
Development of Si3N4/Al composite by pressureless melt infiltration
Akhtar Farid, GUO Shi-ju
Institute of Powder Metallurgy, University of Science and Technology Beijing, Beijing 100083, China
Received 25 August 2005; accepted 20 December 2005
Abstract: Pressureless infiltration process to synthesize Si3N4/Al composite was investigated. Al-2%Mg alloy was infiltrated into Si3N4 and Si3N4 containing 10% Al2O3 preforms in the atmosphere of nitrogen. It is possible to infiltrate Al-2%Mg alloy in Si3N4 and Si3N4 containing 10% Al2O3 preforms. The growth of the dense composite of useful thickness was facilitated by the presence of magnesium powder at the interface and by flowing nitrogen. During infiltration Si3N4 reacted with aluminium to form Si and AlN, the growth of composite was found to proceed in two ways, depending on the Al2O3 content in the initial preform. Firstly, preform without Al2O3 content gives rise to AlN, Al3.27Si0.47 and Al type phases after infiltration. Secondly, perform with 10% Al2O3 content gives rise to AlN-Al2O3 solid solution phase (AlON), MgAl2O4, Al and Si type phases. AlON phase was only present in composite, containing 10% Al2O3 in the Si3N4 preforms before infiltration.
Key words: Si3N4 composite; infiltration; AlON; AlN-Al2O3 solid solution
1 Introduction
Composites of ceramic/metal or metal/ceramic are expected to have properties superior to their constituents alone[1-4]. However, the fundamental difference in atomic bonding between metals and ceramics results in quite different physical and chemical properties, such as surface energy, thermal expansion, chemical activity. These differences pose restrictions in fabrication of metal ceramic composites. For example non-wetting between ceramic surfaces and molten metals requires pressure or interfacial reactions to ensure good bonding between metals and ceramics[4-6]. The infiltration of molten metal into the porous ceramic preforms is an attractive processing route to prepare ceramic metal composites. The metal phase fraction, shape, size and distribution can be controlled through consolidation and densification processing of ceramic preforms. Moreover, porous preforms can be formed easily to complex shapes. Infiltration with metal can yield dense composites without large shrinkage associated with liquid phase sintering.
Pressureless melt infiltration is more attractive due to its cost effectiveness and near net shape capability. The essential requirements for pressureless melt infiltration of aluminium alloys are presence of magnesium in the alloy or infiltrating system and nitrogen in the furnace environment[7]. Binary Al-Mg alloys containing as low as 2% Mg will infiltrate spontaneously into ceramic preform at temperatures as low as 800 ℃ but infiltration stops after 1-2 mm[8].
Increasing the magnesium content or temperature enables the infiltration of thicker section before termination. It has been suggested that magnesium in the alloy evaporates and reacts with N2 in the furnace atmosphere and forms a coating of Mg3N2 on the ceramic reinforcement, which on coming in contact with molten aluminium gets reduced to aluminium nitride then by recycling magnesium[9]. Hence, it is supposed that the reaction between Mg3N2 and Al induces wetting and allows pressureless infiltration of Al-alloys into ceramic perform. Also, use of magnesium at the ceramic/metal interface can continue infiltration for longer period of time[10]. In contrast to the previous work, in this study the pressureless infiltration of Si3N4 with Al is discussed. The aim of this study directs to the synthesis of Si3N4/Al composite by melt infiltration process at temperature higher than 1 200 ℃.
2 Experimental
Silicon nitride preforms with 40% porosity were prepared by cold pressing Si3N4 powder (1 ?m). The procedure for the preparation of preforms is as follows. The Si3N4 powder was mixed with EBS wax and mixture was pored into the die cavity. Approximately 200 MPa pressure was applied to get cylindrical Si3N4 preforms, 10 mm in diameter and 7 mm in height. These green compacts were heated slowly up to 450 ℃ at 2 ℃/min in vacuum, so as to burn out the binder without changing the shape and dimension of the preforms. From 450 ℃ samples were heated to 700 ℃ in air and held there for 30 min to get enough strength for handling in further processing operations. The porosity of the preforms measured by coating the preforms with wax and using Archimedes principle was found to be approximately 39%. Since there is a possibility of wax entering the pores of the preform, this method slightly underestimates the actual porosity. Al and Mg powders were used to prepare Al-2%Mg compacts. The mixture of Al-2%Mg powder was poured into the die cavity and approximately 200 MPa pressure was applied to get cylindrical compacts, 10 mm in diameter and 6mm in height. No binder was used in making of Al-2%Mg compacts. Graphite crucibles were prepared to carry out infiltration experiments. Si3N4 preform was kept on the top of the Al-2%Mg compact with and without 10-15 mg magnesium powder at the Al-2%Mg compact and Si3N4 preform interface in graphite crucible as shown in Fig.1. The crucible containing Si3N4 preform and Al-2%Mg compact was introduced in the furnace and heated to 500 ℃ in vacuum at the rate of 10 ℃/min, then N2 atmosphere was introduced inside the furnace and the pressure of N2 was kept a little higher than atmospheric pressure in order to avoid the air entrance. Then temperature was raised to 1 100-1 400 ℃ at the rate of 10 ℃/min. Infiltration experiments were carried out in static N2 and flowing N2 atmospheres. The samples were held at infiltration temperature for 1 h and cooled inside the furnace.
Infiltrated samples were prepared metallographi- cally (parallel to the infiltration direction) to measure the infiltration depth by optical microscope. Infiltrated samples were ground to a powder and characterized by X-ray diffractometry(XRD) to identify the various phases present in the composite. Some infiltrated samples were mounted and polished metallographically to 0.5 ?m diamond finish for measurement by scanning electron microscopy. The above experimental procedure was repeated for the infiltration experiments on Si3N4-10%Al2O3 preforms.
3 Results and discussion
3.1 Infiltration
Infiltration experiments demonstrate that pressure- less infiltration of molten Al-alloys in Si3N4 preforms could occur if the correct process conditions are employed. The critical process conditions were found to be alloy composition and flowing nitrogen atmosphere. It is observed that the use of magnesium in the alloy and nitrogen atmosphere in conjunction with an appropriate process temperature results in infiltration. No infiltration occurs without magnesium in the alloy. Using the experimental arrangement in Fig.1, alloy Al-2%Mg, a Si3N4 preform and process conditions of 1 h soak at temperature larger than 1 200 ℃, partial infiltration incase of Al-2% Mg alloy and considerable infiltration thickness in case of Al-2%Mg alloy with 10-15 mg magnesium powder at the interface of Si3N4 preform and Al-2%Mg compact is observed. Infiltration experiments carried out on Si3N4-10%Al2O3 compact do not exhibit any measurable difference in the infiltration height. The results of infiltration experiments are summarized in Tables 1-3. The SEM micrographs before and after infiltration are shown in Fig.2.
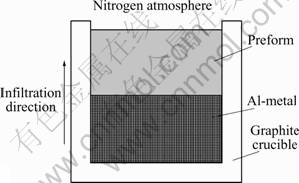
Fig.1 Schematic drawing of experimental arrangement used to fabricate Si3N4-Al Composites
Table 1 Effect of temperature on infiltration of Al-2% Mg alloy in Si3N4 preform

Table 2 Effect of 10-15 mg Mg at interface on infiltration height
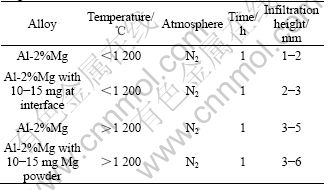
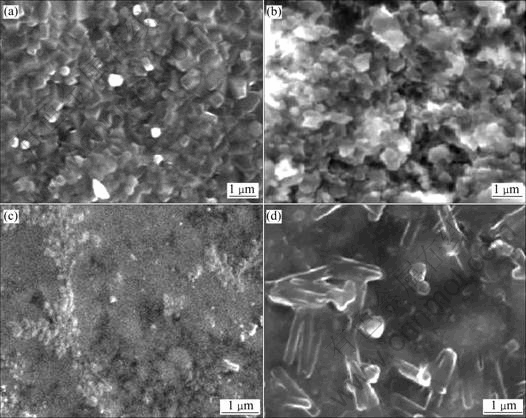
Fig.2 SEM micrographs before and after infiltration: (a) Si3N4 preform before infiltration; (b) Si3N4+10% Al2O3 preform; (c) Si3N4 preform after infiltration showing dense composite; (d) Si3N4+10% Al2O3 preform after infiltration showing dense composite
Table 3 Effect of addition of 10%Al2O3 on infiltration height in atmosphere of nitrogen
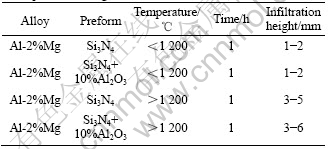
The above results demonstrate that the combination of magnesium in the alloy and at interface decreases the surface tension of the molten Al-alloy[10]. Nitrogen atmosphere causes further reduction of surface tension of Al-2% Mg alloy[11]. Additionally, the reactivity of magnesium induces interfacial reactions with solid ceramic surfaces. These reactions are typically not enough to promote spontaneous wetting, but in combining with nitrogen atmosphere, they may change or be altered thus allowing the observed infiltration. So, the use of magnesium at the interface is an extra support to the infiltration to proceed. Pure aluminium did not wet Si3N4 particles readily, so, no infiltration was observed. Whereas, magnesium level greater than the threshold value, the Si3N4 particles are wetted readily. While working at temperature higher than 1 200 ℃ the threshold magnesium level was found to be 2% by mass. The micrographs in Fig.2 show the developed com- posites are dense as no porosity is observed. The micrographs also show that there is severe chemical reaction between Si3N4 and Al-Mg Alloy because no Si3N4 particles are present in the micrographs. So, it can be concluded that reaction between Si3N4 and Al has been necessarily wetted in the presence of Mg and N2 atmosphere for the observed infiltration.
3.2 Growth of composite
Infiltration experiments were carried out on two types performs, Si3N4 preform and Si3N4-10%Al2O3 preform. 10%Al2O3 was added to increase the wetability of Si3N4 compact with Al. Very less difference in infiltration height was observed in these two types of preforms. But the resultant phases formed after infiltration were different depending on Al2O3 content in the initial preform. Composites developed by using Si3N4 preform without addition of Al2O3 showed AlN, Al2O3, Al3.27Si0.47 and Si phases after infiltration as shown in XRD results in Fig.3. Si3N4 preform with addition of 10% Al2O3 preform showed AlON, Al2O3, MgAl2O4, AlN and Si phases after infiltration as shown in XRD results in Fig.4.
In N2 atmosphere, magnesium can reduce the partial pressure of residual oxygen, therefore near the surface of the Al-Mg alloy melt, the O2 partial pressure is so low that melt will react with N2 and form a nitride. The formation of AlN is favorable above 1 000 ℃. So the surface of melt must be covered with a thin layer of AlN during infiltration[4]. Also liquid aluminium will react with silicon nitride to give AlN above 1 000 ℃ as follows.
4Al +Si3N4→4AlN+Si
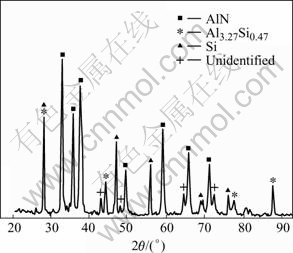
Fig.3 XRD pattern (Cu was used as target) of infiltrated Si3N4 composite

Fig.4 XRD pattern (Cu was used as target) of infiltrated Si3N4-10%Al2O3 composite
The silicon produced in above reactions will consume in formation of Al3.27Si0.47 and Si type phases. It is suggested that less amount of alumina will present in pure Si3N4 preform infiltrated with Al-2%Mg alloy because AlN formation is favored. So, the composite developed with infiltration of Si3N4 preform with Al-Mg Alloy contains AlN, Al2O3, Al3.27Si0.47 and Si type phases as shown in XRD spectra in Fig.3.
Composite developed by the infiltration of Si3N4- 10%Al2O3 compacts showed presence of AlON phase as shown in XRD spectra in Fig.4. In the case of Al2O3-Al-Mg-X the interfacial reaction of leads to the formation of magnesium oxides and mixed oxides (spinel). With small amount of Mg, aluminium alloys do not form any spinel while the wetting of particulate alumina is not extensive. Below 7%Mg, magnesium reacts with alumina to form spinel MgAl2O4 but this reaction decreases from >4% Mg and doesn’t take place over 7%-8% Mg where MgO is exclusively formed[12]. In the presence of additive like MgAl2O4 the AlON can be stabilized at the infiltration temperature T>1 300 ℃[13, 14]. It is suggested that AlON phase will be present only in the areas rich in MgAl2O4 spinel. So, addition of 10% Al2O3 in the initial preform gives rise to AlON, Al2O3, MgAl2O4, AlN and Si type phases as shown in XRD spectra in Fig.4.
References
[1] HANABE M, ASWATH P B. Synthesis of in-situ reinforced Al composites from Al—Si—Mg—O precursors [J]. Acta Materialia, 1997, 45: 4067-4076.
[2] SWAMINATHAN S, SRINIVASA RAO B, JAYARAM V. The production of AlN-rich matrix composites by the reactive infiltration of Al alloys in nitrogen [J]. Acta Materialia, 2002, 50: 3095-3106.
[3] TRAVITZRY N A. Microstructure and mechanical properties of alumina/copper composites fabricated by different infiltration techniques [J]. Materials Letters, 1998, 36: 114-117.
[4] TRAVITZRY N A, GUTMANAS E Y, CLAUOSEN N. Mechanical properties of Al2O3/Si composites fabricated by pressureless infiltration technique [J]. Materials Letters, 1997, 33: 47-50.
[5] TAHA M, EL-MAHALLAWY N A. Metal–matrix composites fabricated by pressure-assisted infiltration of loose ceramic powder [J]. Journal of Materials Processing Technology, 1998, 73: 139-146.
[6] SAIZ E, FOPPIANO S, MOBERLYCHAN W, TOMSIA A P. Synthesis and processing of ceramic–metal composites by reactive metal penetration [J]. Composite-A, 1999, 30: 399-403.
[7] AGHAJANIAN M K, ROCAZELLA M A, BURKE J T, KECK S D. The fabrication of metal matrix composites by a pressureless infiltration technique [J]. Journal of Materials Science, 1991, 26: 447-454.
[8] RAO B S, JAYARAM V. The initiation and continuation of infiltration Al-Mg based alloys into Alumina performs [A]. The Third PRICM Conference of Advanced Materials and Processing [C]. Hawaii, 1998. 367-373.
[9] SCHIROKY G H, MILLER D V, AGHAJANIAN M K, FAREED A S. Fabrication of CMCs and MMCs using novel processes [J]. Key Engineering Materials, 1997, 127-131: 141-152.
[10] RAO B S, JAYARAM V. New technique for pressureless infiltration Al-alloys into Al2O3 preforms [J]. Journal of Materials Research, 2001, 16: 2906-2913.
[11] GOICOECHEA J, GARCIA CORDOVILLA G, LOVIS E, PARMIE A. Surface tension of binary and ternary aluminium alloys of the systems Al-Si-Mg and Al-Zn-Mg [J]. Journal of Materials Science, 1992, 27: 5247-5251.
[12] MCCULLOUGH C, GALUSKA P, PITTMAN S R. Criteria for matrix selection in continuous fiber aluminum matrix composites [A]. Design Fundamentals of High Temperature Composites, Intermetallics and Metal-Ceramics Systems[C]. USA: Anaheim, California, 1996. 15-28.
[13] BOEY F, CAO L, KHOR K A, TOK A. Phase reaction and sintering behavior of a Al2O3-20wt%AlN-5wt%Y2O3 system [J]. Acta Materialla, 2001, 49: 3117-3127.
[14] WEI L Y, NAN L, RUNZHANG Y. The formation and stability of γ-aluminium oxynitride spinel in the carbothermal reduction and reaction sintering processes [J]. Journal of Materials Science, 1997, 32: 979-982.
Corresponding author: Akhtar Farid; E-mail: faridmet22@hotmail.com
(Edited by LONG Huai-zhong)