Trans. Nonferrous Met. Soc. China 24(2014) 1807-1812
Controlled synthesis of one-dimensional Au-Ag porous nanostructures
Li-shan YANG1,2, Xiao-hu GU2
1. National & Local Joint Engineering Laboratory for New Petro-chemical Materials and Fine Utilization of Resources, Key Laboratory of Sustainable Resources Processing and Advanced Materials of Hunan Province College, Key Laboratory of Chemical Biology and Traditional Chinese Medicine Research, College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha 410081, China;
2. School of Materials Science and Engineering, Shandong University, Jin’an 250061, China
Received 29 September 2013; accepted 25 March 2014
Abstract: The fabrication of a new type of one-dimensional Au-Ag porous nanotube (NPT) structure was presented based on a facile combination of nanocrystal growth and surface modification. Ag nanowires with various diameters were firstly served as the chemical plating templates via a polyol-process. Then, one-dimensional (1D) Au-Ag porous nanostructures with tailored structural features could be prepared by controlling the individual steps involved in this process, such as nanowire growth, surface modification, thermal diffusion, and dealloying. Structural characterizations reveal these Au-Ag porous nanotubes, non-porous nanotubes and porous nanowires possess novel nano-architectures with multimodal open porosity and excellent structural continuity and integrity, which make them particularly desirable as novel 1D nanocarriers for biomedical, drug delivery and sensing applications.
Key words: one-dimension; Ag alloy; thermal diffusion; dealloying; porous nanostructure; nanotube
1 Introduction
One-dimensional (1D) nanostructures as a kind of important nanomaterials [1-5], have stimulated great and increasing interest due to their various potential applications, such as catalysis, optics, biomedicine, surface enhanced Raman scattering (SERS), and sensors [6-12]. A number of synthesis methods have been developed for the construction of 1D nanostructures (nanorods, nanowires and nanotubes), such as chemical vapor deposition (CVD) [13], lithography [14], template- directed synthesis [15], solvothermal synthesis [16], and surfactant assisted chemical synthesis [17].
Among these techniques, template-based synthesis is considered a facile and effective method to synthesize 1D noble metal nanomaterials [18-22]. For example, Ag nanowires can also be used as chemical templates to synthesize nanowires of other metals, such as, Au/Ag, Pd/Ag, and Pt/Ag alloys, through galvanic replacement reactions between the Ag nanowires with appropriate precursors [23]. In our previous study, Ag nanowires were served as “physical templates” followed a dealloying process to fabricate Au porous nanotubes (Au-PNTs) [24]. Later, we systematically investigated the effects of the reaction parameters on the growth of Au-Ag nanostructures, such as reaction temperature, species, concentration and molar ratios of precursors.
Herein, we present a detailed investigation on the controlled synthesis of 1D Au-Ag porous nanotubes (PNTs). The preparation and structural modulation process of 1D nanotubular structures is clearly shown in Scheme 1. Ag nanowires are used as “seeds”/templates to fabricate alloy composite nanowires. Then, with the modulation of various parameters, alloy nanotubes with smooth exterior and interior surfaces, gradient porous nanotubes with thicker exterior inert metal layer, 3D porous nanotubes, 3D porous nanowires (F) are readily produced. Au-Ag PNTs maintain the original 1D structure and dimension of the Ag nanowires and their walls are composed of interweaved ligaments and pores. Resemble to the nanoporous gold (NPG) structure prepared from dealloying bulk Au/Ag alloys [25], the PNTs show a bicontinuous structure and the ligaments are not made of nanoparticles but rather adopt a locally single crystalline structure with continuous lattice spanning from one ligament to the other. Based on their high surface area-to-volume ratio, PNTs can be exploited as an excellent candidate platform for the applications in optics, catalysis, sensing and electronics.
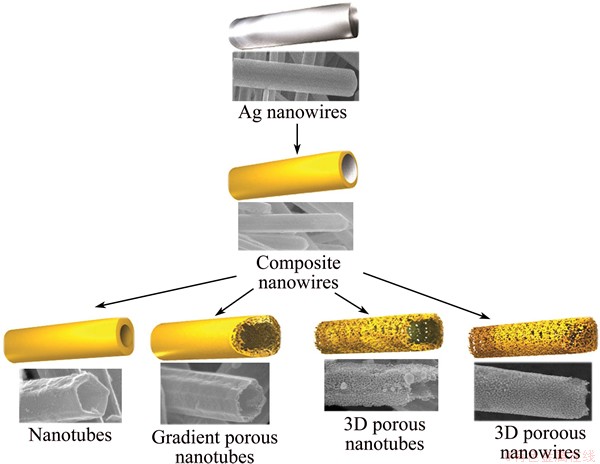
Scheme 1 Schematic illustration of preparation process of 1D hierarchical nanotubes
2 Experimental
2.1 Materials
AgNO3 (AR), ethylene glycol (EG, AR), HAuCl4·4H2O (AR), ascorbic acid (AR), cetyltrimethyl- ammonium bromide (CTAB; AR), concentrated nitric acid (HNO3; 67%, AR), C2H5OH (AR) and NaOH (AR) were purchased from Shanghai Sinopharm Chemical Reagent Co., Ltd., China Polyvinylpyrrolidone (PVP, 55000) was purchased from Sigma-Aldrich. All chemicals were used without further purification.
2.2 Synthesis of Ag nanowires
Ag nanowires were synthesized via a modified polyol process in our previous study [24]. In a typical experiment, 10 mL of ethylene glycol (EG) was first placed in a three-neck flask, and heated at 160 °C for 15 min. Then, 6 mL of an EG solution of AgNO3 (0.5 mol/L) and 6 mL of an EG solution of PVP (0.75 mol/L) were simultaneously added through a two-channel syringe pump at a rate of 0.375 mL/min to the flask, and kept in the oil bath for another 4 h. The final products (Ag nanowires) were thoroughly washed with ethanol several times, then collected by centrifugation, and finally re-dispersed in ultrapure water for further use.
2.3 Fabrication of Au-Ag alloy composite nanowires
In a typical synthesis process, 1 mL solution of the as-prepared Ag wires (0.02 mol/L) was dispersed in 17 mL mixed water solution containing 25 mmol/L CTAB and 6 mmol/L ascorbic acid (AA for short) under magnetic stirring, and heated at 40 °C for 10 min. Then 2 or 4 mL of 1 mmol/L HAuCl4 solution was added to the vial. The solution was kept stirring for another 40 min until the solution color became stable. The samples were washed with ultrapure water and centrifuged to remove excess Cl-, CTAB and ascorbic acid.
2.4 Preparation of hierarchical Au-Ag porous nanotubes
In a typical synthesis process, the Au-Ag composite nanowire samples were re-dispersed in 10 mL of ultrapure water, then transferred to a 20 mL autoclave, sealed and maintained at 400 °C for 2 h. After the thermal treatment, the products were etched with specific amount of concentrated HNO3 for 15 min, and finally washed with ultrapure water and NH3·H2O before characterization. For example, the 3D Au-Ag porous nanotubes can be synthesized by using Au-Ag composite nanowires fabricated from 2 mL of HAuCl4 as raw materials, followed with a thermal treatment at 450 °C and a dealloying process. Various kinds of hierarchical Au-Ag porous nanotubes could be obtained by tuning the amount of HAuCl4 and the thermal treatment temperature. Detailed results were described in the results and discussion.
2.5 Characterizations
Scanning electron microscopy (SEM) images were taken using a JEOL JSM-6700F field-emission scanning electron microscope operated at an accelerating voltage of 10 kV. Composition measurements were conducted with an Oxford INCA x-sight energy dispersive X-ray spectrometer (EDS) attached to the same microscope. Transmission electron microscopy (TEM) images and selected-area electron diffraction (SAED) patterns were taken using a JEOL JEM-2100 high-resolution transmission electron microscope (HRTEM) operated at an accelerating voltage of 200 kV. The UV-vis spectra were recorded using a Shimadzu UV-1700 UV-vis spectrophotometer.
3 Results and discussion
3.1 Synthesis of Ag nanowires
By adjusting both the concentrations and ratio of Ag precursor salt and PVP, Ag nanowires were synthesized with a series of diameters from 50 to 500 nm (Fig. 1). It was reported that at the initial stage of the process, small Ag seeds formed with the slow injection of new precursor, and heterogeneous nucleation occurred by enclosing a mixture of {111} and {100} facets to lower the total interfacial free energy. And Ag nanowires (or nanorods) can be produced as a result of the faster growth rate of {100} planes than {111} planes [6]. Here, Ag nanowires with smaller diameters can be synthesized at lower precursor concentration and slower precursor adding rate (Figs. 1(a) and (b)). Thus, it may be concluded that Ag {111} planes grow faster with higher precursor concentration and faster precursor adding rate.
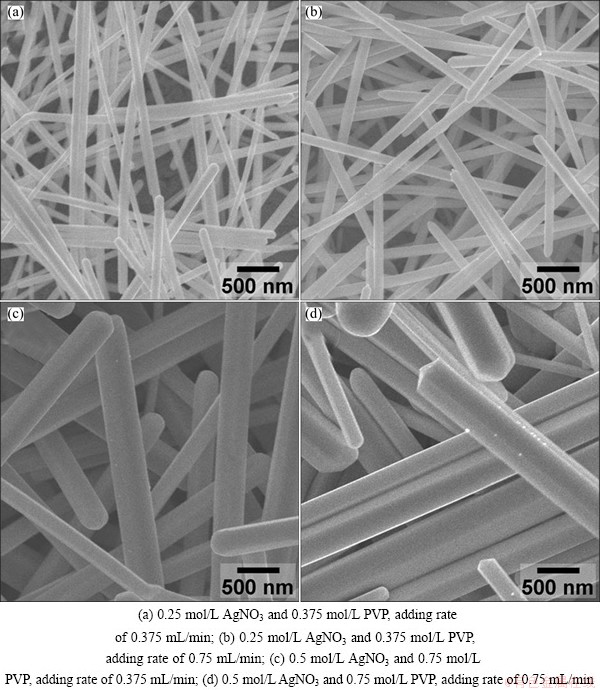
Fig. 1 SEM images of Ag nanowires fabricated under different conditions
3.2 Preparation of Au-Ag alloy composite nanowires
To produce the composite nanostructures, a quasi- seed mediated (i.e., Ag nanowires served as “seeds”/ templates during the chemical plating/surface modification process) approach is adopted here [26]. In this work, CTAB and AA are used as surfactant and reduction reagent, respectively. Due to the large difference in standard electrochemical potentials between [AuCl4]-/Au pair and Ag+/Ag pair, spontaneous galvanic displacement reaction also occurs at certain defect portions of the template surfaces. We hypothesize that the existence of galvanic displacement at the early stage of plating process can help the formation of small active Au sites on the Ag nanowire surface. With more reaction precursors added, more Au atoms are reduced by AA, and directed by CTAB to the pre-formed surface active sites. Alloy composite nanowires synthesized with different amounts of HAuCl4 are shown in Fig. 2. When the amount of HAuCl4 is less than 4 mL, the alloy composite nanowires well preserve the original morphologies of Ag nanowires (Figs. 2(a) and (b)). With the increasing amount of HAuCl4, the diameter of the composite nanowires increases and more depositions can be clearly found on the surface (Figs. 2(c) and (d)).
Noble metal nanomaterials exhibit distinctive surface plasmon resonance (SPR) features that are strongly dependent on the shape and structure. Thus the preparation process can be conveniently investigated by the UV-vis spectroscopic method. Ag nanowires have a characteristic absorption band around 392 nm with a small shoulder peak at 385 nm due to their structural anisotropy, and a series of UV-vis spectra followed the preparation process are shown in Fig. 3. At the early stage, obvious red-shift of the absorption peak can be seen. A few amount of HAuCl4 can make the characteristic absorption peak of Ag nanowires shift to long wavelength direction. When the amount of HAuCl4 increases to above 2 mL, the absorption peak of Au nanostructures can be found.
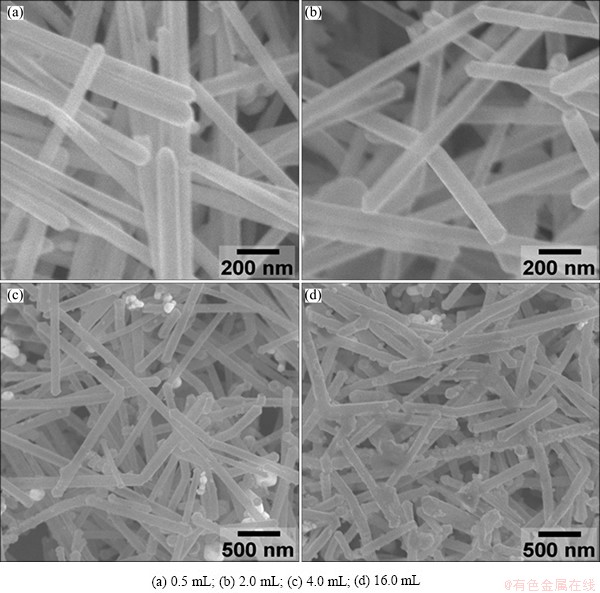
Fig. 2 SEM images of Au-Ag composite nanowires obtained with different amount of HAuCl4
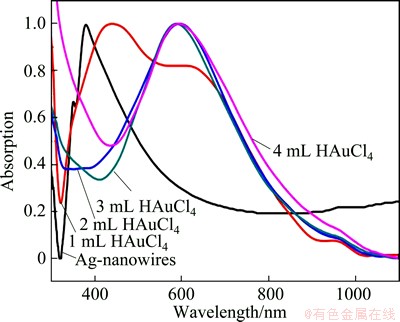
Fig. 3 UV-vis spectra of preparation process of Au-Ag composite nanowires with different amount of HAuCl4
3.3 Preparation of hierarchically structured Au-Ag porous nanotubes
Typical SEM and TEM images of Au-Ag porous nanotubes are shown in Fig. 4. Compared with Au-Ag porous nanotubes obtained from 4 mL HAuCl4, nanotubes fabricated from 2 mL HAuCl4 have a thicker tube wall and complete porous structures. Since both Ag and Au have a face-centered cubic structure and little mismatch in cell lattice parameters (4.086 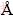 and 4.079
and 4.079  for Ag and Au, respectively), Au atoms tend to deposit on the Ag templates epitaxially. Here, thick Au layers can prevent the dissolution of internal Ag atoms, and finally result in different tubular structures with a smooth surface and smaller pores inside (Fig. 4(d)).
for Ag and Au, respectively), Au atoms tend to deposit on the Ag templates epitaxially. Here, thick Au layers can prevent the dissolution of internal Ag atoms, and finally result in different tubular structures with a smooth surface and smaller pores inside (Fig. 4(d)).
Besides the thickness of Au layers, the annealing temperature during the treatment of the Au-Ag composite nanowires can also influent the alloying process (or interdiffusion between exterior Au atoms and interior Ag atoms). Figure 5(a) shows a typical SEM image of Au-Ag PNTs with smaller pore diameter (compared to Fig. 4(c)), which are prepared with 4 mL HAuCl4 and annealed at 400 °C. However, when we reduce the annealing temperature below 350 °C, only tubular nanostructures with no porous structure can be obtained no matter how adjusting the amount of HAuCl4 (Fig. 5(b)). Moreover, if the Au-Ag composite nanowires are developed with 8 mL HAuCl4 followed by annealing at 500 °C for 2 h, Au-Ag porous nanowires without hollow structures inside are finally obtained (Fig. 5(c)). EDS analysis of the Au-Ag PNTs (samples in Figs. 4 and 5) indicates that the mole ratio of Ag to Au is in the range of 1:9-1:10. The exact alloying and dealloying processes during the formation of these Au-Ag one-dimensional porous structures is still on investigation.
Fig. 4 Typical SEM (a, c) and TEM (b, d) images of Au-Ag PNTs fabricated from 2 mL HAuCl4 (a, b), and 4 mL HAuCl4 (c, d)
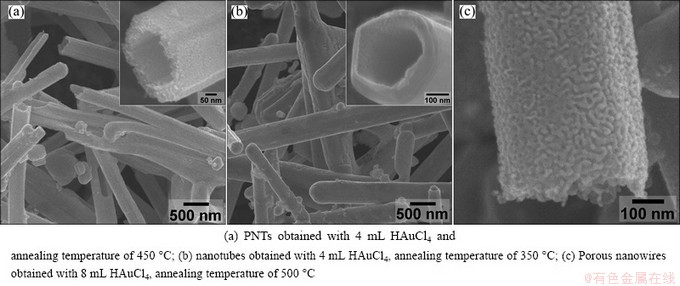
Fig. 5 Typical SEM images of Au-Ag PNTs under different annealing conditions
4 Conclusions
1) Novel 1D Au-Ag PNTs were fabricated based on an effective combination of nanocrystal growth and surface modification.
2) By controlling the individual steps involved in this process, such as nanowire growth, surface modification, thermal diffusion, and dealloying process, a new class of 1D metallic nanostructures can be prepared with tailored structural features and pre-designed functionalities.
3) With porous surfaces and hollow interiors, these tubular nanostructures show high surface areas, which make them particularly attractive for biomedical and sensing applications.
References
[1] XIA Y N, YANG P D, SUN Y G, WU Y D, MAYERS B , GATES B , YIN Y D, KIM F, YAN H Q. One-dimensional nanostructures: Synthesis, characterization, and application [J]. Advanced Materials, 2003, 15(5): 353-389.
[2] LIU W C, CAI W, MENG X L. Effects of temperature and pressure on morphologies of quasi-one-dimensional ZnO nanostructures fabricated via thermal evaporation [J]. Transactions of Nonferrous Metals Society of China, 2006, 16: 337-340.
[3] VAZQUEZ-MENA O, VILLANUEVA G, SAVU V, SIDLER K, van den BOOGAART M A F, BRUGGER J. Metallic nanowires by full wafer stencil lithography [J]. Nano Letters, 2008, 8 (11): 3675-3682.
[4] WIRTZ M, PARKER M, KOBAYASHI Y, MARTIN C R. Template- synthesized nanotubes for chemical separations and analysis [J]. Chemistry-A European Journal, 2002, 8(16): 3572-3578.
[5] WU M Z, QUAN G Y, LIU Y M, MA Y Q, DAI P, ZHANG L D. Comparative study of one-dimensional NiCo alloy nanostructures assembled by in situ and ex situ applied magnetic fields [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 1562-1566.
[6] SUN Y G, XIA Y N. Multiple-walled nanotubes made of metals [J]. Advanced Materials, 2004, 16(3): 264-268.
[7] KIJIMA T, YOSHIMURA T, UOTA M, IKEDA T, FUJIKAWA D, MOURI S, UOYAMA S. Noble-metal nanotubes (Pt, Pd, Ag) from lyotropic mixed-surfactant liquid-crystal templates [J]. Angewandte Chemie International Edition, 2004, 43(2): 228-232.
[8]  N, BARANOV D, IRSEN S, HILGENDORFF M,
N, BARANOV D, IRSEN S, HILGENDORFF M,  L M, GIERSIG M. Synthesis of flexible, ultrathin gold nanowires in organic media [J]. Langmuir, 2008, 24(17): 9855-9860.
L M, GIERSIG M. Synthesis of flexible, ultrathin gold nanowires in organic media [J]. Langmuir, 2008, 24(17): 9855-9860.
[9] XIA Y N, XIONG Y J, LIM B K, SKRABALAK S E. Shape- controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? [J]. Angewandte Chemie International Edition, 2009, 48(1): 60-103.
[10] LIANG H W, LIU S, GONG J Y, WANG S B, WANG L, YU S H. Ultrathin Te nanowires: An excellent platform for controlled synthesis of ultrathin platinum and palladium nanowires/nanotubes with very high aspect ratio [J]. Advanced Materials, 2009, 21(18): 1850-1854.
[11] DENG Q, LI X M, PENG Z S, LONG Y F, XIANG L M, CAI T J. Catalytic performance and kinetics of Au/λ-Al2O3 catalysts for low-temperature combustion of light alcohols [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 437-442.
[12] SHEN H X, YAO J L, GU R A. Fabrication and characteristics of spindle Fe2O3@Au core/shell particles [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 652-656.
[13] CHEN Z W, WAJE M, LI W Z, YAN Y S. Supportless Pt and PtPd nanotubes as electrocatalysts for oxygen-reduction reactions [J]. Angewandte Chemie International Edition, 2007, 46(22): 4060-4063.
[14] PYAYT A L, WILEY B, XIA Y N, CHEN A T, DALTON L. Integration of photonic and silver nanowire plasmonic waveguides [J]. Nature Nanotechnology, 2008, 3: 660-665.
[15] DEMIROK U K, LAOCHAROENSUK R, MANESH K M, WANG J. Ultrafast catalytic alloy nanomotors [J]. Angewandte Chemie International Edition, 2008, 47(48): 9349-9351.
[16] TAO A R, HUANG J X, YANG P D. Langmuir-blodgettry of nanocrystals and nanowires [J]. Accounts of Chemical Research, 2008, 41(12): 1662-1673.
[17] MURPHY C J, SAU T K, GOLE A M, ORENDORFF C J, GAO J X, GOU L F, HUNYADI S E, LI T. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications [J]. The Journal of Physical Chemistry B, 2005, 109(29): 13857-13870.
[18] CASWELL K K, BENDER C M, MURPHY C J. Seedless, surfactantless wet chemical synthesis of silver nanowires [J]. Nano Letters, 2003, 3(5): 667-669.
[19] PIETROBON B, McEACHRAN M, KITAEV V. Synthesis of size-controlled faceted pentagonal silver nanorods with tunable plasmonic properties and self-assembly of these nanorods [J]. ACS Nano, 2009, 3(1): 21-26.
[20] KIM, T Y, KIM W J, HONG S H, KIM J E, SUH K S. Ionic-liquid-assisted formation of silver nanowires [J]. Angewandte Chemie International Edition, 2009, 48(21): 3806-3809.
[21] GUO S J, DONG S J, WANG E K. Rectangular silver nanorods: Controlled preparation, liquid-liquid interface assembly, and application in surface-enhanced Raman scattering [J]. Crystal Growth & Design, 2009, 9(1): 372-377.
[22] SUKANTA D E, HIGGINS T M, LYONS P E, DOHERTY E M, NIRMALRAJ P N, BLAU W J, BOLAND J J, COLEMAN J N. Silver nanowire networks as flexible, transparent, conducting films: Extremely high dc to optical conductivity ratios [J]. ACS Nano, 2009, 3: 1767-1774.
[23] SUN Y G, XIA Y N. Mechanistic study on the replacement reaction between silver nanostructures and chloroauric acid in aqueous medium [J]. Journal of the American Chemical Society, 2004, 126(12): 3892-3901.
[24] GU X H, XU L Q, TIAN F, DING Y. Au–Ag alloy nanoporous nanotubes [J]. Nano Research, 2009, 2(5): 386-393.
[25] DING Y, KIM Y J, ERLEBACHER J. Nanoporous gold leaf: Ancient technology/advanced material [J]. Advanced Materials, 2004, 16(21): 1897-1900.
[26] BURDA C, CHEN X B, NARAYANAN R, EL-SAYED M A. Chemistry and properties of nanocrystals of different shapes [J]. Chemical Reviews, 2005, 105(4): 1025-1102.
可控合成一维金银合金多孔纳米结构
杨立山1,2,谷小虎2
1. 湖南师范大学 化学与化工学院,化学生物学及中药分析教育部重点实验室,
资源精细化与先进材料湖南省高校重点实验室,石化新材料与资源精细利用国家地方联合工程实验室,长沙 410081;
2. 山东大学 材料科学与工程学院,济南 250061
摘 要:报道了一种基于纳米晶体生长及表面修饰技术制备一维金银合金多孔纳米管的合成方法。实验采用多元醇热反应制备多种直径的银纳米线为前驱物模板,通过调控银线尺寸、表面修饰、热扩散、脱合金工艺等实验参数获得结构可控的一维金银多孔纳米结构。运用多种测试方法观察了无孔纳米管、多孔纳米管、多孔纳米线等多种一维结构的孔分布及连续结构。
关键词:一维;银合金;热扩散;去合金化;多孔结构;纳米管
(Edited by Hua YANG)
Foundation item: Project (2012CB932800) supported by the National Basic Research Program of China; Project (2012M521330) supported by China Postdoctoral Science Foundation
Corresponding author: Xiao-hu GU; Tel: +86-15064076660; E-mail: xhu533@163.com
DOI: 10.1016/S1003-6326(14)63257-X