)
The effect of various ammonia/ammonium sulphate concentration for the leaching of malachite was investigated in ammonia/ammonium sulphate solution. These results of copper extraction are given in Fig. 4. These results proclaim that the copper extraction rate increases with leaching time for different ammonia and ammonium concentrations, respectively. The copper extraction rate increases with increasing ammonia concentration (Fig. 4(a)) at ammonium sulphate concentration of 1.5 mol/L. With the increase of ammonia concentration, more reaction molecules attack the solid. The copper extraction rate increases with the concentration of ammonium sulphate (Fig. 4(b)) at ammonia concentration of 3.0 mol/L. At the same ammonia concentration, the copper extraction rate of copper in ammonia/ammonium solution is higher than that of copper in ammonia solution. This can be explained by the fact that ammonia/ammonium solution is a buffer solution and provides constant pH at a certain value for better forming copper-ammonia complexes. Considering ammonia volatility and low-cost production, 3.0 mol/L ammonia + 1.5 mol/L ammonium sulphate solution was determined as lixiviant for the following experiments.

Fig. 4 Effect of ammonia and ammonium sulphate concentration on copper extraction in ammonia/ammonium solution (Temperature 25 oC; particle size 0.100-0.150 mm; liquid-to-solid ratio 20/1 mL/g; stirring speed 500 r/min): (a) Ammonia; (b) Ammonium sulphate
3.2.4 Effect of particle size of samples
The effect of the particle size of samples on the leaching of malachite was studied. The results are given in Fig. 5. It is obvious that the extraction rate of copper increases as the particle size decreases. It can be taken for granted that the increase in the surface area causes better exposure of the malachite ore to solution with the decrease of the particle size.
3.2.5 Effect of liquid-to-solid ratio
The effect of liquid-to-solid ratio from 5 to 30 mL/g on the leaching of malachite was investigated at 25 °C. The results are given in Fig. 6. The result indicates that the increase of liquid-to-solid ratio can enhance the dissolution of copper. The larger the liquid-to-solid ratio, the faster the leaching rate. This can be explained by the fact that the amount of fluid reactant per unit surface of the solid increases with the increase of liquid-to-solid ratio. However, there is no obvious change in copper recovery when liquid-to-solid ratio is over 25 mL/g.
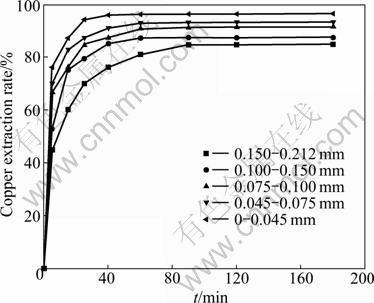
Fig. 5 Effect of particle size on copper extraction of malachite (Ammoniacal concentration 3.0 mol/L NH4OH + 1.5 mol/L (NH4)2SO4; temperature 25 °C; liquid-to-solid ratio 20 mL/g; stirring speed 500 r/min)

Fig. 6 Effect of liquid-to-solid ratio on copper recovery of malachite (Ammonia concentration 3.0 mol/L NH4OH + 1.5 mol/L (NH4)2SO4; temperature 25 °C; particle size 0.100- 0.150 mm; stirring speed 500 r/min; time 180 min)
3.2.6 Effect of temperature
The effect of reaction temperature on the dissolution of malachite was investigated at 15, 25, 35, 45 and 55 °C. In order to dissolve malachite completely, the liquid-to- solid ratio of 25 mL/g was adopted. As can be seen from Fig. 7, the extraction rate of copper increases with the increase of temperature. The extraction rate of copper after 120 min reaches 96.8% and 98.2% for 25 °C and 55 °C, respectively. But the effect of temperature on the copper extraction is insignificant after 90 min of leaching.
3.3 Discussion
XRD analyses of the leaching residues show that malachite is completely dissolved, whereas dolomite and quartz are not dissolved in the ammonia/ammonium sulphate solution. Removal of malachite by dissolution increases the content of other elements in the ore.
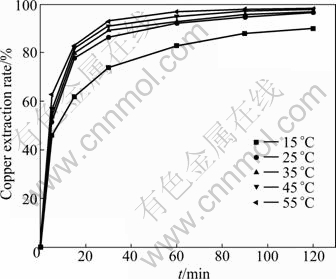
Fig. 7 Effect of temperature on copper extraction of malachite (Ammoniacal concentrations 3.0 mol/L NH4OH+1.5 mol/L (NH4)2SO4; particle size 0.100-0.150 mm; stirring speed 500 r/min)
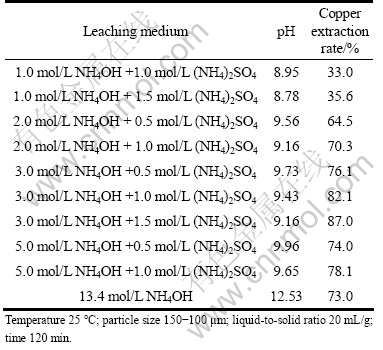
NH4OH+(NH4)2SO4 solution was applied as leaching medium in these experiments for the dissolution of malachite ore. The copper extraction rate and pH value of ammonia leaching medium for the leaching of malachite are listed in Table 2. When 3 mol/L NH4OH is used as leaching medium, maximum copper extraction is only 35% at 180 min (Fig. 3). And when the ammoniacal reagents (3.0 mol/L NH4OH + 1.5 mol/L (NH4)2SO4 solution) are used, the recovery can reach 87.0% at 120 min. When 13.4 mol/L NH4OH solution is used as leaching medium, the copper extraction is 73%.
Table 2 Relationship between pH and copper extraction in various ammonia leaching media
Kinetic evaluation of reactions which appear in the ammonia leaching of malachite illuminates that the leaching reactions are confirmed neither with chemical nor diffusion kinetic models of dissolution. These models are insufficient to account for the reaction of malachite at the inception of dissolution (i.e. 5-10 min) and for the total leaching period investigated, because no precision curves could be obtained from these two models. A new model, which is based on the interface transfer and diffusion across the product layer [21], is enough to explain the ammonia leaching of malachite. Equation of this model is written as follows:
![]() (4)
(4)
where x is the reacted fraction of malachite, k is the rate constant, and t is the leaching time.
Plot of ![]() versus leaching
versus leaching
time (t) at different temperatures from Eq. (4) is given in Fig. 8(a). Obviously, the model is fitted well to all date sets.
In order to determine the effect of experimental variables, including ammonia concentration and ammonium sulphate concentration, temperature and particle size (dp) on the dissolution kinetics, the following semi-empirical model is established:
![]()
![]() (5)
(5)
where T is the temperature, K; c(NH3·H2O) is the ammonia concentration; c[(NH4)2SO4] is the ammonium sulphate concentration; k0 is the apparent reaction rate coefficient; dp is the particle diameter, mm.
![]() (6)
(6)
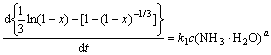 (7)
(7)
For different ammonia concentration, when other parameters are kept constant, Eq. (5) could be written as is the slope of the straight line corresponding to different ammonia concen-tration in Fig. 8(b). Similarly,
is the slope of the straight line corresponding to different ammonia concen-tration in Fig. 8(b). Similarly, ![]()
![]() is the slope of the straight line corresponding to ammonium sulphate concentration and particle size in Figs. 8(c) and (d), respectively. The value of ln[d[(1/3)ln(1-x)-[1-(1-x)-1/3]]/dt] versus ln[c(NH3·H2O)] is plotted to get a straight line in Fig. 9(a), and the slope is calculated to be 2.983, that is, the value of a is equal to 2.983. Similarly, the value of b and c could be estimated as 0.941 1 and -0.945 1, respectively, as given in Fig. 9.
is the slope of the straight line corresponding to ammonium sulphate concentration and particle size in Figs. 8(c) and (d), respectively. The value of ln[d[(1/3)ln(1-x)-[1-(1-x)-1/3]]/dt] versus ln[c(NH3·H2O)] is plotted to get a straight line in Fig. 9(a), and the slope is calculated to be 2.983, that is, the value of a is equal to 2.983. Similarly, the value of b and c could be estimated as 0.941 1 and -0.945 1, respectively, as given in Fig. 9.
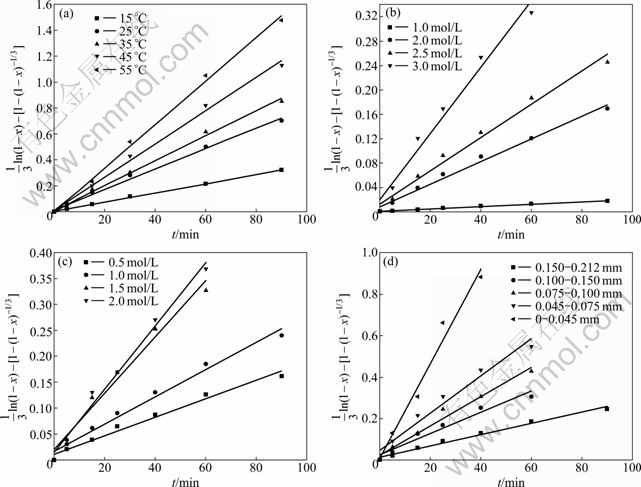
Fig. 8 Plots of![]() versus time at different operation parameters: (a) Temperature; (b) Ammonia concentration;(c) Ammonium sulphate concentration; (d) particle size
versus time at different operation parameters: (a) Temperature; (b) Ammonia concentration;(c) Ammonium sulphate concentration; (d) particle size
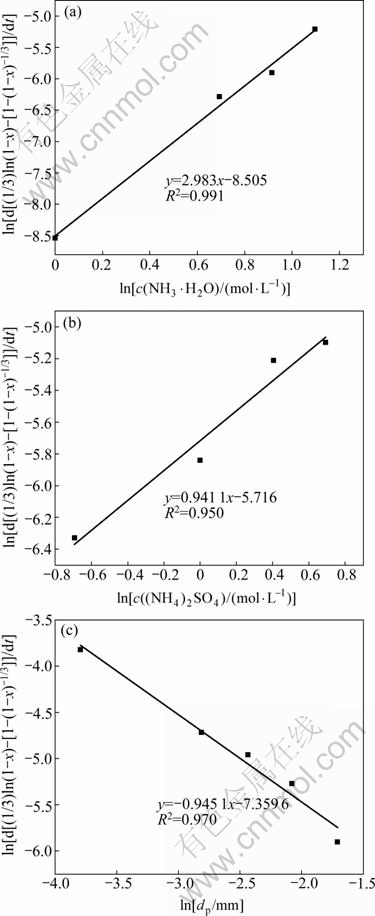
Fig. 9 Plots of ln[d[1/3ln(1-x)-(1-(1-x)-1/3)]/dt] versus ln[c(NH3·H2O)] (a), ln[c(NH4)3SO4] (b) and ln dp (c)
When the other parameters are fixed with temperature as the variable factor, the equation can be written as
![]() (9)
(9)
Using the fitted k value (Fig. 8(a)), Arrhennius curve for the dissolution of malachite is shown in Fig. 10. The calculated activation energy is 26.75 kJ/mol.
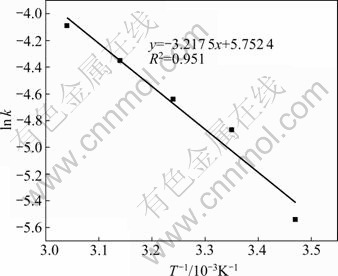
Fig. 10 Arrhenius curve obtained for dissolution of malachite ore
Substituting the value of a, b c and E into Eq. (5), and the value of k0 is calculated to be about 72.37 min-1 when the equation fits different straight lines in Fig. 8. Consequently, the model equation can be expressed as
![]()
![]()
4 Conclusions
1) The dissolution kinetic of malachite in ammonia/ ammonium sulphate solutions is investigated. It is observed that the main important parameters are determined as leaching time, ammonia/ammonium concentration, leaching temperature and particle size.
2) The optimal leaching conditions of malachite ore are found as ammonia/ammonium sulphate concentrations are 3.0 mol/L NH4OH + 1.5 mol/L (NH4)2SO4, liquid-to- solid ratio is 25 mL/g, leaching time is 120 min, leaching temperature is 25 °C, stirring speed is 500 r/min and particle size is finer than 45 μm. Under these conditions, the copper extraction rate could be as high as 96.8%.
3) The dissolution process of malachite was found to be controlled by mixture kinetics. The reaction rate is affected by both the interface transfer and diffusion across the product layer. The activation energy was found to be 26.75 kJ/mol. The model equation can be expressed as
![]()
![]()
References
[1] PEACEY J, GUO Xian-jian, ROBLES E. Copper hydrometallurgy: Current status, preliminary economics, future direction and positioning versus smelting [J]. Transactions of Nonferrous Metals Society of China, 2004, 14(3): 560-568.
[2] JU Shao-hua, TANG Mo-tang, YANG Sheng-hai, TANG Chao-bo. Thermodynamics of Cu(II)-NH3–NH4Cl-H2O system [J]. Transactions of Nonferrous Metals Society of China, 2005, 15(6): 1414-1419.
[3] ZHANG Jie, WU Ai-xiang, WANG Yi-ming, CHEN Xue-song. Experimental research in leaching of copper-bearing tailings enhanced by ultrasonic treatment [J]. J China Univ Ming and Technol, 2008, 18(1): 98-102.
[4] WATLING H R. The bioleaching of sulphide minerals with emphasis on copper sulphites—A review [J]. Hydrometallurgy, 2006, (84): 81-108.
[5] ANTONIJEVI? M M, DIMITRIJEVI? M D, STEVANOVI? Z O. Investigation of possibility of copper recovery from the floatation tailing by acid leaching [J]. Journal of Hazardous Materials, 2008, 158(1): 23-34.
[6] SUN Xi-liang, CHEN Bai-zhen, YANG Xi-yun, LIU You-yuan. Technological conditions and kinetics of leaching copper from complex copper oxide ore [J]. Journal of Central South University of Technology, 2009, 16(6): 936-941.
[7] STEVANOVI? Z, ANTONIJEVI? Z, AVRAMOVI? L, MARKOVI? R, BUGARIN M, TRUJI? V. Leach-SX-EW copper revalorization from overburden of abandoned copper mine Cerovo, Eastern Serbia [J]. Journal of Mining and Metallurgy Section B: Metallurgy, 2009, 45(1): 45-57.
[8] BING?L D, CANBAZO?LU M. Dissolution kinetics of malachite in sulphuric acid [J]. Hydrometallurgy, 2004, 72(1/2): 159-165.
[9] ATA O N, ?OLAK S, EKINCI Z, ?OPUR M. Determination of the optimum conditions for leaching of malachite ore in H2SO4 solutions [J]. Chemical Engineering and Technology, 2001, 24(4): 409-413.
[10] NAVARRO P, ALGUACIL F J. Extraction of copper from sulphate solutions by LIX 864 in escaid100 [J]. Technical Note Minerals Engineering, 1999, 12(3): 323-327.
[11] AMORES M, COEDO A G, ALUACIL F J. Extraction of copper from sulphate solutions by MOC 45: Application to Cu separation from leachates of a copper flue dust [J]. Hydrometallurgy, 1997, 47(1): 99-112.
[12] HABASHI F. Trends in the hydrometallurgical treatment of copper oxides ores [J]. Arab Mining Journal, 1983, 3(4): 46-52.
[13] ALGUACIL F J. Recovery of copper from ammoniacal/ammonium carbonate medium by LIX 973N [J]. Hydrometallurgy, 1999, 52(1): 55-61.
[14] ARZUTUG M E, KOCAKERIM M M, COPUR M. Leaching of malachite ore in NH3-saturated water [J]. Industrial & Engineering Chemistry Research, 2004, 43(15): 4118-4123.
[15] OUDNNE P D, OLSON F A. Leaching kinetic of malachite in ammonium carbonate solution [J]. Metallurgical Transactions B, 1983, 14(1): 33-40.
[16] WANG Xi, CHENG Qi-Yang, HU Hui-Ping, YIN Zhou-lan, XIAO Zhong-liang. Solubility prediction of malachite in aqueous ammoniacal ammonium chloride [J]. Hydrometallurgy, 2009, 99(3/4): 231-237.
[17] BING?L D, CANBAZO?LU M, AYD?AN S. Dissolution kinetic of malachite in ammonia/ammonium carbonate leaching [J]. Hydrometallurgy, 2005, 76(1/2): 55-62.
[18] YARTA?I A, ?OPUR M. Dissolution kinetic of copper (II) oxide in ammonium chloride solutions [J]. Minerals Engineering, 1996, 9(6): 639-698.
[19] K?NK?L A, KOCAKERIM M M, YAPICI S, DEMIRBA? A. Leaching kinetic of malachite in ammonia solution [J]. International Journal of Mineral Processing, 1994, 41(3/4): 167-182.
[20] EKMEKYAPAR A, OYA R. Dissolution kinetics of an oxidized copper ore in ammonium chloride solution [J]. Chemical and Biochemical Engineering Quarterly, 2003, 17(4): 261-266.
[21] DICKINSON C F, HEAL G R. Solid-liquid diffusion controlled rate equations [J]. Thermochimica Acta, 1999, 340/341: 89-103.
(Edited by HE Yun-bin)
Foundation item: Project(2007CB613601) supported by the National Basic Research Program of China; Project(10C1095) supported by the Foundation of Hunan Educational Committee, China
Received date: 2011-05-20; Accepted date: 2011-09-19
Corresponding author: YIN Zhou-lan, Professor, PhD; Tel: +86-731-88877364; E-mail: yzllxh@gmail.com

